Back to Journals » Journal of Pain Research » Volume 8
Low-dose naloxone provides an abuse-deterrent effect to buprenorphine
Authors Webster L , Smith M, Unal C, Finn A
Received 19 June 2015
Accepted for publication 20 August 2015
Published 4 November 2015 Volume 2015:8 Pages 791—798
DOI https://doi.org/10.2147/JPR.S90780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Lynn R Webster,1 Michael D Smith,1 Cemal Unal,2 Andrew Finn3
1PRA Health Sciences, Salt Lake City, UT, USA; 2Biometrical Solutions LLC, Raleigh, NC, USA; 3BioDelivery Sciences International, Inc., Raleigh, NC, USA
Abstract: In developmental research, plasma buprenorphine concentrations comparable to a 2 mg buprenorphine–naloxone (BN) sublingual tablet have been achieved with a 0.75 mg dose of BN buccal film, a small, bioerodible polymer film for application to mucosal membranes. This was a randomized, double-blind, placebo-controlled, single-dose, four-period crossover study in opioid-dependent subjects with chronic pain receiving >100 mg oral morphine equivalents daily who experienced withdrawal following a naloxone challenge dose. The objective of the study was to determine if intravenous (IV) naloxone doses of 0.1 and 0.2 mg would produce a withdrawal response when coadministered with a 0.75 mg IV dose of buprenorphine. Fifteen subjects receiving 90–1,260 mg oral morphine equivalents per day enrolled and completed the study. Precipitated withdrawal occurred in 13% (2/15) of placebo-treated subjects and 47% (7/15) of buprenorphine-treated subjects. When combined with the 0.75 mg dose of buprenorphine, a 0.1 mg dose of naloxone increased the incidence of precipitated withdrawal to 60%, and a 0.2 mg dose of naloxone increased the incidence to 73%. By 15 minutes postdose, the mean change in Clinical Opioid Withdrawal Scale (COWS) score from predose was 3.0 for placebo, 6.9 for buprenorphine, 9.8 for BN 0.1 mg, and 12.4 for BN 0.2 mg. The mean COWS score with each active treatment was significantly greater than placebo (P<0.001), and the mean COWS score for each of the naloxone-containing treatments was significantly greater than for buprenorphine alone (P<0.001). Naloxone doses as low as 0.1 mg added an abuse-deterrent effect to a 0.75 mg IV dose of buprenorphine.
Keywords: opioid dependence, withdrawal symptoms, abuse-deterrent, buprenorphine, naloxone, intravenous
Introduction
Opioid use and dependence has reached epidemic proportions in the United States. Recent estimates suggest that more than 2 million Americans are dependent on prescription analgesics, and nearly 500,000 are dependent on heroin.1 As a consequence, drug misuse or abuse accounts for approximately 2.5 million emergency department visits annually,2 and death among opioid users is 5.7 times more common than in age- and sex-matched controls.3 Despite being highly prevalent and imposing a burden of illness that includes elevated risks of mortality due to suicide, homicide, infectious disease, and liver-related disease;3 increased probability of mental illness; poor quality of life; social stigma; and unemployment,4 fewer than half of the patients with opioid dependence receive medical care for their condition, often because they are not ready to stop using or they have no health coverage and cannot afford it.1
Treatment of opioid-dependent patients often involves substitution therapy with buprenorphine or methadone in combination with psychosocial modalities.5,6 The abuse potential and risk of death from overdose with buprenorphine may be lower than with full μ-agonists (heroin, oxycodone),7–9 but it is present. In order to deter efforts to extract buprenorphine from commercial products for intravenous (IV) abuse, buprenorphine is combined with naloxone, an opioid receptor antagonist that is poorly absorbed from the oral mucosa, but completely active on IV administration. Coadministration of IV naloxone and buprenorphine occupies the μ-receptors first and reduces the pleasant effects of buprenorphine. In subjects receiving full μ-agonists, IV naloxone will displace the substance from the μ-receptor with resultant precipitated withdrawal. The inclusion of naloxone in buprenorphine–naloxone (BN) combinations reduces the potential for parenteral abuse while maintaining the benefits of buprenorphine alone.10,11
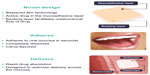
Buprenorphine/naloxone buccal film (BBN; Bunavail®, BioDelivery Sciences International, Inc., Raleigh, NC, USA) consists of a small, bilayered dissolvable polymer film that adheres to the buccal mucosa, using the BioErodible MucoAdhesive (BEMA®; BioDelivery Sciences International, Inc.) technology (Figure 1). The BEMA® technology considerably increases buprenorphine bioavailability and, compared with other routes of administration, BBN requires substantially lower doses of buprenorphine to manage patients with opioid dependence. Unpublished research associated with the development of BBN suggested that a buprenorphine dose as low as 0.75 mg may produce plasma concentrations comparable to published concentrations with a 2 mg dose of BN sublingual tablets (~1.0 ng/mL). Such a dose of buprenorphine would require the use of a naloxone dose lower than the 0.5 mg dose that is combined with the 2 mg dose of buprenorphine. This study was conducted to determine 1) the minimum effective dose of naloxone that produces a withdrawal response when administered with buprenorphine 0.75 mg in opioid-dependent subjects and 2) whether administration of buprenorphine 0.75 mg without naloxone produces a withdrawal response in opioid-dependent subjects.
