Back to Journals » Clinical Ophthalmology » Volume 16
Long-Term Visual Function After Fractionated Stereotactic Radiotherapy for Primary Optic Nerve Sheath Meningioma: A Retrospective Analysis of 34 Subjects
Authors Vanikieti K, Chaiwithooanukul C, Puataweepong P, Jindahra P, Padungkiatsagul T
Received 25 July 2022
Accepted for publication 8 September 2022
Published 22 September 2022 Volume 2022:16 Pages 3119—3128
DOI https://doi.org/10.2147/OPTH.S383702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kavin Vanikieti,1 Chaloemwong Chaiwithooanukul,1 Putipun Puataweepong,2 Panitha Jindahra,3 Tanyatuth Padungkiatsagul1
1Department of Ophthalmology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Radiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Tanyatuth Padungkiatsagul, Department of Ophthalmology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Bangkok, 10400, Thailand, Tel +662 201 1526, Email [email protected]
Objective: To evaluate long-term visual function after fractionated stereotactic radiotherapy (FSRT) for primary optic nerve sheath meningioma (PONSM).
Methods: This 22-year retrospective study included 34 subjects (34 affected eyes) with PONSM who were treated with FSRT exclusively. Subjects with a history of biopsy/resection were excluded. Visual function, including visual acuity (VA) and visual field mean deviation (VF MD), was evaluated at presentation (pre-radiotherapy; pre-RT) and at the final follow-up (post-radiotherapy; post-RT); treatment complications were also evaluated. Treatment success was defined as either stabilization or improvement of visual function.
Results: The median pre-RT VA and pre-RT VF MD were 0.70 logarithm of the minimum angle of resolution (logMAR; range: 0.0– 2.9 logMAR) and − 15.4 decibels (dB) (range: − 31.4 to − 3.2 dB), respectively. The median total dose of FSRT was 50 Gy (range: 45– 54 Gy) and the median number of fractions was 25 (range: 25– 30). The median follow-up interval was 89 months (range: 6– 251 months). The median post-RT VA and post-RT VF MD were 0.48 logMAR (range: 0.0– 2.9 logMAR) (p = 0.010) and − 6.8 dB (range: − 20.6 to − 1.6 dB) (p = 0.005), respectively. Among the 34 included eyes, VA was successfully treated in 29 eyes (85.3%) and worsened in 5 eyes (14.7%). Of the 14 eyes with both VA and reliable VF MD at pre-RT and post-RT time points, VF MD was successfully treated in 13 eyes (92.8%) and worsened in one (7.2%); overall visual function was successfully treated in 13 eyes (92.8%) and worsened in 1 eye (7.2%). Complications occurred in one subject (2.9%; radiation retinopathy).
Conclusion: Approximately 90% of PONSM subjects exhibited long-term treatment success in terms of VA, VF MD, and overall visual function after FSRT. Additionally, the incidence of complications was low. Therefore, FSRT is effective and safe treatment for PONSM.
Keywords: primary, optic nerve sheath meningioma, fractionated stereotactic radiotherapy, visual acuity, visual field, optic nerve tumor
Introduction
Primary optic nerve sheath meningioma (PONSM) is a rare benign tumor that arises from the proliferation of meningoepithelial cells lining the sheath of the intraorbital or intracanalicular optic nerve. In contrast, secondary optic nerve sheath meningioma (SONSM) arises from intracranial meningioma (eg, sphenoid wing meningioma) that extends through the optic canal and orbit to the optic nerve.1–3 PONSM represents approximately 10% of all optic nerve sheath meningiomas (ONSMs), 1–2% of all meningiomas, and 2% of all orbital tumors.4,5 The classic clinical triad of PONSM includes painless, slowly progressive monocular vision loss; optic nerve atrophy; and the presence of optociliary shunt vessels. Other clinical findings may also include proptosis, ophthalmoplegia, and facial numbness. Clinical characteristics and magnetic resonance imaging (MRI) findings are usually sufficient for diagnosis of PONSM. Furthermore, biopsy is generally not recommended due to the potential of substantial visual loss from the procedure.1,3,6,7
Although the natural course of PONSM is benign, it can progress to irreversible blindness in 85% of untreated affected eyes.8–10 Surgical management is not recommended because of the high risk of blindness associated with disrupting blood flow to the optic nerve. In previous studies, radiotherapy (RT) has shown great potential for stabilizing or improving visual acuity (VA) in up to 90% of PONSM subjects.7,11,12 In current clinical practice, various RT modalities are used to treat PONSM; such modalities include intensity-modulated RT,13 stereotactic radiosurgery,14 proton beam therapy,15 fractionated stereotactic RT (FSRT),5 two-dimensional RT,16 and three-dimensional conformal RT.17 The majority of existing literatures indicate that FSRT is the treatment of choice for PONSM because of its high efficacy and low incidence of complications.1,10,12,18–20
There were some limitations in previous studies.1,3,5,18,19 First, they did not exclude PONSM subjects who had a history of tumor biopsy/resection. Second, they generally did not adequately describe the visual field (VF) programs and VF change criteria. Therefore, the primary goal of our study was to evaluate long-term visual function, including VA and VF, in subjects with PONSM who had no history of tumor biopsy/resection and were treated with FSRT exclusively.
