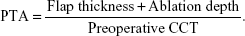Back to Journals » Clinical Ophthalmology » Volume 10
Long-term evaluation of eyes with central corneal thickness <400 µm following laser in situ keratomileusis
Authors Reza Djodeyre M, Beltran J, Ortega-Usobiaga J , Gonzalez-Lopez F, Ruiz-Rizaldos A, Baviera J
Received 17 November 2015
Accepted for publication 12 January 2016
Published 29 March 2016 Volume 2016:10 Pages 535—540
DOI https://doi.org/10.2147/OPTH.S100690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mohammad Reza Djodeyre,1 Jaime Beltran,2 Julio Ortega-Usobiaga,3 Felix Gonzalez-Lopez,4 Ana Isabel Ruiz-Rizaldos,1 Julio Baviera2
1Department of Refractive Surgery, Clinica Baviera, Zaragoza, 2Department of Refractive Surgery, Clinica Baviera, Valencia, 3Department of Research and Development, Clinica Baviera, Bilbao, 4Department of Refractive Surgery, Clinica Baviera, Madrid, Spain
Purpose: To study long-term refractive and visual outcomes of laser in situ keratomileusis (LASIK) in eyes with a postoperative thin central cornea.
Methods: In this retrospective observational case series, we studied 282 myopic eyes with a normal preoperative topographic pattern and postoperative thin corneas (<400 µm) that had at least 3 years of follow-up after LASIK in three private clinics. The main outcome measures were safety, efficacy, predictability, percent tissue altered, and complications.
Results: The mean postoperative central corneal thickness was 392.05 µm (range: 363.00–399.00 µm). After a mean follow-up of 6.89±2.35 years (standard deviation), the safety index was 1.17, the efficacy index was 0.94, and predictability (±1.00 diopter [D]) was 73.49. The mean residual stromal bed thickness was 317.34±13.75 µm (range: 275–356 µm), the mean flap thickness was 74.76±13.57 µm (range: 55–124 µm), and the mean percent tissue altered was 37.12%±3.62% (range: 27.25%–49.26%). No major complications were recorded.
Conclusion: LASIK with a resultant central cornea thickness <400 µm seems to be effective, safe, and predictable provided that preoperative topography is normal and the residual stromal bed thickness is >275 µm.
Keywords: LASIK, thin, cornea, ectasia, myopia, pachymetry, topography
Introduction
Laser in situ keratomileusis (LASIK) is an effective, predictable, stable, and safe technique for the correction of refractive errors.1,2 Since corneal laser refractive surgery was first introduced in ophthalmology, many studies have tried to establish risk factors for one of its most dreaded complications, keratectasia. These risk factors include age <30 years, residual stromal bed thickness <300 μm, preoperative central corneal thickness (CCT) <500 μm, preoperative mean keratometry readings >47.00 D, manifest preoperative refraction spherical equivalent (SE) >−8.00 D, against-the-rule astigmatism, Orbscan posterior float >50 μm, topography (inferior/superior ratio ≥1.40 D), retreatment surgery, and percent of tissue altered (PTA) >40%.3–6 Randleman et al4 suggested the Ectasia Risk Score System in 2008. Although an irregular topographic pattern seems to be the main risk factor, ectasia in eyes with normal preoperative corneal topography still present a conundrum.7,8 Santhiago et al6 recently proposed that the PTA at the time of LASIK was significantly associated with ectasia in eyes with normal preoperative topography. Febbraro et al9 considered a postoperative CCT <400 μm to be the cutoff value for laser refractive surgery; however, to our knowledge, there are no published data to support this hypothesis.
Postoperative corneal ectasia is most likely a manifestation of biomechanical integrity reduced to below the threshold required to maintain corneal shape and curvature. Corneal tensile strength gradually decreases in the deeper 60% throughout the central corneal stroma.10,11 Flap thickness plays a direct role in this decrease, as the anterior lamellar flap does not contribute significantly to postoperative corneal tensile strength.12 On the other hand, as only 50% of cases of post-LASIK corneal ectasia develop during the first year after surgery, a long-term analysis is needed to evaluate this complication.13 The present study aims to evaluate the long-term visual outcome and complications of LASIK in eyes with a postoperative CCT <400 μm.