Methods
Subjects
Men and women at least 21 years of age and generally in good health (ie, American Society of Anesthesiologists Physical Status Classification System Class I or II12) were enrolled between July 18, 2011 and September 9, 2011. Since patients receiving chronic opioid therapy are physically dependent and therefore can serve as appropriate assays for the investigation of legal and illegal opioids, subjects were eligible to participate if they had chronic moderate-to-severe noncancer pain and were being treated with a stabilized dose of opioid >100 mg morphine equivalents per day for at least 3 months. They were also eligible for participation if they displayed signs and symptoms of withdrawal (ie, Clinical Opioid Withdrawal Scale [COWS13] total score ≥5) following naloxone challenge; were fluent in English and able to provide meaningful written informed consent for the study; were not pregnant, lactating, or of childbearing potential; and agreed to abide by all study restrictions and comply with all study procedures.
Subjects were excluded if they had clinically unstable cardiac disease, a QTc interval >450 ms, a history of or an immediate family member with Long QT syndrome; were taking Class IA antiarrhythmic medications (eg, quinidine, procainamide, disopyramide) or Class III antiarrhythmic medications (eg, sotalol, amiodarone, dofetilide); a known allergy or history of significant adverse reaction to buprenorphine, naloxone, or related compounds; a body mass index >45 kg/m2; had been diagnosed with cancer within the 5 years before screening; a documented history of alcohol and/or substance abuse; a positive alcohol breath test or a positive urine drug screen for nonopioids; a history or current diagnosis of a significant psychiatric disorder; clinically significant abnormalities in clinical chemistry, hematology, or urinalysis at screening; participated in any investigational product or device study within 30 days before screening or were scheduled to participate in an investigational device or another investigational drug study during the course of this study; tested positive for Hepatitis B surface antigen, Hepatitis C antibody, or human immunodeficiency virus; donated blood or had a significant blood loss within 30 days before screening.
Institutional review board (IRB)-approved informed consent was obtained from all eligible subjects by Compass IRB (Mesa, AZ, USA) prior to any assessments being conducted, in accordance with written consent guidelines and the mandates of Good Clinical Practice and the Declaration of Helsinki.14
Design
This was a randomized, double-blind, placebo-controlled, four-period crossover study. Eligible subjects entered an inpatient facility on two separate occasions for 6 days and 5 nights each. During the inpatient periods, subjects received two of the four study treatments according to a random code at 8 am on day 1 and day 4. Subjects were discharged from the inpatient facility on day 5 and returned to the study center for the second inpatient period the evening of day 6 and repeated the process from the first inpatient period with administration of study treatments three and four. Subjects were discharged from the inpatient facility on day 11 and contacted by telephone for a follow-up evaluation approximately 7 days later. During the entire study period, subjects continued to take their background opioid medication at the same dose and on the same schedule as before they entered the study.
Study visits
Screening
Each subject received naloxone HCl (increments of 0.05 mg IV at 5 minute intervals, up to 0.2 mg total) and was monitored for signs of mild opioid withdrawal (ie, COWS ≥5). Mild withdrawal was medically managed with IV midazolam (1–2 mg IV every 2–3 minutes, with no upper limit) and hydromorphone (1–2 mg IV every 5 minutes). Those with mild opioid withdrawal completed the full screening evaluation.