Materials and Methods
This study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (IRB number: COA. MURA2022/116), which waived the need for written informed consent from the subjects. All data were kept confidential in our database. We retrospectively reviewed the electronic medical records of 34 subjects who were diagnosed with PONSM and treated with FSRT exclusively in Ramathibodi Hospital between January 2000 and September 2021.
Subject Selection
All subjects were diagnosed on the basis of clinical characteristics and MRI findings. Inclusion criteria were diagnosis with PONSM and treatment with FSRT exclusively in Ramathibodi Hospital between January 2000 and September 2021. Exclusion criteria were one or more of the following: SONSM defined as intracranial meningioma (eg, sphenoid wing meningioma) that extends through the optic canal and orbit to the optic nerve; age < 20 years at symptom onset; history of tumor biopsy/resection; presence of visually significant cataract and/or ocular diseases (other than PONSM) that could affect VA and/or VF.
Treatment Protocol
FSRT was performed using a two-linear accelerator (Linac)-based system, which included the frame-based Linac stereotactic system (X-Knife) and the Edge® Radiosurgery System. The frame-based Linac stereotactic system consisted of a 6-MV dedicated Linac with fixed circular cones and the X-Knife forward-planning system (versions 3 and 4; Integra Radionics Inc., Burlington, MA, USA). The Edge® Radiosurgery System (Varian Medical Systems, Palo Alto, CA, USA) consisted of a megavoltage Linac with 120 multileaf collimators. For X-Knife treatment, a bite block with a removable Gill–Thomas–Cosman (GTC) frame was used, while the Edge system used a thermoplastic facemask. Individual treatment planning was conducted at a workstation using a set of images from a computed tomography (CT) simulation scan (slice thickness of 1.25 mm), with or without gadolinium-enhanced MRI. Target and critical organ contouring was performed by physicians, and treatment plans were generated by a medical physicist. Gross tumor volume (GTV) and critical structures were contoured in each consecutive slice of CT and MRI scans. A margin of 0–3 mm was added to the GTV to obtain the planning target volume (PTV). The prescribed radiation dose was the conventional 45–54 Gy in 25–30 fractions, with five fractions applied each week.
Demographic Data, Clinical Assessment, and Radiographic Evaluation
We recorded demographic data (sex, age at symptom onset, and history of neurofibromatosis type 2 [NF2]) and clinical characteristics (laterality, side of affected eye, duration of symptoms, length of time from symptom onset to initiation of RT, and signs and symptoms at presentation).
Visual function examinations included VA and VF at presentation (pre-radiotherapy; pre-RT) and at the final follow-up (post-radiotherapy; post-RT). VA was measured with the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. VF assessment was performed using the 30–2 SITA program (Humphrey Field Analyzer, Carl-Zeiss Meditec, Dublin, CA, USA). Visual field mean deviation (VF MD) was used as a proxy for the degree of VF defect. Eyes with VA worse than or equal to 20/200 were not subjected to VF MD evaluation because poor VA may reduce the validity of such an evaluation. A reliable VF examination was defined as a VF examination that met all of the following conditions: VA better than 20/200, fixation losses < 20%, false negative errors < 33%, and false positive errors < 33%. We recorded VF MD findings only for eyes with both reliable pre-RT VF examination data and reliable post-RT VF examination data. Thus, the VF MD data of 14 eyes were included.