Materials and methods
In this retrospective case series, we reviewed the medical records of 60,325 consecutive myopic eyes that had undergone LASIK at three centers of Clinica Baviera (Zaragoza, Bilbao, and Valencia, Spain) from January 2003 to April 2010. Clinica Baviera is a private ophthalmology institution with 49 centers located throughout Spain that performs a high volume of refractive surgical procedures. Clinical records and surgical data are stored in a database. The inclusion criteria were myopic refractive error, defined as a negative SE, postoperative CCT <400 μm, more than 3 years of follow-up, and normal preoperative topography. These search criteria were entered into the database. Written informed consent was obtained, clinical charts were reviewed, and the relevant fields of the case report form were completed. The Clinica Baviera review board ruled that ethics approval was not required for this study. All patients had stable refraction for at least 1 year before the procedure. No significant ocular pathology, family history of keratoconus, and historical findings (eg, corneal transplantation) were reported.
All the procedures were performed by experienced surgeons. The preoperative regimen was similar for all patients and included anesthesia with tetracaine hydrochloride 0.01% eyedrops and oral sedation (alprazolam 0.25 mg). Keratectomy was performed using a Moria LSK-One microkeratome (Moria, Antony, France; distributed by Microtech Inc, Doylestown, PA, USA). In our institution, surgeons were trained to achieve flaps thinner than 80 μm in special cases using 80 or 100 μm footplates. A relative fast pass of the manual microkeratome helps to achieve an intended thin flap. The excimer lasers used were the Technolas Keracor 217C or Z (Bausch & Lomb) and the MEL 80 (Zeiss-Meditec, Jena, Germany).
CCT (preoperative and postoperative) and corneal bed thickness were obtained by taking at least three pachymetry measurements (DGH 5100 Technology, Inc, Exton, PA, USA), of which the smallest value was recorded. Flap thickness was measured by subtracting the corneal bed thickness (before laser application) from the CCT. The residual stromal bed thickness was calculated by subtracting the laser ablation depth from the measured corneal bed thickness. The postoperative CCT was calculated by subtracting the laser ablation depth from the preoperative CCT.
Preoperative and postoperative examinations included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), refraction, cycloplegic refraction, slit-lamp evaluation, intraocular pressure, indirect ophthalmoscopy, keratometry, pachymetry, and topography (Orbscan II, Bausch & Lomb, Rochester, NY, USA). All eyes were targeted for distance vision. The patients were usually examined immediately after surgery and at 12 hours, 1 week, and 1 and 3 months. Annual follow-up visits were recommended. Postoperative corneal topography was usually assessed at 3 months and at each annual follow-up visit. The PTA, which is the percentage of anterior tissue that is modified during LASIK, was obtained using the equation:
|
Predictability was defined based on the percentage of eyes that achieved a postoperative SE of ±1.00 D and of ±0.50 D. The indicators of visual outcomes were the efficacy index (postoperative UDVA/preoperative CDVA) and the safety index (postoperative CDVA/preoperative CDVA).
Continuous data were expressed as mean ± standard deviation (SD) and range (minimum and maximum). As most variables did not follow a normal distribution, the median and interquartile range were also calculated. In order to calculate 95% confidence intervals, mean ±1.96 SD was used for normally distributed variables, and the percentiles 0.025 and 0.975 were used in the remaining cases. Normality was confirmed using the Kolmogorov–Smirnov test. Categorical variables were expressed as frequency (n) and percentage (%). Statistical analysis was performed using SPSS 19.0 for Windows (IBM Corporation, Armonk, NY, USA).
Results
Out of a total of 60,325 LASIK procedures performed on myopic eyes, 282 eyes from 146 patients had a postoperative CCT <400 μm and a follow-up of at least 3 years (6.89±2.35, range: 3.02–11.41 years) that were included in this study. LASIK was performed by 15 surgeons. The mean (SD) age of the patients included was 33.26±8.78 years (range: 19–62 years); 67 patients (45.89%) were men. Age was <30 years in 117 eyes (41.48%). Of the 16 eyes with keratectasia from the 60,325 myopic LASIK procedures (0.0003%), one had a postoperative CCT <400 μm. This eye was not included in the study because of preoperative irregular topography suggesting subclinical keratoconus. Corneal ectasia also developed in the fellow eye of the same patient with postoperative CCT >400 μm. The MEL 80 excimer laser was used in 71 eyes (25.18%), the Technolas Keracor 217Z laser (tissue-saving program) in 64 eyes (22.69%), and the Technolas Keracor 217C or Z laser (standard program) in the remaining 147 eyes (52.13%). Preoperative mean keratometry reading was >47.00 D in ten eyes (3.54%). Preoperative manifest refraction SE was >−8.00 D in 111 eyes (39.36%) and astigmatism >3.00 D in 21 (7.44%). Three eyes (1.06%) had a preoperative Orbscan posterior float >50 μm. Preoperative CCT was <500 μm in 84 eyes (29.78%) and postoperative residual stromal bed thickness <300 μm in 28 eyes (9.92%). All eyes had normal topography before surgery. Preoperative clinical data are shown in Table 1.
