Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei
Authors Dos Santos Ramos MA, Calixto G , Gaspar de Toledo L, Vidal Bonifácio B, Campaner dos Santos L, Gottardo de Almeida MT, Chorilli M, Bauab TM
Received 18 July 2015
Accepted for publication 7 October 2015
Published 16 December 2015 Volume 2015:10(1) Pages 7455—7466
DOI https://doi.org/10.2147/IJN.S92638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Matheus Aparecido dos Santos Ramos,1 Giovana Calixto,2 Luciani Gaspar de Toledo,3 Bruna Vidal Bonifácio,1 Lourdes Campaner dos Santos,4 Margarete Teresa Gottardo de Almeida,3 Marlus Chorilli,2 Taís Maria Bauab1
1Department of Biological Sciences, School of Pharmaceutical Sciences, 2Department of Drugs and Medicine, School of Pharmaceutical Sciences, São Paulo State University, Araraquara, 3Department of Infectious Diseases, Faculty of Medicine of São José do Rio Preto, São José do Rio Preto, 4Department of Organic Chemistry, Chemistry Institute, São Paulo State University, Araraquara, São Paulo, Brazil
Abstract: Vaginal infections caused by Candida krusei are a problem of extreme complexity due to the intrinsic resistance to azole drugs. The species Syngonanthus nitens (Bong.) Ruhland is a plant of the Eriocaulaceae family that has demonstrated promising antifungal activity. In phyto-formulation research, liquid crystal precursor mucoadhesive systems (LCPM) stand out as drug delivery systems for vaginal administration because they increase the activity and overcome the problems associated with plant-based medicines. Therefore, the objective of this study was to evaluate the potential of the methanolic extract of scapes of S. nitens (S. nitens extract [SNE]) and an SNE-loaded LCPM against C. krusei as prophylaxis for vulvovaginal candidiasis. LCPM formulation developed consisted of oleic acid as the oil phase (50% w/w), polyoxypropylene (5) polyoxyethylene (20) cetyl alcohol (40% w/w) as the surfactant and a polymeric dispersion containing 2.5% Carbopol® 974P and 2.5% polycarbophil (10% w/w) as the aqueous phase. LCPM formulation developed was characterized using polarized light microscopy, rheological analysis, and in vitro mucoadhesive studies. Different strains of C. krusei, including one standard strain (American Type Culture Collection 6258) and three clinically isolated strains from the vaginal region (CKV1, 2, and 3), were used to determine the minimum inhibitory concentration, inhibition of biofilms, and time kill. The in vivo prophylaxis assay was performed using the standard strain (American Type Culture Collection 6258). The analyses of F by polarized light microscopy and rheology showed isotropy; however, the addition of 100% artificial vaginal mucus (F100) made it more viscous and anisotropic. Moreover, the mucoadhesive strength was modified, which makes F an excellent formulation for vaginal applications. SNE was active against all strains studied, with minimum inhibitory concentration values ranging from 125 to 62.5 µg/mL; after incorporating SNE into F (FE), these values decreased to 62.5 to 31.2 µg/mL, demonstrating that incorporation into the formulation potentiated the action of SNE. Additionally, the time kill assays showed that both forms of SNE were capable of controlling growth, thereby suggesting a possible fungistatic mechanism. Unloaded SNE was not active against C. krusei biofilms, but FE was active against a clinical strain (CKV2). In vivo analysis showed that FE was able to prevent the development of infection following 10 days of administration. We concluded that the formulation developed in this study was an important vehicle for the delivery of SNE based on the improved antifungal activity in all in vitro and in vivo analyses. Furthermore, the extract incorporated into the system may serve as an important prophylactic agent against vaginal infections caused by C. krusei.
Keywords: precursor system of mucoadhesive liquid crystal, nanostructured system, prophylaxis, Candida krusei
Introduction
Infections in the vaginal environment caused by yeast of the genus Candida are common in patients in different age groups. These episodes cause the infection known as vulvovaginal candidiasis (VVC), which is characterized as true inflammation of the vulva and vagina with evolution capability for more advanced stages of the disease, such as recurrent VVC and even the development of cervical cancer.1
The distribution of Candida spp. in cases of VVC varies greatly depending on geographical locations and the study population. However, while Candida albicans is the most common cause of VVC (80%–90%), other species (known as non-albicans Candida) can also induce vulvovaginitis, including Candida krusei, Candida glabrata, Candida tropicalis, and Candida parapsilosis. Recently, cases of non-albicans Candida have become a matter of concern because some of these yeast strains are resistant to the drugs available for therapeutics, such as C. krusei.2,3 This yeast species recently emerged as an opportunistic fungus with a high degree of complexity in antifungal therapy for infected patients, especially immunocompromised patients. C. krusei presents an intrinsic resistance profile to derived drug azoles such as fluconazole, the primary drug used in cases of fungal infections.4 Thus, the treatment of cases of infection by this yeast is often weak and unsatisfactory for adherence to treatment therapy, thereby generating high costs and exposing the host to drugs that have a higher risk of side effects (ie, amphotericin B, voriconazole, posaconazole, and ravuconazole) when the infectious agent is resistant to fluconazole.5
Episodes of multidrug resistance to antifungal agents have led to investigations of natural products during the search for a new therapeutic arsenal,6 resulting in a multitude of research aimed at elucidating this potential. The study by de Freitas Araújo et al7 showed the therapeutic applicability of the methanolic extract of scapes of Syngonanthus nitens (Bong.) Ruhland (Eriocaulaceae) in combating VVC caused by C. albicans. This plant species was demonstrated to be attractive based on its antifungal mechanism, which relied on the production of secondary metabolites (ie, flavones and xanthones) that were active against microorganisms. Pacífico et al8 identified 17 compounds (ie, flavones, xanthones, and six new molecules) in S. nitens extracts (SNEs) that contributed to the expansion of biological studies on this species. The estrogenic and mutagenic profiles of this species were evaluated by de Oliveira et al.9 The authors found that xanthones isolated from the S. nitens methanolic extract might be useful as phytoestrogens, thereby providing an opportunity to develop new hormonal agents. Additionally, the presence of flavones and xanthones suggest that it could be used as a new antimutagenic agent based on the achievement of satisfactory results in research.