Regarding the responses to RT, improved/worsened VA was defined as a change of ≥ 2 lines in the ETDRS chart; stable VA was defined as a change of < 2 lines in the ETDRS chart.1,4,12,21–23 Improved/worsened VF MD was defined as a change of ≥ 3 decibels (dB); stable VF MD was defined as a change of < 3 dB.23 Responses to RT in terms of overall visual function are defined and summarized in Table 1. Treatment success was defined as either stabilization or improvement of visual function.10
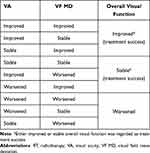 |
Table 1 Definitions of Responses to RT in Terms of Overall Visual Function |
The tumor control rate was defined as stable or reduced tumor size according to official radiological report at the final MRI follow-up.10 RT complications were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.24
Statistical Analysis
Categorical data were summarized using frequencies and percentages, then analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Continuous data were summarized as means ± standard deviations (SDs) for normally distributed data or medians (ranges) for non-normally distributed data; they were analyzed using the two-sample t-test or Mann–Whitney U-test, depending on the distribution of the data. ETDRS VA values were converted to logarithm of the minimum angle of resolution (logMAR) values for statistical analysis. VA categories of counting fingers (CF), hand motion, light perception, and no light perception were converted to 2.6, 2.7, 2.8, and 2.9 logMAR, respectively.25,26 Spearman correlation analysis was used to evaluate the relationship between the change in VA (post-RT VA – pre-RT VA) (logMAR) and the change in VF MD (post-RT VF MD – pre-RT VF MD) (dB). Statistical analyses were performed using STATA software, version 17.0 (StataCorp LLC, College Station, TX, USA). p-values < 0.05 were considered statistically significant.
Results
Demographic Data and Clinical Characteristics
The demographic data and clinical characteristics are summarized in Table 2. Thirty-four affected eyes of 34 subjects were included in this study. Majority of subjects were women (29/34, 85.3%). The mean age at symptom onset was 46.3 ± 9.8 years. The median duration of symptoms was 10.5 months (range: 0.5–120 months). The median interval from symptom onset to initiation of RT was 13 months (range: 0.5–121 months). None of the subject had a previous history of NF2 diagnosis.
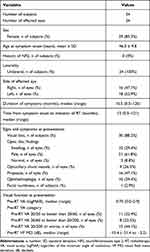 |
Table 2 Demographic Data and Clinical Characteristics |
All subjects had unilateral PONSM. The most common signs and symptoms at presentation were visual loss (30 subjects, 88.2%), proptosis (16 eyes, 47.1%), ophthalmoplegia (10 eyes, 29.4%), and facial numbness (one subject, 2.9%). Regarding optic disc findings at presentation, a pale optic disc was most common (21 eyes, 61.8%); other findings included a swollen optic disc (10 eyes, 29.4%) and a normal optic disc (three eyes, 8.8%). Optociliary shunt vessels were observed in nine eyes (26.5%) at presentation. Visual function at presentation included median pre-RT VA and pre-RT VF MD of 0.7 logMAR (range: 0.0–2.9 logMAR) and −15.4 dB (range: −31.4 to −3.2 dB), respectively. Pre-RT VA was 20/200 or worse in 15 eyes (44.1%), while it ranged from 20/20 to better than 20/60 in 11 eyes (32.4%) and from 20/60 to better than 20/200 in eight eyes (23.5%).
Dose of RT and Number of Fractions
The median total dose of FSRT was 50 Gy (range: 45–54 Gy) and the median number of fractions was 25 (range: 25–30).