Treatment
Subjects received a single IV bolus dose of each of the following treatments: buprenorphine 0.75 mg (2.5 mL of concentrated 0.3 mg/mL buprenorphine [to equal 0.75 mg of buprenorphine] with 0.5 mL of 5% dextrose for a total volume of 3 mL); buprenorphine 0.75 mg + naloxone 0.1 mg (2.5 mL of concentrated 0.3 mg/mL buprenorphine [to equal 0.75 mg of buprenorphine] with 0.25 mL of concentrated 0.4 mg/mL naloxone [to equal 0.1 mg of naloxone] and 0.25 mL of 5% dextrose for a total volume of 3 mL); buprenorphine 0.75 mg + naloxone 0.2 mg (2.5 mL of concentrated 0.3 mg/mL buprenorphine [to equal 0.75 mg of buprenorphine] with 0.5 mL of concentrated 0.4 mg/mL naloxone [to equal 0.2 mg of naloxone] for a total volume of 3 mL); or placebo (3 mL of 5% dextrose). They were randomly assigned to one of four double-blind treatment sequences according to the four-treatment, four-period Williams design shown in Table 1. Each study treatment was prepared from the commercial product under sterile conditions by the study site’s pharmacist and administered in the study center in a double-blind manner as a single IV bolus dose of study drug. In the event subjects experienced mild or greater precipitated withdrawal, rescue medication was provided. Additionally, all doses of background opioid medication were taken in the study center.
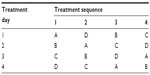  | Table 1 Randomization of study treatments |
Treatment assessments
Table 2 shows the overall timing of key events during the treatment phase of the study. Additionally, 3-lead ECG and pulse oximetry were monitored for 8 hours following each study dose. Adverse events (AEs) and concomitant medications were assessed and recorded at any time.
  | Table 2 Key study events during the treatment phase |
Pharmacodynamic measurements
Serial assessments (ie, physiological measurements, COWS, Drug Effects Questionnaire,15 and the Opioid Agonist Scale16) were made on each day of study treatment administration predose and at 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0, 12.0, and 24.0 hours postdose. Regardless of the scheduled evaluation time, the COWS score was obtained immediately before rescue medication was administered.
Physiological measurements
Following acclimation to the dark for a minimum of 1 minute, pupil diameter was measured with a NeurOptic® VIP-200 pupillometer (NeurOptic, Inc., Irvine, CA, USA) using the same eye throughout the study. Heart rate, blood pressure, respiratory rate, and oxygen saturation were collected after the subject had been sitting for 3 minutes.
COWS
Opioid withdrawal symptoms including pulse rate, gastrointestinal upset, sweating, tremor, restlessness, yawning, pupil size, anxiety or irritability, bone or joint aches, gooseflesh skin, runny nose, and tearing were assessed on individual scales. Total COWS scores range from 0 to 48, with scores of 5–12 indicating mild withdrawal; scores of 13–24 indicating moderate withdrawal; scores of 25–36 indicating moderately severe withdrawal; and scores >36 indicating severe withdrawal.13 In this study, the primary efficacy assessment was the COWS.
Drug Effects Questionnaire
Subjects self-rated the following characteristics using a series of 10 cm visual analog scales: Any Drug Effect, Good Effects, Bad Effects, Drug Liking, High, Sick, Nausea, Sleepy, and Dizzy. Their responses were recorded by making a vertical mark along a horizontal line labeled with “None” at one end and “Extremely” at the other.15
Opioid Agonist Scale
Subjects self-rated their current feelings of Carefree, Coasting, Friendly, Heavy or Sluggish Feeling, Nervous, Nodding, Relaxed, and Turning of Stomach using a 5-point Opioid Agonist Scale: 0= not at all, 1= a little, 2= moderately, 3= quite a bit, and 4 =extremely.17
Safety assessments
During each of the two inpatient periods, the following evaluations were performed: vital signs, AEs, and concomitant medication review.
Statistical methods
No formal sample size and power calculations were performed for this study. A sufficient number of subjects were screened to account for dropouts and ensure that at least 12 subjects completed the four-treatment, four-period crossover.