Medicinal plants can present solubility problems. These issues can be overcome by developing drug delivery nanostructured systems that enhance the solubility and stability and decrease the toxicity of the plants to improve their pharmacological parameters.10
Among these nanostructured systems, the precursors system of liquid crystals has been demonstrated to be a formulation with good adhesiveness in the mucosae.11 The crystals are formed by surfactant molecules that aggregate in the presence of water to form a variety of structures. When these surfactants incorporate water, they may localize at the interface between oil and water, resulting in various liquid crystal structures that can be used for different types of applications. The liquid crystal precursor mucoadhesive systems (LCPMs) have numerous applications. LCPMs are formed by molecules of surfactant and oil that aggregate in the presence of water. Thus, when these surfactants incorporate water from, for example, vaginal mucus, they may localize at the interface between oil and water, resulting in various liquid crystalline structures that can be used for different types of applications. Once these structures have presented mucoadhesive properties, they are advantageous for the topical treatment of diseases of microbial origin and VVC.12,13
Due to its mucoadhesive property, the LCPM promotes better physical stability and a large potential solubilization of drugs, which is advantageous for the incorporation of plant extracts. The process can be performed in both the polar and nonpolar parts of the system, depending on their solubility and the possibility of merger between the surfactant molecules.10
Considering the benefits of the SNE and its antifungal activity and the use of nanotechnology for drug delivery systems with mucoadhesive properties, this study evaluated the potential of this extract loaded or not loaded in a precursor mucoadhesive liquid crystal system to serve as prophylaxis for VVC caused by C. krusei.
Materials and methods
Materials
Polyoxypropylene (5) polyoxyethylene (20) cetyl alcohol (PPG-5-CETETH-20) was purchased from Croda (Campinas, Sao Paulo, Brazil). Oleic acid (OA) was purchased from Synth (Diadema, Sao Paulo, Brazil). Polycarbophil® and Carbopol® 974P were purchased from Lubrizol® AdvancedMaterials (Cleveland, OH, USA). High-purity water was prepared with a Millipore Milli-Q Plus purification system (EMD Millipore, Billerica, MA, USA); its resistivity was 18.2 MΩ-cm. Mucin from porcine stomach type II, amphotericin B, fluconazole, estradiol, cyclophosphamide, 2,3-bis(2-methoxy-4-nitro-5- sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) sodium salt, triphenyltetrazolium chloride, methanol and acid 3-(N-morfolino) propanosulfônic were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Vegetable plant and preparation of the SNE
The collection of plant material was performed in Serra do Jalapão in the state of Tocantins, Brazil. A number of SPF 189975 voucher specimens were deposited in the IB-USP (Instituto de Biociências- Universidade de São Paulo) São Paulo, Brazil. The plant extract was prepared by an exhaustive extraction method of simple percolation using methanol as the solvent.14 The extract was concentrated under reduced pressure in a rotary evaporator (48 hours) at a temperature below 40°C and lyophilized.
Ternary phase diagram construction and preparation of liquid crystal precursor formulations
Ternary phase diagram construction was performed by weighing and mixing different combinations of OA as the oil phase, PPG-5-CETETH-20 as the surfactant, and a polymeric dispersion containing 2.5% Carbopol® 974P and 2.5% Polycarbophil® as the aqueous phase (w/w) at room temperature (25°C±0.5°C). Each final formulation had a pH of 5.5.
The ternary phase diagram was constructed using Sigma Plot Software based on the structures identified by polarized light microscopy (PLM) described below.
Formulation F was selected as the precursor system of the mucoadhesive liquid crystals. SNE was added to F, resulting in formulation FE.