Long-Term Visual Function
The median visual function follow-up interval was 89 months (range: 6–251 months). The median post-RT VA and post-RT VF MD were 0.48 logMAR (range: 0.0–2.9 logMAR) (p = 0.010) and −6.8 dB (range: −20.6 to −1.6 dB) (p = 0.005), respectively. Post-RT VA ranged from 20/20 to better than 20/60 in 17 eyes (50%), and from 20/60 to better than 20/200 in four eyes (11.8%); it was 20/200 or worse in 13 eyes (38.2%). These post-RT VA values significantly differed from pre-RT VA values (p < 0.001). A strong inverse correlation was observed between the change in VA (post-RT VA – pre-RT VA) and the change in VF MD (post-RT VF MD – pre-RT VF MD) (r = −0.612, p = 0.020). Long-term visual function findings and comparisons of visual function between pre-RT and post-RT time points are summarized in Tables 3 and 4, respectively.
 |
Table 3 Long-Term Visual Function Findings |
 |
Table 4 Comparisons of Visual Function Between Pre-RT and Post-RT Time Points |
Responses to RT
Among the 34 included eyes, VA was considered successfully treated in 29 eyes (15 eyes were improved, 14 eyes were stable; 85.3%) and was considered worsened in 5 eyes (14.7%). Of the 14 eyes with both VA and reliable VF MD at pre-RT and post-RT time points, VF MD was considered successfully treated in 13 eyes (10 eyes were improved, 3 eyes were stable; 92.8%) and worsened in only one eye (7.2%). Overall visual function was considered successfully treated in 13 eyes (10 eyes were improved, 3 eyes were stable; 92.8%) and worsened in only one eye (7.2%). Responses to RT are summarized in Table 5.
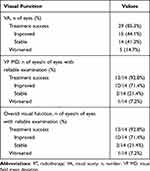 |
Table 5 Responses to RT |
Tumor Control Rate
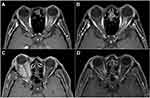
In our center, radiographic follow-up with MRI was performed annually after the completion of FSRT in subjects without complications. Follow-up MRI was not conducted in 5 subjects (14.7%) because their follow up duration was less than 1 year. The remaining 29 subjects (85.3%) had a median MRI follow-up duration of 90 months (range: 13–228 months); their tumor control rate was 100% (Figure 1).
RT Complications
RT complications occurred in only one subject (2.9%). A healthy 38-year-old woman who despite having a presenting VA of 20/20 had a VF defect before FSRT. MRI revealed involvement of the tumor in intraocular, intraorbital, and intracanalicular portions of the left optic nerve. The most anterior extension of the tumor was adjacent to the posterior margin of the globe. In total, 54 Gy of FSRT was applied in 27 fractions over 5 weeks. At the end of FSRT, the subject’s VA was 20/20 and the VF defect was improved. However, 24 months after the completion of FSRT, her VA had worsened to CF. Fundus examination revealed a pale left optic disc. While fundus fluorescein angiography (FFA) showed macular ischemia in the left eye, MRI showed a stable tumor size without other notable findings. The subject received intravenous methylprednisolone (1 g/day) for 3 days, followed by oral prednisolone for 1 month; she did not experience any improvement. After the exclusion of all possible causes of impaired VA, the subject was diagnosed with radiation retinopathy (grade 4, CTCAE version 5.0);24 her VA was CF after 251 months of follow-up.
Discussion
In this study, we retrospectively reviewed the medical records of 34 subjects with PONSM-affected eyes. We sought to characterize long-term visual function, including VA and VF, in subjects with PONSM who had no history of tumor biopsy/resection and were treated with FSRT exclusively.
In our cohort, majority of subjects were female (29/34, 85.3%) and were middle-aged at symptom onset (mean age at symptom onset 46.3 ± 9.8 years), similar to previous studies.5,6,9,11,12,14,15,21,27–34 Pale optic disc was the most common optic disc finding at presentation in our study. This common finding is presumably related to a lack of disease awareness among patients during the early stages of PONSM because of its benign and slow-growing nature. We also observed optociliary shunt vessels in nine eyes (26.5%) at presentation, which was comparable with the findings in a large review where optociliary shunt vessels were observed in fewer than one-third of affected eyes at presentation.20 These collateral vessels are not specific to PONSM; they were also reported in conditions such as SONSM, optic pathway glioma, central retinal vein occlusion, and chronic papilledema.35–37 Although our subjects most frequently presented with visual loss, nearly half and nearly one-third of the affected eyes had proptosis and ophthalmoplegia at presentation, respectively. These findings suggest that comprehensive ophthalmic examinations, including exophthalmometer and ocular motility assessments, should be conducted to establish an early and correct diagnosis in all patients who present with visual loss.