The mean flap thickness (intraoperative measurements after flap lifting) was 74.76±13.57 μm (range: 55–124 μm). In none of the thin flaps did the surgeon mention the presence of an epithelial flap (as in epithelial LASIK) or a Bowman layer in the stromal bed. The consistency of the flap indicated that the stromal layer was included in it. The mean stromal ablation was 113.23±16.88 μm (range: 50–157 μm), resulting in a mean residual stromal bed thickness of 317.34±13.75 μm (range: 275–356 μm). The mean PTA was 37.12%±3.62% (range: 27.25%–49.26%). More than 40% of tissue was altered in 59 eyes (20.92%). Postoperative clinical data at the last follow-up visit are shown in Table 2.
The predictability index in eyes that achieved a postoperative SE within ±1.00 D and within ±0.50 D was 73.49 and 55.80, respectively. The efficacy index was 0.94, and the safety index was 1.17. Retreatment was for undercorrection in 23 eyes (8.15%), which was treated with LASIK enhancement. No loss of CDVA was observed after retreatment. Three eyes lost ≥1 line of CDVA during follow-up owing to cataract formation.
Intraoperative complications were detected in six eyes (2.12%) and comprised one buttonhole, one free flap, and four epithelial defects. Postoperative complications such as diffuse lamellar keratitis, epithelial ingrowth, delayed epithelial healing, infection, or keratectasia were not recorded.
Discussion
Post-LASIK cornea <400 μm is a rare finding. In order to ensure that a sufficient number of samples are available for analysis, data must be obtained from several surgeons. Consequently, the inclusion of 15 surgeons was not considered to lead to bias. On the other hand, in Clinica Baviera, ophthalmologists have a standard protocol and training for their refractive procedures. For the same reason, the analyzed eyes are not as homogeneous as desired according to parameters such as age or preoperative refraction.
Our results provide evidence that postoperative thin cornea alone may not be a risk factor for the development of ectasia after LASIK. Corneal thickness after LASIK comprises flap and residual stromal bed thickness. As corneal tensile strength is not uniform throughout the central stroma, with a progressive weakening in the deeper 60%, the relative extent of biomechanical alteration after refractive surgery, expressed as depth, plays a clear role in postoperative weakening.10,11
Flap thickness is a key variable in this alteration, as the anterior lamellar flap does not contribute significantly to postoperative corneal tensile strength.12 Therefore, the thinner the flap, the safer the LASIK procedure in terms of ectasia. Thin flaps have been thought to predispose to flap folds and postoperative irregular astigmatism, with an increasing risk for loss of visual acuity and poorer quality of vision.14 In our study, flap thickness ranged from 55 to 124 μm (mean, 74.76±13.57 μm) and was measured by 15 surgeons. Rainer et al15 reported that the correlation coefficients for the intraobserver variability were between 0.987 and 0.995 for ultrasound pachymetry measurements. Thin flaps up to 40 μm have been reported and can be explained by the margin of error of ultrasound pachymetry and the fact that thin corneas can have thin central epithelial thickness.16,17 Borderie18 found that the results of three consecutive measurements of CCT by ultrasound pachymetry were within 10 μm. In their study using optical coherent tomography, Wirbelauer et al19 showed that mean geometrical central epithelial thickness ranged from 45 to 92 μm. Using very high-frequency digital ultrasound, Reinstein et al20 reported that corneal vertex epithelial thickness ranged from 43.5 to 63.6 μm in 110 eyes. The human corneal epithelium represents ~10% of the total corneal thickness.21 Lin et al22 reported visual acuity and anatomic results in a large sample of thin-flap LASIK cases (45–130 μm) and found no significant anatomic complications. Femtosecond lasers have also been used to create a thin flap (90–120 mm), with reproducible results.23 The safety of the techniques for creating very thin flaps described here should be verified in studies with larger samples. Femtosecond laser may play a greater role in this type of surgery in the future.
Preoperative CCT was <500 μm in 84 eyes (29.78%). There are various reports of successful LASIK in patients with corneas thinner than 500 or 470 μm.16,24,25 Consequently, thin cornea is a major risk factor for ectasia, but is not the only factor.