Artificial vaginal mucus (AVM) was prepared and added to the F and FE formulations in a 1:1 (w/w) ratio to generate F100 and FE100, which were used to evaluate the in situ phase behavior of the formulations. A volume of 1 L of AVM was prepared by mixing 3.51 g of NaCl, 1.40 g of KOH, 0.222 g of Ca(OH)2, 0.018 g of bovine serum albumin, 2.00 g of lactic acid, 1.00 g of acetic acid, 0.16 g of glycerol, 0.4 g of urea, 5.0 g of glucose, and 15 g of mucin. After complete mixing, the pH was adjusted to 4.2 using 0.1% HCl.15
Structural characterization of selected formulations
PLM
A drop of each formulation was placed on a glass slide, covered with a coverslip, and examined under polarized light at room temperature (25°C±0.5°C) using an Olympus BX41 polarized light microscope coupled with a Q-Color3 camera (Olympus Corporation, Tokyo, Japan). The magnification was 20×.16
In vitro evaluation of the mucoadhesive force
The mucoadhesive force between the vaginal mucosa and the formulations was assessed in a detachment test using a TA-XT plus texture analyzer (Stable Micro Systems, Surrey, UK). Freshly excised pig vaginal mucosae were obtained from a local slaughterhouse, cleaned, and frozen at −30°C until the day of the experiment. After defrosting, a 2 mm thick section was taken from the inner part of the surface of the vaginal mucosa and fitted onto the mucoadhesion test rig. Then, 50 μL of AVM was applied to the surface of the tissue prior to the experiment.16 The formulations were packed into shallow cylindrical vessels, and the analytical probe was lowered to begin the test. The probe, which contained the mucosa, moved at a constant speed (1 mm·s−1) on the surface of the formulation. The mucosa and the formulation were kept in contact for 60 seconds, and no force was applied during this interval. After 60 seconds, the mucosa was drawn upward (0.5 mm·s−1) until the contact between the surfaces was broken. The mucoadhesive force of the formulations was measured in the maximum detachment force as the resistance to the withdrawal of the probe, which reflects the mucoadhesion characteristics. Seven replicates were analyzed at 37°C±0.5°C.17
Determination of flow properties
The rheological measurements were performed at 37°C±0.1°C in triplicate using a controlled-stress AR2000 rheometer (TA Instruments, New Castle, DE, USA) with parallel plate geometry (40 mm diameter) and a sample gap of 200 μm. The samples of the formulations were carefully applied to the lower plate to minimize sample shearing and were allowed to equilibrate for 3 minutes prior to analysis.
The flow properties determined using a controlled shear rate procedure ranged from 0.01 to 100 s−1 and back. Each stage lasted 120 seconds with an interval of 10 seconds between the curves. The consistency and flow indices were determined from the power law described in Equation 1 for a quantitative analysis of flow behavior:
t = κ·γη | (1) |
where “t” is the shear stress, “γ” is the shear rate, “κ” is the consistency index, and “η” is the flow index.18
Biological assays
In vitro antifungal assays
Fungal strains
We employed one strain from the American Type Culture Collection (C. krusei ATCC 6258) and three clinical strains isolated from a female genital tract diagnosed positive for VVC and presenting multidrug resistance to azole drug derivatives (CKV1, CKV2, and CKV3. The clinical strains were donated to the Microbiology Laboratory of the Faculty of Medicine of Sao Jose do Rio Preto for purposes of scientific research through a written consent of the donors. The use of these strains was approved by the Human Research Ethics Committee CEP-FAMERP (Comitê de Ética em Pesquisa - Faculdade de Medicina de São José do Rio Preto) and received the protocol number 152/2006.
The yeast was maintained on Sabouraud dextrose broth plus 20% glycerol and frozen at −20°C. For use, they were subcultured in 2 mL of Sabouraud dextrose broth and incubated at 37°C for 48 hours.
Determination of the minimum inhibitory concentration (MIC)
The evaluation of the antifungal activity by MIC determination was accomplished by the dilution in microplate technique19 with modifications. The SNE was dissolved in 20% dimethyl sulfoxide at an initial concentration of 2,000 μg/mL. Roswell Park Memorial Institute (RPMI) 1640 medium was added to each well of the microplate (96 wells) and the pH was adjusted to 7.0 with acid 3-(N-morfolino) propanosulfônic buffer. A total of 100 μL of unloaded SNE solution (SNES) and FE (with SNE at 2× concentration of the MIC) were added at concentrations ranging from 1,000 to 7.8 μg/mL. Cultures of yeast incubated for 48 hours were transferred to sterile phosphate-buffered saline (PBS), adjusted to a 0.5 McFarland scale (106 CFU/mL) and confirmed by the spectrophotometric reading at 530 nm and cell counting in a Neubauer chamber. One 1/1,000 dilution was made to provide a suspension of 103 CFU/mL, and 100 μL of the microorganism suspensions were distributed into each well of the microplate.
Amphotericin B and fluconazole were used as the positive controls. Culture medium, yeast growth, plant extracts, and solvent were also used as controls. The microplates were incubated at 37°C for 48 hours. After incubation, 20 μL of aqueous 2% solution of 2,3,5-triphenyltetrazolium chloride was added, and the plates were incubated at 37°C for 2 hours.7 The tests were performed in triplicate.
Assay of inhibition of the biofilm
The biofilm adhesion method was performed as described by Pitangui et al20 with modifications. Initially, 100 μL of inoculum (5.0×108 cells/mL) suspended in saline (0.9%) was added to the wells in the microplates (96 wells). The samples (ATCC 6258 and CKV1, 2, and 3) were incubated under rotation at 80 rpm at 37°C for 2 hours. After the pre-adhesion period, the supernatant was removed and 100 μL of RPMI medium was added to each microplate well; then, the incubation proceeded at 37°C for 48 hours with RPMI renewed after 24 hours. After all of the incubation periods, the supernatant was removed, and the wells were washed with 100 μL of (0.9%) saline. Next, 100 μL of serial dilutions of SNES and FE were generated over a range of 20 to 0.6 mg/mL. Amphotericin B was used as a positive control. Culture medium, yeast growth, SNES, F, FE, and dimethyl sulfoxide were also used as controls.
The microplates were re-incubated for 24 hours at 37°C. The XTT® was used as the developer.