We found a significant improvement in post-RT VA, compared with pre-RT VA (median [range]: 0.48 [0.0–2.9] vs 0.70 [0.0–2.9] logMAR, respectively; p = 0.010). This significant improvement in VA after FSRT was consistent with the findings in previous studies of FSRT for subjects with PONSM.1,23,38 Additionally, after FSRT, most eyes had VA ranging from 20/20 to better than 20/60, while most eyes had VA 20/200 or worse before FSRT (p < 0.001).
Furthermore, there was a significant improvement in post-RT VF MD, compared with pre-RT VF MD (median [range]: −6.8 [−20.6 to −1.6] vs −15.4 [−31.4 to −3.2] dB, respectively; p = 0.005). This finding indicates that FSRT improves both VA and VF MD in PONSM subjects. Moreover, we found a parallel between the extent of improvement in VA and the extent of improvement in VF MD, with a strong inverse correlation between the change in VA (post-RT VA – pre-RT VA) and the change in VF MD (post-RT VF MD – pre-RT VF MD) (Spearman r = −0.612, p = 0.020).
After FSRT, 29 of the 34 eyes (85.3%) were considered treatment success in terms of VA. This was consistent with the findings in previous FSRT studies, where 76.9–100% of eyes exhibited treatment success in terms of VA.4,12,21 The remaining 5 eyes had worsened VA; excluding the one eye that was affected by radiation retinopathy, 3 of 4 eyes (75%) had presenting VA of CF or worse. Thus, we speculate that poor pre-RT VA of CF or worse is associated with a worse response to FSRT in terms of VA. With regards to VF MD, 13 of 14 eyes (92.8%) with reliable VF examination at pre-RT and post-RT time points were considered treatment success after FSRT, which was comparable with the VF treatment success rates of 89.5–93% in previous studies.11,12
In our study, the tumor control rate was 100%. This was consistent with the findings in many previous studies, where the tumor control rates were 95.4–100% after FSRT.4–6,9,11,12,14,15,21,27–34 Our absolute tumor control rate indicates that FSRT can achieve both clinical and radiographic success in the treatment of PONSM.
RT complications during follow-up occurred in only one subject (2.9%; radiation retinopathy). This finding was comparable with the results of previous studies, where radiation retinopathy occurred in 3.8–4.9% of PONSM subjects who were treated with FSRT.4,5,11 We hypothesized that there were two risk factors for radiation retinopathy in our subject: close proximity between the anterior extension of the tumor and the posterior region of the retina, which may have caused the retina to receive the full radiation dose;21,39,40 and a total radiation dose > 50 Gy, which has been reported to cause retinal damage.4,40,41 Radiation retinopathy usually develops 2–4 years after RT.11,21,39,42 This is comparable with the timing in our subject, who exhibited radiation retinopathy 24 months after FSRT. Despite the rarity of radiation retinopathy, PONSM patients should be informed of this complication before FSRT is initiated.
Our study had several strengths. First, to our knowledge, this study included the largest number of PONSM subjects who had no history of tumor biopsy/resection and were treated with FSRT exclusively. Second, we used clear definitions of each response to RT in terms of VA, VF MD, and overall visual function. Finally, our follow-up interval was longer than the intervals used in previous studies.5,6,11,29,34
However, our study had some limitations. First, its retrospective design led to the absence of follow-up MRI data for five subjects (14.7%). Second, we did not evaluate other components of visual function, such as color vision or contrast sensitivity.
Conclusion
In this study, approximately 90% of PONSM subjects exhibited long-term treatment success in terms of VA, VF MD, and overall visual function after FSRT. Additionally, the incidence of complications was low. Therefore, FSRT is effective and safe treatment for PONSM.
Acknowledgments
We thank Miss Sasiporn Sitthisorn, Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, for advice regarding statistical analyses. We also thank Ryan Chastain-Gross, Ph.D., from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Disclosure
The authors declare no competing interests.