The depth of ablation, together with flap thickness, determines the relative amount of biomechanical change after LASIK. Santhiago et al6 recently reported that a percent tissue altered ≥40% at the time of LASIK was significantly associated with ectasia in eyes with normal preoperative topography. However, in our study, 59 eyes (20.92%) had more than 40% of tissue altered with no signs of ectasia after more than 3 years of follow-up.
We found that the lowest postoperative residual stromal bed thickness was 275 μm. Rao and Epstein26 postulate that the main risk factors for ectasia are residual stromal bed thickness and preexisting abnormal corneal topography. From 1999 to 2002, a residual stromal bed thickness of 250 μm was attempted. After 2002, the minimal residual stromal bed thickness was calculated preoperatively to be 300 μm.5 However, ectasia can occur in patients with no obvious risk factors.6,27 Kymionis et al24 measured the residual stromal bed thickness in eyes with thin CCT that underwent LASIK or photorefractive keratectomy to correct myopia. The lowest residual corneal bed thickness was 276 μm in the LASIK group and 308 μm in the photorefractive keratectomy group.22 The authors did not observe complications after a minimum 1-year follow-up.
All the patients in our study had at least 3 years of follow-up (6.89±2.35; range, 3.02–11.41 years). Length of follow-up is important, especially for post-LASIK patients, because corneal ectasia develops during the first year after LASIK in only 50% of cases.13
Rao et al28 suggested that patients with positive keratoconus screening test results have higher anterior and posterior elevation on Orbscan topography. Some authors consider Orbscan posterior float >50 μm a risk factor for corneal ectasia.3,5 We used the anterior and posterior corneal elevation, together with the anterior and posterior best-fit sphere, as additional parameters to evaluate the potential development of postoperative ectasia. In our study, three eyes (1.06%) had a preoperative Orbscan posterior float >50 μm. We will continue to study the occurrence of late-onset keratectasia in the future.
We observed that a postoperative corneal thickness <400 μm might not be a contraindication to LASIK. No major intraoperative or postoperative complications were found. LASIK has been performed on eyes with preoperative thin corneas of which some of the corneas would presumably have postoperative CCT thinner than 400 μm;16,24,25 however, we were unable to find data on outcomes of LASIK in eyes with post-LASIK thin corneas.
In our study, the predictability index in eyes that achieved a postoperative SE within ±0.50 D and within ±1.00 D was 55.80 and 73.49, respectively. Yuen et al29 observed that the overall predictability index over 10 years ranged from 56.4% to 83% for SE within ±0.50 D, while Alió et al2 reported a predictability index of 73% for SE within ±1D. The safety index we recorded was 1.17, which is comparable with findings from other large, long-term studies (Alió et al,2 1.08; Yuen et al,29 1.04). The overall efficacy index in long-term LASIK studies on myopic eyes has been reported to be approximately 0.87.2,29 In our study, the efficacy index was 0.94.
The limited number of eyes in our study may have prevented us from detecting ectasia. A larger sample or a longer follow-up may have uncovered cases of ectasia. However, we did not aim to detect the incidence of ectasia after corneal laser ablation; rather, we evaluated the safety of LASIK in a group of patients for whom laser refractive surgery is controversial.
Conclusion
In summary, our long-term study indicates that post-LASIK corneal thickness <400 μm may not be the cutoff value for LASIK. We detected no severe late-onset or irreversible complications, such as ectasia. We recommend careful selection of candidates for surgery, with emphasis on normal corneal topography, a residual stromal bed thickness >275 μm, and creation of thin flaps.
Disclosure
The authors report no conflicts of interest in this work.