Time kill assay
This test was performed with the standard strain (ATCC 6258) and the clinical strain that was most sensitive in the MIC assay (CKV3). One mL of the SNE (unloaded and loaded in F) at a 2× concentration of the MIC was added to a test tube containing 9 mL of RPMI 1640 culture medium with the previously standardized yeast (2.5×103 cells/mL). The mixture was incubated at 37°C. After 0, 0.5, 1, 2, 4, 8, 12, 24, 36, and 48 hours, 500 μL of the media was removed, and the contents were diluted in buffer solution in sterile PBS at a 1:1 ratio. The samples were subjected to centrifugation at 5,000 rpm for 2 minutes; then, the entire supernatant was discarded, and the precipitate was resuspended in 1 mL of PBS. From this suspension, a 1:10 dilution was performed with PBS and 100 mL was seeded onto the surface of Sabouraud dextrose agar plates with the aid of a sterile Drigalski handle. After 48 hours of incubation, we proceeded to count the colonies.21
In vivo prophylaxis assay of VVC
The prophylactic assay was developed with the strain C. krusei ATCC 6258. This experiment was developed according to the methods reported by de Freitas Araújo et al7 and Zeng et al22 with modifications. Wistar female rats (Rattus norvegicus) that were 8 weeks old and weighed 200–300 g were employed. The animals were maintained throughout the experiment in the bioterium of the School of Pharmaceutical Sciences of Araraquara – UNESP, Brazil, with adequate temperature and ventilation, a 12-hour light/dark cycle, and free consumption of water and food during the course of all experiments. The animals were housed in cages with dimensions of 50×60×22 cm lined with previously sterilized wood shavings. They were moved to the experimental room 7 days prior to the start of the procedures, which is consistent with the ethical standards of research development with animals at the current institution. This assay was approved by the Animal Research Committee CEUA/UNESP (Comitê de ética do uso de animais/Universidade Estadual Paulista Júlio de Mesquita Filho) and received the protocol number 34/2013.
Experimental groups and treatment applied
The animals were divided into six groups of four animals each. Table 1 presents all considerations relating to the experimental groups.
  | Table 1 General considerations of the experimental groups of the prophylactic assay |
Group 2 was used to evaluate the impact of immunosuppression on the development of infection. Group 4 was used as antifungal controls for the use of a vaginal cream based on tetracycline hydrochloride (25 mg/g) + amphotericin B (12.5 mg/g) (EMS®, Sao Paulo, Brazil). Groups 5 and 6 (treatment with S. nitens unloaded/loaded) were administered the extract at a concentration of 2× the MIC value of the unloaded extract obtained in the in vitro screening (250 μg/mL).
Experimental conditions and treatment before infection
Initially, the animals in groups 3, 4, 5, and 6 were brought into a state of immunosuppression with the application of cyclophosphamide solution (Sigma-Aldrich Co.; 50 mg/body weight) by the intraperitoneal route on the 1st day of the experiment so that they would become susceptible to further development of the infection. All animals were treated twice daily with their respective therapeutic agents for 10 days prior to the infection. The time of this treatment was based on the time of death and/or decline of fungal growth obtained in the time kill test. From days 5 to 10 of the pre-infection treatment, the animals were subjected to induction of a pseudo-estrus state with once daily subcutaneous injection of estradiol solution (0.2 mg/mL; Sigma-Aldrich Co.). The change in the course of the estrous cycle of the animals was observed by microscopy from vaginal washes with a buffer solution of sterile PBS (100 mL) according to the method described by Marcondes et al23 where the presence of cornfield anucleate epithelial cells represented the desired estrus.
Treatment after infection and analysis of therapy
After 10 days of pre-infection treatment and the induction of a pseudo-estrus state, the animals were infected with a suspension of C. krusei (5.0×106 cells/mL) intravaginally (100 μL) using a micropipette. The animals were rested for 2 days after the infection to allow fixing of the microorganisms. After this period, vaginal washes with sterile PBS were performed to verify the vaginal fungal burden using microscopic observation of the washes and culture in Sabouraud dextrose agar with chloramphenicol.
The treatment lasted for more than 10 days after the infection of the animals. Vaginal washes were collected on days 2, 4, 6, 8, and 10 for microscopic verification of the presence of yeast cells and culturing of the same in Sabouraud dextrose agar with chloramphenicol.
An additional assay was developed with the strain C. albicans ATCC 10231, due to its high prevalence in cases of VVC.7 Just as in the assay with C. krusei, the animals were treated prior to infection with FE and SNES at a concentration of 2× the MIC value of the unloaded extract obtained in the in vitro screening (500 μg/mL). A group of positive infection was also employed to prove that all animals were able to develop the infection.
After the analysis period, the animals were euthanized in a CO2 chamber.
Statistical analysis
Statistical data were analyzed using analysis of variance. We used the Tukey test to compare the results of the treatments and the Dunnett test to compare the results of the treatment and the control.
Results and discussion
Preparation of the ternary phase diagram
A phase diagram was constructed to evaluate the combination of different proportions of the surfactant, oil phase, and aqueous phase used in the formulations.
After the preparation of the various combinations, the resulting formulations were characterized visually as opaque liquid systems (OLS), transparent liquid systems (TLS), translucent liquid systems (TrLS), transparent viscous systems (TVS), and phase separations. This resulted in the construction of the diagram shown in Figure 1.