All data collected were analyzed and presented, and key outcomes were identified a priori. The primary outcome for this study was the COWS total score. Secondary outcomes included physiological measurements (ie, pupil diameter, oxygen saturation, blood pressure, heart rate, and temperature), the Drug Effects Questionnaire, and the Opioid Agonist Scale.
Descriptive statistics were used to summarize the pharmacodynamic outcomes at each time point for each treatment group. No formal statistical analyses were planned, but selected results were analyzed for statistical significance using SAS® Version 9.3 (SAS Institute Inc., Cary, NC, USA). An appropriate mixed-effect model was fit to the data, and model-based statistics (eg, least squares means and standard errors) were obtained and used to construct 95% confidence intervals for mean differences between the treatment groups. These comparisons were performed at each time point, as well as across time. The full model included factors for the overall mean, fixed effects due to sequence, treatment, period, time, and a random effect for subjects nested within sequence. Prior unpublished clinical research indicated that peak plasma buprenorphine concentrations ≥1.5 ng/mL will be obtained from a 0.3 mg intramuscular dose and decline to <0.1 ng/mL 12 hours later. Therefore, it was assumed that the washout period between treatments was sufficient to avoid carryover effects, and hence they were not evaluated.
AEs were tabulated and classified by system organ class and preferred term using the Medical Dictionary for Regulatory Affairs (MedDRA, version 12.0; MedDRA MSSO, McLean, VA, USA), as well as the overall incidence. The timing of these withdrawal events was frequently prior to the planned 15-minute COWS assessment. No covariate or subgroup analyses were planned in this study.
Results
Subjects
A total of 15 subjects were enrolled in and completed the study. All the 15 subjects received each of the four study treatments; no subjects discontinued; and no subject data were excluded for any reason. A total of five subjects had protocol deviations related to the study inclusion/exclusion criteria. Waivers were granted for each of these deviations, and none was considered a major deviation that affected the results or their interpretation.
As shown in Table 3, study subjects were Caucasian (100%) and predominantly male (60%), with a mean age of 49.5 years and a mean body mass index of 30.4. More than 80% of subjects reported a variety of current conditions consistent with a chronic opioid-using population, including musculoskeletal, nervous system, and psychiatric disorders. Opioid medications identified at screening included morphine, oxycodone, fentanyl pump, fentanyl patch, extended-release morphine, oxycodone/acetaminophen, hydrocodone/acetaminophen, methadone, hydrocodone, and endocet in doses ranging from 90 to 1,260 morphine sulfate equivalents.
  | Table 3 Demographics |
Rescue
Among subjects requiring rescue medication (Figure 2), two placebo-treated subjects (13%) required rescue compared with seven who received buprenorphine alone (47%). A naloxone dose as low as 0.1 mg increased the incidence of precipitated withdrawal over buprenorphine alone (60% vs 47% of subjects), and a naloxone dose of 0.2 mg further increased the incidence of precipitated withdrawal over buprenorphine alone (73% vs 47% of subjects).
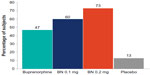  | Figure 2 Subjects requiring rescue medication. |
COWS
At 15 minutes postdose or immediately before rescue treatment (Table 4), mean COWS scores following each active treatment were significantly greater than placebo (P<0.001). Mean COWS scores for subjects receiving BN 0.1 mg and 0.2 mg were significantly greater than buprenorphine alone (P<0.001). The mean change from predose COWS scores was 3.0 for placebo, 6.9 for buprenorphine, 9.8 for BN 0.1 mg, and 12.4 for BN 0.2 mg. The percentage of subjects with COWS total score of ≥13 was 13% for placebo, 47% for buprenorphine alone, 60% for BN 0.1 mg, and 73% for BN 0.2 mg.
Drug Effects Questionnaire
At 15 minutes postdose, subjects receiving BN 0.1 mg had significantly higher scores for Bad Effects and Sick than placebo-treated subjects (P<0.05 for both variables), and subjects in both naloxone-containing treatment groups had numerically higher Bad Effects scores than those receiving buprenorphine alone. Scores for Dizzy with both naloxone-containing treatments were significantly higher than with placebo (P<0.05). Change from baseline in Any Drug Effect was significantly greater with each of the active treatments than with placebo (P<0.05). Scores for Drug Liking, Good Effects, and High were significantly greater for BN 0.2 mg than placebo (P<0.05).
