Back to Journals » International Journal of Nanomedicine » Volume 12
Liposomal curcumin and its application in cancer
Authors Feng T, Wei Y, Lee RJ, Zhao L
Received 14 January 2017
Accepted for publication 12 June 2017
Published 21 August 2017 Volume 2017:12 Pages 6027—6044
DOI https://doi.org/10.2147/IJN.S132434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Ting Feng,1,* Yumeng Wei,1,* Robert J Lee,2 Ling Zhao1
1Department of Pharmaceutics, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 2Division of Pharmaceutics, College of Pharmacy, The Ohio State University, Columbus, OH, USA
*These authors contributed equally to this work
Abstract: Curcumin (CUR) is a yellow polyphenolic compound derived from the plant turmeric. It is widely used to treat many types of diseases, including cancers such as those of lung, cervices, prostate, breast, bone and liver. However, its effectiveness has been limited due to poor aqueous solubility, low bioavailability and rapid metabolism and systemic elimination. To solve these problems, researchers have tried to explore novel drug delivery systems such as liposomes, solid dispersion, microemulsion, micelles, nanogels and dendrimers. Among these, liposomes have been the most extensively studied. Liposomal CUR formulation has greater growth inhibitory and pro-apoptotic effects on cancer cells. This review mainly focuses on the preparation of liposomes containing CUR and its use in cancer therapy.
Keywords: curcumin, liposomes, drug delivery, bioavailability, cancer
Introduction
Cancer is a leading cause of deaths around the world. It is a disease characterized by genetic mutations and epigenetic changes, which can be triggered partly by environmental factors including oxidative stress.1 Treatment of cancer varies and typically involves surgery, radiotherapy and chemotherapy. In recent years, molecular targeted therapy and immunotherapy are gaining traction. Complementary therapeutic modalities such as traditional Chinese medicines are also widely used, especially in Asia.2,3 Chemotherapy is the main approach for the treatment of metastatic tumors.4 However, it is associated with serious side effects, such as bone marrow suppression, neurotoxicity, gastrointestinal reaction and liver and kidney damages.5 In addition, chemotherapeutics can induce multidrug resistance, leading to treatment failure upon recurrence of the disease.6,7
Some plant extracts have shown interesting anticancer properties without the serious side effects of cytotoxic agents. Among them, curcumin (CUR), which has anti-inflammatory activities, has also shown activity against cancer through multiple mechanisms, including inhibition of initiation, progression, invasion and metastasis of cancer cells. Venkata et al8 discussed the anti-inflammatory and anticancer activities of CUR and their interconnectedness. It has been shown that CUR acts on protein kinases MAPK, Akt and Bcl-2;9–12 transcription factors NF-κB, AP-1 and STAT-313–20 and enzymes such as COX-2, matrix metalloproteinases (MMPs) and LOX.21–23 Although CUR has numerous pharmacological activities, its poor aqueous solubility (≤0.125 mg/L), low bioavailability, rapid metabolism and rapid systemic elimination are barriers to its clinical application. Optimal pharmacological effects require an oral dose of >8.0 g/day.24 Improving bioavailability of CUR is a major challenge.
In previous decades, different strategies such as liposomes, solid dispersion, complex, emulsion, micelles, nanogels and microspheres have been employed to overcome poor absorption and other limitations of CUR. Song et al25 developed a solid dispersion formulation of CUR with d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and mannitol and showed that solubility, dissolution rate, oral bioavailability and cell permeability of CUR were increased. A study by Singh et al26 showed that silica nanoparticle–CUR complex conjugated with hyaluronic acid improved stability, uptake and cytotoxicity of CUR on COLO-205 cancer cells. Shinde and Devarajan27 reported that microemulsions composed of docosahexaenoic acid could effectively deliver CUR to the brain and inhibit the growth of human glioblastoma U-87MG cell line in vitro. Wei et al28 demonstrated that cholesteryl-hyaluronic acid nanogel of CUR exhibited excellent solubility and sustained drug release under physiological conditions and effectively inhibited human pancreatic adenocarcinoma MiaPaCa-2 cells. Jyoti et al29 investigated chitosan (CS) microspheres of CUR and showed