Using the data from the diagram it was possible to obtain a TVS when the concentrations of the surfactant, the oil phase, and the aqueous phase were 15%–75%, below 40%, and 25%–85%, respectively. The TLS was formed when the concentrations of the surfactant, the oil phase, and the aqueous phase were greater than 20%, below 70%, and below 40%, respectively. The TrLS was formed when the concentrations of the surfactant, the oil phase, and the aqueous phase were 20%–35%, 25%–65%, and 10%–45%, respectively. The OLS were obtained in the regions where the concentrations of the surfactant, the oil phase, and the aqueous phase were below 25%, below 65%, and greater than 40%–100%, respectively. Phase separation occurred in a small proportion of the formulations in which the concentrations of the surfactant, the oil phase, and the aqueous phase were below 30%, 35%–100%, and below 45%, respectively.
All of the formed TLS and TrLS were isotropic because they appeared dark field when visualized under polarized light. In contrast, the TVS were anisotropic because they showed various structures that were typical of liquid crystalline systems, such as Maltese crosses and striae.
A TLS (F) composed of 40% w/w PPG-5-CETETH-20, 50% w/w OA and 10% w/w polymeric dispersion was selected as the liquid crystal precursor system because its liquid phase facilitated vaginal administration by syringe. Then, a phase transition was observed for the TVS region when AVM was added to F, indicating that its conformation had been changed. This can be observed in the dilution line shown in the diagram in Figure 1. The results obtained by PLM showed that the transition of F to F100 modified the structure. F no longer had characteristics of microemulsion (ME) and started to display striae structures. This transition proves that F has the profile of a liquid crystal precursor.
Salmazi et al24 developed a phase diagram consisting of OA as the oil phase, PPG-5-CETETH-20 as the surfactant and 0.5% chitosan dispersion as the aqueous phase with the addition of 16% poloxamer 407 for vaginal administration of curcumin. They obtained a larger area of TVS that suggested the presence of cationic chitosan polymers.
Chitosan and the anionic polymers polycarbophil and Carbopol 974P are widely used in drug delivery systems, mainly due to their gel forming abilities that change the rheological properties of formulations. However, 0.5% chitosan has shown greater ability to influence the system conformation, possibly due to its cationic property that more significantly interacts with the other components of the formulation compared to anionic polymers.18,24
Regardless, we observed a larger region of TVS when we compared the diagram developed in this study to the diagram without the presence of polymers. Thus, the anionic polymers also have the ability to form systems with more organized structures.
Structural characterization by PLM
The photomicrographs obtained from the PLM analysis of F, FE, F100, and FE100 were illustrated in Figure 2.
The SNE-loaded formulations of F and F100 showed the same patterns as the unloaded formulations.
The F and FE photomicrographs showed a dark field characteristic of MEs that were optically transparent and isotropic systems with ultralow interfacial tension.
The F100 and FE100 photomicrographs showed striated structures that might represent the hexagonal liquid crystalline mesophase.24
These results demonstrated that F behaved as a precursor of a liquid crystal system and was able to form an organized liquid crystal mesophase with the incorporation of AVM.
Rheological analysis
The continuous rheological behavior was plotted on a shear rate (Pa) versus shear stress (1/s) graph (as shown in Figure 3), resulting in ascending and descending curves that indicated the flow behavior of the formulations.
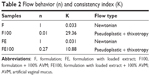
Both formulations without AVM (F and FE) behaved as Newtonian liquids (n=1) without thixotropy in which shear stress was directly proportional to the shear rate. Additionally, the descending curve overlapped with the ascending curve because their initial structure did not change with the shear, which was a characteristic of ME.17,25
These formulations also showed a low viscosity as indicated by the low consistency index (K) in Table 2. Therefore, extract incorporation did not change the flow behavior of the liquid crystal precursor. Indeed, both F and FE have a flow property that renders them suitable for vaginal applications with a syringe.
However, when these formulations (F and FE) came in contact with the AVM, the diluted formulations (F100 and FE100) underwent a change of flow behavior (also shown in the graph of Figure 1). Therefore, these formulations have a pseudoplastic behavior (n<1) with thixotropy (ie, they were able to return to their original structures when the shear stress was removed and they showed an increase in their consistency index [K]). This behavior is characteristic of liquid crystalline systems due to the formation of semisolid crystalline structures.26
The rheological behavior of these formulations is desirable because the FE formulation will flow easily out of the syringe at the moment of administration, thereby facilitating its administration and contact with the vaginal mucus. Moreover, this behavior will increase the formulation viscosity and allow it to subsist for a longer period in the vaginal mucosa and release the extract for a longer time, thereby improving the clinical performance of the treatment.
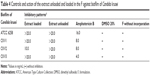
In vitro evaluation of mucoadhesive force
Figure 4 illustrates the mucoadhesive force and mucoadhesive strength of all of the formulations.
Liquid crystalline hexagonal phases have been widely reported in mucoadhesive systems due to their high viscosity. However, this property makes it difficult to apply for vaginal administration. Therefore, the systemic administration of precursor Newtonian liquid crystals that form a liquid crystalline mesophase mucoadhesive in situ has been developed as a strategy to overcome this difficulty.
The results showed that the incorporation of the extract did not cause a significant change in the mucoadhesion of the formulations (P>0.05). However, when the liquid crystal compositions (F and FE) were diluted in AVM there was a significant increase in the mucoadhesive parameters. The F100 and FE100 formulations were able to interact more strongly with the vaginal mucosa and consequently remained for a longer time in the vaginal environment.