References
1. Pitz S, Becker G, Schiefer U, et al. Stereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternative. Br J Ophthalmol. 2002;86:1265–1268. doi:10.1136/bjo.86.11.1265
2. Jeremic B, Pitz S. Primary optic nerve sheath meningioma. Stereotactic fractionated radiation therapy as an emerging treatment of choice. Cancer. 2007;110:714–722. doi:10.1002/cncr.22859
3. Becker G, Jeremic B, Pitz S, et al. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002;54:1422–1429. doi:10.1016/S0360-3016(02)03753-7
4. Ratnayake G, Oh T, Mehta R, et al. Long-term treatment outcomes of patients with primary optic nerve sheath meningioma treated with stereotactic radiotherapy. J Clin Neurosci. 2019;68:162–167. doi:10.1016/j.jocn.2019.07.005
5. Soldà F, Wharram B, Gunapala R, Brada M. Fractionated stereotactic conformal radiotherapy for optic nerve sheath meningiomas. Clin Oncol. 2012;24(8):e106–e112. doi:10.1016/j.clon.2012.03.015
6. Andrews DW, Faroozan R, Yang BP, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51(4):890–902, discussion 903–904. doi:10.1097/00006123-200210000-00007
7. Saeed P, Rootman J, Nugent RA, et al. Optic nerve sheath meningiomas. Ophthalmology. 2003;110(10):2019–2030. doi:10.1016/S0161-6420(03)00787-5
8. Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37(3):167–183. doi:10.1016/0039-6257(92)90135-G
9. Narayan S, Cornblath WT, Sandler HM, Elner V, Hayman JA. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56(2):537–543. doi:10.1016/S0360-3016(03)00005-1
10. Vaishnav YJ, Singh R, Didwania P, et al. Radiotherapy and radiosurgery in the management of optic nerve sheath meningiomas: an international systematic review and meta-analysis of twenty studies. World Neurosurg. 2022;164:e929–e944. doi:10.1016/j.wneu.2022.05.064
11. Baumert BG, Villà S, Studer G, et al. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72(2):169–174. doi:10.1016/j.radonc.2004.04.008
12. Hamilton SN, Nichol A, Truong P, et al. Visual outcomes and local control after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthal Plast Reconstr Surg. 2018;34(3):217–221. doi:10.1097/IOP.0000000000000914
13. Bloch O, Sun M, Kaur G, Barani IJ, Parsa AT. Fractionated radiotherapy for optic nerve sheath meningiomas. J Clin Neurosci. 2012;19:1210–1215. doi:10.1016/j.jocn.2012.02.010
14. Marchetti M, Bianchi S, Milanesi I, et al. Multisession radiosurgery for optic nerve sheath meningiomas–an effective option: preliminary results of a single-center experience. Neurosurgery. 2011;69(5):1116–1122, discussion 1122–1123. doi:10.1227/NEU.0b013e31822932fe
15. Arvold ND, Lessell S, Bussiere M, et al. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2009;75(4):1166–1172. doi:10.1016/j.ijrobp.2008.12.056
16. Kennerdell JS, Maroon JC, Malton M, Warren FA. The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988;106:450–457. doi:10.1016/0002-9394(88)90882-3
17. Augspurger ME, Teh BS, Uhl BM, et al. Conformal intensity modulated radiation therapy for the treatment of optic nerve sheath meningioma. Int J Radiat Oncol. 1999;45:324. doi:10.1016/S0360-3016(99)90361-9
18. de Melo LP, Arruda Viani G, de Paula JS. Radiotherapy for the treatment of optic nerve sheath meningioma: a systematic review and meta-analysis. Radiother Oncol. 2021;165:135–141. doi:10.1016/j.radonc.2021.10.009
19. Turbin RE, Thompson CR, Kennerdell JS, et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890–899. doi:10.1016/S0161-6420(02)01017-5
20. Solli E, Turbin RE. Primary and secondary optic nerve sheath meningioma. J Neurol Surg B Skull Base. 2021;82(1):27–71. doi:10.1055/s-0041-1723801
21. Saeed P, Blank L, Selva D, et al. Primary radiotherapy in progressive optic nerve sheath meningiomas: a long-term follow-up study. Br J Ophthalmol. 2010;94(5):564–568. doi:10.1136/bjo.2009.166793
22. Paulsen F, Doerr S, Wilhelm H, Becker G, Bamberg M, Claßen J. Fractionated stereotactic radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2012;82:773–778. doi:10.1016/j.ijrobp.2010.11.018
23. Abouaf L, Girard N, Lefort T, et al. Standard-fractionated radiotherapy for optic nerve sheath meningioma: visual outcome is predicted by mean eye dose. Int J Radiat Oncol Biol Phys. 2012;82:1268–1277. doi:10.1016/j.ijrobp.2011.04.010