Opioid Agonist Scale
At 15 minutes postdose, BN 0.2 mg produced substantially higher scores for the Nervous and Turning of Stomach items than buprenorphine alone or placebo. Small potential treatment differences between the active treatments and placebo were recorded on the Carefree, Heavy or Sluggish Feeling, Nervous, and Turning of Stomach items, but none of them was significant.
Safety
An overview of AEs is provided in Table 5. Most were similar to the symptoms of opioid withdrawal, such as fatigue, somnolence, nausea, and dizziness. Most subjects reported at least one AE in each treatment group, and most AEs were considered by the investigator to be related to study treatment. The majority of subjects reporting AE severity as moderate were in the naloxone-containing active treatment groups. There were no AEs rated as severe and no serious AEs.
  | Table 5 Adverse events by MedDRA body system |
Discussion
This study was conducted to determine if a 0.75 mg IV dose of buprenorphine would produce an opioid abstinence-like response in opioid-dependent subjects and ascertain whether the addition of naloxone doses of 0.1 and 0.2 mg would increase the withdrawal response. The 0.75 mg dose of buprenorphine was selected after the results of developmental research suggested that this dose in the BEMA® delivery system could produce plasma concentrations similar to a 2 mg dose of BN sublingual tablets. Naloxone doses of 0.1 and 0.2 mg were chosen based on the precipitated withdrawal symptoms observed at these doses in methadone-maintained opioid-dependent subjects.18–20
In our clinical experience, we have noted that there is often an inverse relationship between the opioid maintenance dose and the withdrawal-inducing naloxone dose; higher maintenance doses require lower doses of naloxone to induce withdrawal. There is, however, a minimum dose of antagonist required, and that may vary based on the type and dose of opioid; the duration of dependence; concomitant medications; age; and probably genotype.
Our results demonstrate that in opioid-dependent subjects, precipitated withdrawal can occur with a 0.75 mg IV dose of buprenorphine and that the frequency and intensity of withdrawal are enhanced by coadministration of naloxone doses of 0.1 mg (an 8:1 ratio) and 0.2 mg (a 4:1 ratio). Combining naloxone 0.1 or 0.2 mg with buprenorphine 0.75 mg appears to substantially decrease the potential for abuse compared with buprenorphine alone. During the initial 15-minute postdose treatment period, the median change from baseline in COWS scores for both naloxone-containing treatments was similar (14.0 and 16.0) and greater than both buprenorphine alone (6.0) and placebo (0.0). The mean COWS total score changes were significantly superior to placebo for each of the active treatments (P<0.001), and the naloxone-containing regimens (9.8 and 12.4) were significantly higher than buprenorphine alone (P<0.001).
The outcomes of the safety analysis were predictable. With respect to the incidence of AEs, BN 0.1 mg and buprenorphine alone were similar, and the risk of having an AE with both was slightly higher than placebo and slightly lower than BN 0.2 mg. Adding naloxone 0.1 or 0.2 mg to buprenorphine increased the incidence of moderately severe AEs and drug withdrawal syndrome compared with buprenorphine alone. While subjects in the naloxone-containing treatment groups had numerically higher Bad Effects scores than those receiving buprenorphine alone at 15 minutes postdose, overall results trended toward more AEs with higher naloxone doses, and the result was probably due to small sample size.
Our pharmacodynamic results partially align with prior work. For the naloxone-containing treatments, this study concurs with Strain et al,19 who found significant withdrawal effects from naloxone 0.1 and 0.2 mg in methadone-maintained subjects. On the other hand, Strain et al19 did not identify intramuscular buprenorphine (dose range 0.5–8.0 mg) as an opioid agonist or antagonist and suggested a low potential for abuse, whereas our findings indicate that a 0.75 mg IV dose of buprenorphine can precipitate withdrawal. Since subjects in Strain et al’s study19 used methadone 20 hours before taking buprenorphine and took no other treatments in the predose period, it is possible that buprenorphine acted primarily as an opioid replacement for methadone. Although both studies demonstrated that small amounts of naloxone can induce withdrawal in opioid-dependent subjects, it is important to note that because our study included subjects on several different opioids, while Strain et al19 studied subjects taking only methadone, the results are not directly comparable.