Thus, the system designed here stands out as a novel system for vaginal delivery because it combines the advantage of forming a strong matrix liquid crystal in situ with high vaginal mucoadhesive strength.
Biological analysis
Determination of MIC
Table 3 shows the MIC values obtained with the extract unloaded and loaded in F.
The intrinsic resistance to fluconazole and increase in resistance to amphotericin B is a concern for the therapeutic inefficiency presented in cases of C. krusei. Therefore, the search for new drugs that promote inhibition of the fungus in question is extremely relevant.27 In this study, the antifungal screening results used to determine the MIC were innovative because the plant extract showed an inhibitory capacity against all of the strains used. These data confirmed the results observed by de Freitas Araújo et al7 who assessed the in vitro activity of SNE against Candida spp. strains. The potential of plant extracts to exhibit antimicrobial activity is supported by the presence of secondary metabolites in their constitution (ie, phenols and flavonoids). Thus, the presence of these components in plants belonging to the Eriocaulaceae28 family can be indicative of antifungal activity.
The finding that their incorporation into the drug delivery system promoted increased antifungal activity (decreased MIC) proved that F could behave as a potentiating agent of the antifungal activity of the plant extract. Several studies showed that the in vitro antimicrobial activity of natural products was enhanced following the incorporation of drug delivery systems in the nano-scale because they have dynamic interactions with the application route.10
The F components may be responsible for the enhancement of antimicrobial activity because they could possess direct interaction capability with fungal membranes and promote increased cell permeability to facilitate the action of the extract. The use of OA as the oil phase of the system (50%) may have triggered a direct interaction with the yeast cell membranes, thereby increasing the speed of the SNE action. It is classified as an essential fatty acid (omega 9) that is directly involved in human metabolism and plays a key role in hormone synthesis. Based on these characteristics, OA is considered an important component of pharmaceutical formulations and is widely used in cosmetics to present favorable softness.29 In addition to these parameters, OA has been widely used in drug delivery systems to promote the improvement of active principles according to their interactions with membranes, especially the skin.30
Assay of inhibition of the biofilm
According to the results shown in Table 4, the unloaded SNE does not possess action against biofilms. The inhibitory pattern was observed in a strain (CKV2) when the extract was loaded into F. It is possible that the observed action is linked to the increased permeability of the fungal membrane that promotes an increase in the substantivity of the SNE. Similarly, it is also possible that the mucoadhesive property interfered directly with the inhibition of the biofilms because it maintained direct contact with the system containing the extract in a uniform manner with more intense delivery and was fixed in place. The mucoadhesive components (Carbopol 974P, PPG-5-CETETH-20, and Polycarbophil) may have been the main factors underlying this result because we were able to fix the formulation containing the extract directly on the biofilm formed in the well of the microplate, which presented themselves as fixed and uniform films.
Shaikh et al31 showed that the use of mucoadhesive drug delivery systems on biofilms was more suitable for employing polymers that promoted direct adhesion with the biofilm and represented the most promising and effective treatment. This finding demonstrates a role for the use of mucoadhesive polymers as the aqueous phase of the formulation in dispersion. A study by Donnelly et al32 evaluated the action of toluidine blue in a mucoadhesive system to evaluate the applicability of the same in cases of oropharyngeal biofilms caused by Candida spp. The authors observed that the adhesive profile exerted on the biofilm was the primary factor underlying the observed inhibitory action.
Time kill assay
Figures 5 and 6 show the interference of the unloaded and loaded extract on the growth of the strains tested over the course of 48 hours.
  | Figure 5 Time kill assay with Candida krusei ATCC 6258. |
  | Figure 6 Time kill assay with Candida krusei CKV3. |
The observed data demonstrated that the results were different. A comparison of the results between the unloaded and loaded SNE in the assay with C. krusei ATCC 6258 demonstrated that the growth inhibitory action exerted by the SNE loaded into F was higher than the unloaded extract, where the growth was controlled and constant for 8 hours. After this period, the growth increased at a higher intensity; however, the growth remained lower compared to the growth control at the end of 48 hours. The CKV3 strain was more resistant to both forms of extract administration, which was expected because it exhibited the most virulent profile of the clinical strains used in this study. The unloaded extract controlled fungal growth more effectively than the loaded extract, although its growth was checked for 8 hours; after this period, the highly intensive growth rate did not match the growth control at the end of the experiment.
Prophylaxis of VVC
Morphological analysis of the vaginal epithelium established that the animals were in the pseudo-estrus phase after 5 days of application of the estradiol solution. The epithelium displayed cornified cells without nuclei according to the considerations reported by Marcondes et al.23 The vaginal fluid cultures of the infected groups were positive, confirming that all of the animals were under the same conditions prior to the post-infection treatment period.
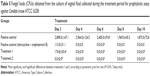
Table 5 presents the general characteristics observed in the experimental assay, including the number of infected animals and the fungal burden of the experimental groups during the experiment.
The treatment performed with the extract loaded into F (group 6) prior to infection was able to prevent the outbreak of the infectious state, showing a successful prophylactic profile for treatment. The animals in this experimental group showed no vaginal fungal burden after intravaginal inoculation of C. krusei; this pattern remained fixed until the end of the experiment, proving that the extract activity loaded into the system could prevent infection.