that the microspheres improved solubility and therapeutic index of CUR in HT-29 cells. In addition, it was reported that a series of mesoporous silica material-loaded CUR exhibited higher oral bioavailability and cellular uptake and significantly increased A549, MCF-7 and B16F10 cell apoptosis compared to CUR.30–32 A novel drug carrier composed of sodium alginate, hydroxyapatite bilayer-coated iron oxide nanoparticle composite (IONP/HAp-NaAlg) was synthetized via the co-precipitation approach and used to delivery CUR over 7 days.33 CUR encapsulated in polymeric nanoparticles showed higher solubility and enhanced bioavailability.34 CUR was encapsulated into bovine serum albumin nanoparticles by co-precipitation method and showed higher cytotoxicity on A549 cells, HepG2 cells and RAW264.7 cells compared with the free CUR at the same drug concentration.35 Among drug carriers, liposomes have been extensively studied for many years and shown rather promising prospects for in vivo delivery of CUR. Liposomes are phospholipid bilayer vesicles that can carry both hydrophobic and hydrophilic drugs.36 Liposomes have been used in the delivery of anticancer drugs and are able to alter the biodistribution and clearance of drug molecules.37,38 Intravenously (iv) administered liposomes are taken up by the reticuloendothelial system (RES) after entering the body. Liposomal drugs mainly accumulate in the liver, spleen, lung, bone marrow or other tissues and organs so as to improve the therapeutic index of drugs and reduce their side effects.39 Besides, liposomes are prepared easily and the preparation technology is mature. With the broad application of liposomes, more novel liposomes such as long-circulating liposomes and ligand-modified liposomes have been designed to prolong action time of drugs in blood and target different cancers. A study showed that PEGylated liposomes of mitomycin C were captured by cells and collected at the target site due to enhanced permeability and retention (EPR) effect.40 Recently, folate-modified doxorubicin-loaded nanoliposomes were shown to effectively inhibit the growth of B16F10 melanoma cells. The folate-modified liposomes improved antitumor efficacy and reduced systemic toxicity of doxorubicin.41 Thus, combination of CUR and liposomes should enhance the stability, bioavailability, targeting property and anticancer efficacy of CUR. The purpose of this review is to introduce the preparation method and application of liposomes in CUR and their effects on cancer therapy.
CUR
Physical property
CUR is a natural yellow-color compound extracted from rhizome of turmeric plant Curcuma longa, which belongs to the Zingibaraceae family and is widely grown in Southeast Asia. It is a lipophilic molecule that can permeate the cell membrane easily. There are three main types of curcuminoids including 1,7-bis-4-hydroxy-3-methoxyphenyl-hepta-1,6-diene-3,5-dione (CUR I, ~77%), 1,4-hydroxy-3-methoxyphenyl-7,4-hydeoxyphenyl-hepta-1,6-diene-3,5-dione (demethoxy CUR II, ~17%) and 1,7-bis-4-hydeoxyphenyl-hepta-1,6-diene-3,5-dione (bisdemethoxy CUR III, ~3%) (Figure 1).42 The main commercial CUR is CUR I, which belongs to Biopharmaceutical Classification System (BCS) Class IV.43 The molecular formula, molecular weight and melting point of CUR I are C21H20O6, 368.37 g/mol and 183°C, respectively. And it is extremely sensitive to light, while temperature has little influence on its stability even at 250°C. In solution, when the pH value is >5, CUR I is unstable and its degradation rate significantly speeds up with increasing the pH value of the solution. Trans-6-(4′-Hydroxy-3′-methoxyphenyl)-2,4-dioxo-5-hexenal is a major degradation product, and vanillin, ferulic acid and feruloyl methane are minor degradation products.44,45
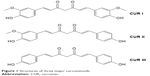  | Figure 1 Structures of three major curcuminoids. |
Pharmacology
CUR is a multifunctional compound from a nature plant and has various therapeutic activities, such as wound-healing, anti-inflammatory, anticarcinogenic, antibacterial, antispasmodic, anticoagulant and anticancer (Figure 2).46,47 In fact, CUR was shown to strongly inhibit NF-κB activity and NF-κB-related pathways to induce cellular apoptosis. NF-κB plays a very important role in establishing the relation between inflammation and cancer.14,48 Moreover, CUR is safe and well tolerated even at very high doses. Cheng et al49 studied safety of CUR in humans and showed that CUR when taken at 8 g daily for 3 months did not produce any toxic effect.