24. US Department of Health and Human Services, National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0; 2017.
25. Grover S, Fishman GA, Anderson RJ, et al. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999;106(9):1780–1785. doi:10.1016/S0161-6420(99)90342-1
26. McAnany JJ, Genead MA, Walia S, et al. Visual acuity changes in patients with Leber congenital amaurosis and mutations in CEP290. JAMA Ophthalmol. 2013;131(2):178–182. doi:10.1001/2013.jamaophthalmol.354
27. Adams G, Roos DE, Crompton JL. Radiotherapy for optic nerve sheath meningioma: a case for earlier intervention? Clin Oncol. 2013;25(6):356–361. doi:10.1016/j.clon.2013.02.004
28. Browner JV, Amdur RJ, Kirwan J, Mendenhall WM, Friedman W. Radiation therapy for optic nerve sheath meningioma. Pract Radiat Oncol. 2013;3(3):223–228. doi:10.1016/j.prro.2012.06.010
29. Milker-Zabel S, Huber P, Schlegel W, Debus J, Zabel-du Bois A. Fractionated stereotactic radiation therapy in the management of primary optic nerve sheath meningiomas. J Neurooncol. 2009;94(3):419–424. doi:10.1007/s11060-009-9874-8
30. Pandit R, Paris L, Rudich DS, Lesser RL, Kupersmith MJ, Miller NR. Long-term efficacy of fractionated conformal radiotherapy for the management of primary optic nerve sheath meningioma. Br J Ophthalmol. 2019;103(10):1436–1440. doi:10.1136/bjophthalmol-2018-313135
31. Jin J, Joo JD, Han JH, et al. Optic nerve sheath meningioma: preliminary analysis of the role of radiation therapy. Brain Tumor Res Treat. 2018;6(1):8–12. doi:10.14791/btrt.2018.6.e2
32. Eckert F, Clasen K, Kelbsch C, et al. Retrospective analysis of fractionated intensity-modulated radiotherapy (IMRT) in the interdisciplinary management of primary optic nerve sheath meningiomas. Radiat Oncol. 2019;14(1):240. doi:10.1186/s13014-019-1438-2
33. Smee RI, Schneider M, Williams JR. Optic nerve sheath meningiomas–non-surgical treatment. Clin Oncol. 2009;21(1):8–13. doi:10.1016/j.clon.2008.10.010
34. Sitathanee C, Dhanachai M, Poonyathalang A, Tuntiyatorn L, Theerapancharoen V. Stereotactic radiation therapy for optic nerve sheath meningioma; an experience at Ramathibodi Hospital. J Med Assoc Thai. 2006;89(10):1665–1669.
35. Turbin RE, Pokorny K. Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Contr. 2004;11(5):334–341. doi:10.1177/107327480401100508
36. Ling JD, Chao D, Al-Zubidi N, Lee AG. Big red flags in neuro-ophthalmology. Can J Ophthalmol. 2013;48(1):3–7. doi:10.1016/j.jcjo.2012.08.016
37. Okamoto N, Suzuki A, Ohnishi M, Fukuda M. The formation and involution of optociliary veins during the course of central retinal vein occlusion. Jpn J Ophthalmol. 2000;44(3):312–313. doi:10.1016/S0021-5155(99)00212-9
38. Kheir V, Faouzi M, Borruat F-X. Visual outcomes of fractionated radiotherapy in optic nerve sheath meningioma: a retrospective study. Klin Monatsbl Augenheilkd. 2019;236(4):526–529. doi:10.1055/a-0828-7335
39. Subramanian PS, Bressler NM, Miller NR. Radiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthalmology. 2004;111:565–567. doi:10.1016/j.ophtha.2003.06.025
40. Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:765–773. doi:10.1016/0360-3016(94)90347-6
41. Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982;89(12):1494–1501. doi:10.1016/S0161-6420(82)34611-4
42. Krishnan R, Kumar I, Kyle G, John Husband D. Radiation retinopathy after fractionated stereotactic conformal radio-therapy for primary intraorbital optic nerve sheath meningioma. J Neuroophthalmol. 2007;27(2):143–144. doi:10.1097/WNO.0b013e318064e5b0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.