The animals in experimental group 5 (not treated with the loaded SNE) developed infection after inoculation with the fungal inoculum; however, their fungal burden was reduced compared to the positive controls for infection (groups 2 and 3). The animals were considered cured after 4 days of post-infection treatment, similar to the behavior of experimental group 4 (treated with amphotericin B).
Significant differences were noted compared to the behavior of the positive controls for infection (groups 2 and 3). As expected, group 2 (animals with no immunosuppressive infected state) developed a weak infection that lasted just 4 days because VVC was considered to be an opportunistic infection. The animals in the positive group that was immunosuppressed prior to infection (group 3) remained infected at the end of the experiment.
The results of the control test with C. albicans were significant. The animals of the positive infection group (infected without previous treatment) developed a severe infection (5,305±6.56 CFU/mL). After 10 days of treatment prior to administration of the C. albicans suspension the animals treated with FE did not developed infection (0±0.00 CFU/mL) which shows the ability to prevent the development and proliferation of the strain in the vaginal environment. The animals treated with the SNES developed a discrete infection (211±1.15 CFU/mL). These data show the effectiveness of the FE to prevent a vaginal infection caused by the most prevalent species in cases of VVC. This behavior can be related to the hyphal inhibition capacity performed by SNE, according to data collected in previous studies,7 since the formation of these structures in the vaginal environment is an important factor in the establishment of infection by C. albicans.
We placed emphasis on the results of the prophylactic profile shown by the extract incorporated into F because it may be related to adhesion. The system was developed based on the fixation characteristics of the vaginal environment; thus, materials with adhesive properties were used. The advantage of working with drug delivery systems with this profile is that it results in a more intense delivery because it promotes direct contact with the application site. Thus, there is a complete delivery that prevents the expulsion of the contents by the patient after administration, which is important for a delivery system for the intravaginal route. The choice of surfactant (PPG-5 Ceteth-20 Procetyl®) was based on these characteristics because it exhibits adhesiveness when it comes into contact with water. Therefore, we aimed to trigger adhesion when the system came into contact with vaginal mucus. The adhesive materials in the pharmaceutical formulations may be hydrophilic molecules of natural or synthetic origin that contain numerous organic components (ie, carboxyl groups, hydroxyl groups, and amines) that form chemical bonds with the biological surface.11
The aqueous phase of the system (polymeric dispersion) was also developed to promote adhesion in the vaginal environment. Carbopol 974P and Polycarbophil (constituents of polymeric dispersion) are examples of synthetic polymers that are negatively charged polyacrylic acid derivatives. The bioadhesive behavior is justified based on physical and chemical processes (ie, hydrophobic interactions, hydrogen bonds, and Van der Waals forces) that are controlled by pH and the ionic composition.33 In addition to this characteristic, the use of polymers in the aqueous phase was intended to promote sustained delivery of the plant extract because it is retained in the polymer network and exhibits a slower liberation and controlled manner. In systems that have polymeric network compositions, the drug may be homogenously dispersed in the polymer matrix or adsorbed on their surface or within a reservoir. This phenomenon involves the liberation of the same physical and chemical processes, such as water penetration into the matrix, diffusion of the drug through the pores of the matrix, polymer degradation or a combination of the last two mechanisms.13,34
The use of nanotechnology for drug delivery systems for the incorporation of plant extracts has been shown to have great advantages for vaginal applications. Recently, Bonifácio et al35 evaluated the antifungal potential of the ethanolic extract of the leaves of Astronium urundeuva loaded or not in a nanostructured lipid system to treat VVC caused by C. albicans. The authors found that the incorporation of the extract in the system significantly improved its activity in all in vitro analyses. Additionally, the therapeutic profile was developed when the extract was subjected to an in vivo model of VVC where the animals were cured with only 6 days of treatment. This was superior to treatment with the unloaded extract, which was not effective during the 8 days of treatment.
Conclusion
According to our in vitro and in vivo analyses, we can conclude that the incorporation of the methanolic extract of S. nitens into the precursor system of mucoadhesive liquid crystals increased its antifungal activity. The results demonstrate the possibility of using this extract loaded in the system for prophylaxis of vaginal infections caused by C. krusei. Furthermore, the system can be an effective vehicle for the delivery of drugs using the intravaginal route.
Acknowledgments
We thank grant #2013/25432-0, São Paulo Research Foundation (FAPESP), and the Programa de Apoio ao Desenvolvimento Científico of the Faculdade de Ciências Farmacêuticas de Araraquara, UNESP, Brazil (PADC).
Disclosure
The authors report no conflicts of interest in this work.