This study has some limitations. Specifically, no physiological measurements were made, and neither the Drug Effects Questionnaire nor the Opioid Agonist Scale was administered, immediately before the administration of IV rescue medication. Assessments for these parameters were made only at the protocol-specified time points even though many subjects were rescued before the 15-minute assessment. Therefore, potentially meaningful results – such as the ratings for Turning of Stomach and Nervous for the active treatments versus placebo on the Opioid Agonist Scale, which might signal precipitated withdrawal – probably reflect the combined effects of study treatment with hydromorphone and midazolam.
The results of this study demonstrate that among opioid-dependent chronic pain subjects who were receiving at least 100 mg oral morphine equivalents per day, a 0.75 mg IV dose of buprenorphine can precipitate withdrawal, and the incidence of buprenorphine-associated precipitated withdrawal increases over a period of hours after administration. However, a naloxone dose as low as 0.1 mg increases the incidence and severity of precipitated withdrawal over buprenorphine alone, providing an additional abuse-deterrent effect that is most significant in the first 15 minutes postdose. With respect to safety, the incidence of AEs with BN 0.1 mg was similar to buprenorphine alone, slightly greater than placebo, and slightly lower than with BN 0.2 mg. Compared with buprenorphine alone, the addition of naloxone to buprenorphine increased the incidence of moderately severe AEs (eg, fatigue, chills) and drug withdrawal syndrome.
Acknowledgments
BioDelivery Sciences International, Inc. funded this study and paid consulting fees to LW, MS, and CU for its conduct and analysis. AF is an employee and shareholder of BioDelivery Sciences International, Inc. The authors acknowledge Christopher Caiazza for assistance with preparation and submission of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
US Department of Health and Human Services. 2012 National Survey on Drug Use and Health: Summary of National Findings. Washington, DC: US Department of Health and Human Services. Available from: http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.pdf. Accessed August 19, 2015. | |
Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Available from: http://www.Samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Accessed August 19, 2015. | |
Pierce M, Bird SM, Hickman M, Millar T. National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005–2009. Drug Alcohol Depend. 2015;146:17–23. | |
Mauger S, Fraser R, Gill K. Utilizing buprenorphine-naloxone to treat illicit and prescription-opioid dependence. Neuropsychiatr Dis Treat. 2014;10:587–598. | |
US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Center for Substance Abuse Treatment. Detoxification and Substance Abuse Treatment Training Manual – Based on a Treatment Improvement Protocol (TIP) Series 45. DHHS Publication No (SMA) 08-4331. Rockville, MD: US Department of Health and Human Services; 2008. Available from: http://162.99.3.213/products/trainingcurriculums/tip45.html. Accessed August 19, 2015. | |
Fiellin DA, O’Connor PG. Office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347:817–823. | |
Maxwell JC, McCance-Katz EF. Indicators of buprenorphine and methadone use and abuse: what do we know? Am J Addict. 2010;19:73–88. | |
Clark RE, Samnaliev M, Baxter JD, Leung GY. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff (Millwood). 2011;30: 1425–1433. | |
Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33: 1179–1191. | |
Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–958. | |
Sullivan JG, Webster L. Novel buccal film formulation of buprenorphine-naloxone for the maintenance treatment of opioid dependence: a 12-week conversion study. Clin Ther. 2015;37:1064–1075. | |
American Society of Anesthesiologists. Physical Status Classification System. Schaumburg, IL: American Society of Anesthesiologists. Available from: http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed August 19, 2015. | |
Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105:154–159. | |
World Medical Association. Declaration of Helsinki (2008). Ferney-Voltaire, France: World Medical Association. Available from: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed August 19, 2015. | |
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl). 2013;227:177–192. | |
Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. | |
Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. | |
Preston KL, Bigelow GE, Liebson IA. Buprenorphine and naloxone and in combination in opioid dependent humans. Psychopharmacology. 1988;94:484–490. | |
Strain EC, Preston KL, Liebson IA, Bigelow GE. Acute effects of buprenorphine, hydromorphone and naloxone in methadone-maintained volunteers. J Pharmacol Exp Ther. 1992;261:985–993. | |
Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend. 2007;90:261–269. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.