References
Sugar A, Rapuano CJ, Culbertson WW, et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy; a report by the American Academy of Ophthalmology (Ophthalmic Technology Assessment). Ophthalmology. 2002;109:175–187. | ||
Alió J, Muftuoglu O, Ortiz D, et al. Ten-year follow-up of laser in situ keratomileusis for myopia of up to – 10 diopters. Am J Ophthalmol. 2008;145:46–54. | ||
Tabbara KF, Kotb AA. Risk factors for corneal ectasia after LASIK. Ophthalmology. 2006;113:1618–1622. | ||
Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37–50. | ||
Binder PS, Trattler WB. Evaluation of a risk factor scoring system for corneal ectasia after LASIK in eyes with normal topography. J Refract Surg. 2010;26(4):241–250. | ||
Santhiago MR, Smadja D, Gomes BF, et al. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014;158(1):87–95. | ||
Randleman JB, Trattler WB, Stulting RD. Validation of the Ectasia Risk Score System for preoperative laser in situ keratomileusis screening. Am J Ophthalmol. 2008;145(5):813–818. | ||
Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25(4):388–403. | ||
Febbraro JL, Buzard KA, Friedlander MH. Reoperations after myopic laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(1):41–48. | ||
Schmack I, Dawson DG, McCarey BE, Waring GO 3rd, Grossniklaus HE, Edelhauser HF. Cohesive tensile strength of human LASIK wounds with histologic, ultrastructural, and clinical correlations. J Refract Surg. 2005;21(5):433–445. | ||
Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54(2):1418–1425. | ||
Dawson DG, Edelhauser HF, Grossniklaus HE. Long-term histopathologic findings in human corneal wounds after refractive surgical procedures. Am J Ophthalmol. 2005;139(1):168–178. | ||
Guirao A. Theoretical elastic response of the cornea to refractive surgery: risk factors for keratectasia. J Refract Surg. 2005;21:176–185. | ||
Jacobs JM, Taravella MJ. Incidence of intraoperative flap complications in laser in situ keratomileusis. J Cataract Refract Surg. 2002;28:23–28. | ||
Rainer G, Petternel V, Findl O, et al. Comparison of ultrasound pachymetry and partial coherence interferometry in the measurement of central corneal thickness. J Cataract Refract Surg. 2002;28(12):2142–2145. | ||
Djodeyre MR, Ortega-Usobiaga J, Beltran J, Baviera J. Long-term comparison of laser in situ keratomileusis versus laser surface ablation in corneas thinner than 470 μm. J Cataract Refract Surg. 2012;38(6):1034–1042. | ||
Prandi B, Baviera J, Morcillo M. Influence of flap thickness on results of laser in situ keratomileusis for myopia. J Refract Surg. 2004;20(6):790–796. | ||
Borderie V. J’ai testé pour vous. Les pachymètres [I have tested for you. Pachymetry devices]. J Fr Ophthalmol. 2006;29:40–44. French. | ||
Wirbelauer C, Scholz C, Engelhardt R, Laqua H, Pham DT. Biomorphometrie des Hornhautepithels mittels spaltlampenadaptierter optischer Kohärenztomographie [Biomorphometry of corneal epithelium with slit lamp-adapted optical coherence tomography]. Ophthalmologe. 2001;98:848–852. | ||
Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24:571–581. | ||
Erie JC, Patel SV, McLaren JW, et al. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness: a confocal microscopy study. Ophthalmology. 2002;109:1447–1452. | ||
Lin RT, Lu S, Wang LL, Bradley J. Safety of laser in situ keratomileusis performed under ultra-thin corneal flaps. J Refract Surg. 2003;19:S231–S236. | ||
Moshirfar M, Hatch BB, Chang JC, Kurz CJ, Eugarrios MF, Mifflin MD. Prospective, contralateral comparison of 120-μm and 90-μm LASIK flaps using the IntraLase FS60 femtosecond laser. J Refract Surg. 2011;2:7251–7259. | ||
Kymionis GD, Bouzoukis D, Diakonis V, et al. Long-term results of thin corneas after refractive laser surgery. Am J Ophthalmol. 2007;144(2):181–185. | ||
Tomita M, Watabe M, Mita M, Waring GO. Long-term observation and evaluation of femtosecond laser-assisted thin-flap laser in situ keratomileusis in eyes with thin corneas but normal topography. J Cataract Refract Surg. 2014;40(2):239–250. | ||
Rao SN, Epstein RJ. Early onset ectasia following laser in situ keratomileusis: case report and literature review. J Refract Surg. 2002;18:177–184. | ||
Ambrósio R, Dawson DG, Salomão M, Guerra FP, Caiado AL, Belin MW. Corneal ectasia after LASIK despite low preoperative risk: tomographic and biomechanical findings in the unoperated, stable, fellow eye. J Refract Surg. 2010;26:906–911. | ||
Rao SN, Raviv T, Majmudar PA, Epstein RJ. Role of Orbscan II in screening keratoconus suspects before refractive corneal surgery. Ophthalmology. 2002;109:1642–1646. | ||
Yuen LH, Chan WK, Koh J, Mehta JS, Tan DT; SingLasik Research Group. A 10-year prospective audit of LASIK outcomes for myopia in 37932 eyes at a single institution in Asia. Ophthalmology. 2010;117(6):1236–1244. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.