References
Sobel JD. Genital candidiasis. Medicine. 2010;38(6):286–290. | ||
Deorukhkar S, Saini S. Non albicans Candida species: its isolation pattern, species distribution, virulence factors and antifungal susceptibility profile. Int J Public Health. 2013;2(3):533–538. | ||
Schuster MG, Meibohm A, Lioyd L, Strom B. Risk factors and outcomes of Candida krusei bloodstream infection: a matched, case-control study. J Infect. 2013;66(3):278–284. | ||
Abbas J, Bodey GP, Hanna HA, et al. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch Intern Med. 2000;160(17):2659–2664. | ||
Kathiravan MK, Salake AB, Chothe AS, et al. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem. 2012;20(19):5678–5698. | ||
Newman DJ, Gragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. | ||
de Freitas Araújo MG, Pacífico M, Vilegas W, et al. Evaluation of Syngonanthus nitens (Bong.) Ruhl. extract as antifungal and in treatment of vulvovaginal candidiasis. Med Mycol. 2013;51(7):673–682. | ||
Pacífico M, Napolitano A, Masullo M, et al. Metabolite fingerprint of “capim dourado” (Syngonanthus nitens), a basis of Brazilian handcrafts. Ind Crop Prod. 2011;33:488–496. | ||
de Oliveira AP, de Sousa JF, da Silva MA, et al. Estrogenic and chemopreventive activities of xanthones and flavones of Syngonanthus (Eriocaulaceae). Steroids. 2013;78(11):1053–1063. | ||
Bonifácio BV, Silva PB, Ramos MA, Negri KM, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines – a review. Int J Nanomedicine. 2014;9:1–15. | ||
Balogu E, Ay Senyigit Z, Karavana SY, et al. In vitro evaluation of mucoadhesive vaginal tablets of antifungal drugs prepared with thiolated polymer and development of a new dissolution technique for vaginal formulations. Chem Pharm Bull (Tokyo). 2011;59(8):952–958. | ||
Carvalho FC, Calixto G, Hatakeyama IN, Luz GM, Gremião MP, Chorilli M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev Ind Pharm. 2013;39(11):1750–1757. | ||
da Silva PB, Ramos MA, Bonifácio BV, et al. Nanotechnological strategies for vaginal administration of drugs – a review. J Biomed Nanotechnol. 2014;10(9):2218–2243. | ||
Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Pedrovick PR. Farmacognosia: da planta ao medicamento [Pharmacognosy: from the plant to the drug]. Porto Alegre: UFRGS; 2010. Portuguese. | ||
Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–95. | ||
Cevher E, Sensoy D, Zloh M, Mulazimoglu L. Preparation and characterisation of natamycin: γ-cyclodextrin inclusion complex and its evaluation in vaginal mucoadhesive formulations. J Pharm Sci. 2008;97(10):4319–4335. | ||
Carvalho FC, Rocha e Silva H, Luz GM, et al. Rheological, mechanical and adhesive properties of surfactant-containing systems designed as a potential platform for topical drug delivery. J Biomed Nanotechnol. 2012;8(2):280–289. | ||
Calixto G, Yoshi AC, Rocha e Silva H, Stringhetti Ferreira Cury B, Chorilli M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: preparation and characterization. Phar Dev Technol. 2015;20(4):490–496. | ||
Clinical and Laboratory Standards Institute. Reference methods for broth dilution antifungal susceptibility tests for yeasts; approved standards. CLSI document (M27-A3). Wayne, PA: Clinical and Laboratory Standards Institute; 2008. | ||
Pitangui NS, Sardi JC, Silva JF, et al. Adhesion of Histoplasma capsulatum to pneumocytes and biofilm formation on an abiotic surface. Biofouling. 2012;28(7):711–718. | ||
Zore GB, Thakre AD, Jadhav J, Karuppayil SM. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011;18(13):1181–1190. | ||
Zeng H, Tian J, Zheng Y, et al. In vitro and In vivo activities of essential oil from the seed of anethum graveolens L. against Candida spp. Evid Based Complement Alternat Med. 2011;2011:659704. | ||
Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–614. | ||
Salmazi R, Calixto G, Bernegossi J, Ramos MA, Bauab TM, Chorilli M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int J Nanomedicine. 2015;10:4815–4824. | ||
Singh R, Tiwari S, Tawaniya J. Review on nanotechnology with several aspects. Int J Res Comput Eng Electron. 2013;2(3):1–8. | ||
Jones DS, Bruschi ML, de Freitas O, Gremião MP, Lara EH, Andrews GP. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and Carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int J Pharm. 2009;373(1–2):49–58. | ||
Li H, Zhang C, Chen Z, Shi W, Sun S. A promising approach of overcoming the intrinsic resistance of Candida krusei to fluconazole (FLC)-combining tacrolimus with FLC. FEMS Yeast Res. 2014;14(5):808–811. | ||
de Freitas Araújo MG, Hilário F, Vilegas W, et al. Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. Int J Mol Sci. 2012;13(7):9260–9277. | ||
Mendes DB, Lemes LS, Cruz RC, Moraes Filho AV, Castro ML, Carneiro LC. Teor de ácido óleico nos óleos de girassol, milho e soja [Oleic acid content in sunflower oils, corn and soybeans]. Rev Trab Acad. 2012;3(6):19–25. Portuguese. | ||
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–618. | ||
Shaikh R, Raghu T, Singh R, Garland MJ, Woolfson AD, Donelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3(1):89–100. | ||
Donnelly RF, McCarron PA, Tunney MM, David Woolfson A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem Photobiol B. 2007;86(1):59–69. | ||
Khutoryanskiy VV. Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci. 2011;11(6):748–764. | ||
Lages CA, Silva JN, Silva Filho EC, Nunes LC, Silva BB. Polímeros mucoadesivos para uso vaginal: uma prospecção tecnológica [Mucoadhesive polymers for vaginal use: a technological prospecting]. Geintec. 2014;4(1):622–631. Portuguese. | ||
Bonifácio BV, Ramos MA, da Silva PB, et al. Nanostructured lipid systems as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int J Nanomedicine. 2015;10:5081–5092. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.