  | Figure 2 Pharmacological activities of CUR. |
Pharmacokinetic properties of CUR have been studied extensively. It has been reported to be difficult to dissolve in water (≤0.125 mg/L) and have very poor bioavailability.50 In an earlier article, Schiborr et al51 investigated the absorption behavior of CUR in mice by oral administration and intraperitoneal injection. It was found that CUR’s content of the oral administration group (at the dose of 50 mg/kg) was below the detection limit in plasma, liver and brain after 30 min while the injection group (at the dose of 100 mg/kg) was 4–5 μg/g. In another study, 100 mg/kg of CUR was injected by caudal vein in mice. Concentrations at 20 min of CUR in liver, kidney, lung and heart determined by high performance liquid chromatography (HPLC) were 8.00 μg/g, 0.35 μg/g, 0.17 μg/g and 0.06 μg/g, respectively. After 40 min, CUR was only measurable in the liver with 0.04 μg/g. After 100 min, CUR was not detected in any tissues.52 Thus, it was suggested that the metabolism of CUR was fast in mice. More recently, accumulated urine of healthy male rats from 0 h to 16 h was analyzed after administrating 500 mg/kg of CUR by liquid chromatography tandem mass spectrometry (LC/MS-MS), and it was found that major metabolites of CUR were tetrahydrocurcumin, dihydrocurcumin, hexahydrocurcumin and their glucuronic acid derivatives (Figure 3).53 At the same time, CUR is absorbed after oral dosing and can be detected by HPLC. After oral administration of CUR, a small amount of CUR existed in the plasma while the contents of CUR glucoside acid and sulfate were higher than in human.54,55 Another study showed that high oral doses of CUR (equivalent to oral CUR for a 1.2 g/kg) only contained CUR at nanomolar concentration in plasma, liver and colon mucosa tissue, which demonstrated that bioavailability of CUR is very low. Bioavailability of CUR by the way of oral administration in rats (200 mg/kg) was only 4.13%.56 A study from Kaminaga et al57 reported that CUR levels in serum remained at 50 ng/mL when 10–12 g/mL of CUR was administered orally in humans.
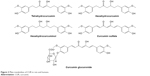  | Figure 3 Main metabolites of CUR in rats and humans. |
Preparation methods for CUR liposomes
Liposomes should be an effective carrier to enhance the stability, bioavailability and targeting property of CUR (Figure 4). Preparation methods for CUR liposomes include thin-film method, freeze–thawing method, injection method, reversed-phase evaporation method, etc.
Traditional preparation methods of CUR liposomes
Thin-film method
Thin-film dispersion method
First, phospholipids, cholesterol (CH) or other lipoids and fat-soluble drugs are mixed in an organic solvent. Second, the solvent is evaporated on a rotary evaporator in a vacuum. When a uniform film appears, aqueous buffer is added to hydrate the lipids to form liposomes. Lin et al58 prepared CUR liposomes for injection using this method. The liposomes were yellow powder. When gently added water for injection, the powder quickly dispersed in water and formed yellow translucent colloidal suspension. Then, characteristics of the CUR liposomes were evaluated, and it was found that the average particle size was 120.1 nm, zeta potential was −50.5 mV, entrapment efficiency was 95.45%±0.86% and drug-loading amount was 4.1%±0.1%. Comparing with CUR injection, the indicators were not observed significant change in the accelerated test. Therefore, the liposomes effectively improved the stability of CUR. In another report, Chen et al59 designed CUR liposomes coated with N-trimethyl chitosan chloride (TMC) by a thin-film dispersion method. The liposomes were composed of soybean phosphatidylcholine (PC), CH and D-α-tocopheryl polyethylene glycol 1000 succinate. The results showed that entrapment efficiency, drug-loading efficiency, particle size and zeta potential of TMC-CUR liposomes were 86.67%±1.34%, 2.33%±0.09%, 657.7 nm and +15.64 mV, respectively. Pharmacokinetic parameters of TMC-CUR liposomes were Cmax =46.13 μg/L, t1/2 =12.05 h and AUC =416.58 μg/L·h, respectively, while those for uncoated CUR liposomes were Cmax =32.12 μg/L, t1/2 =9.79 h and AUC =263.77 μg/L·h. It was demonstrated that the TMC-CUR liposomes enhanced bioavailability of CUR. Gu et al60 prepared carbopol-coated CUR liposomes using thin-film dispersion method. The encapsulation efficiency of CUR liposomes (88.00%±2.7%) decreased slightly compared to that of noncoated CUR liposomes (89.21%±2.3%). However, carbopol-coated CUR liposomes after oral administration in rats showed that the relative bioavailability was 281%, which was 2.22 times as the non-coated CUR liposomes.
Thin-film ultrasonic dispersion method
Thin-film dispersion method yields large and multilamellar vesicles (MLV) that can be processed into 0.25–1 μm small unilamellar vesicles (SUV) through sonication. Sun et al61 prepared novel CUR liposomes with CUR, hydrogenated lecithin, CH and poly(amidoamine) dendrimers and dendrons (PAMAM) lipid globules at the ratio of 1:20:3.26:1.6 (v/v) using thin-film ultrasonic dispersion method. The results showed that the average particle size of CUR liposomes was 97.08 nm and the entrapment efficiency was 99.37%±0.89%. The stability was favorable that there was not any visible change even it was placed at room temperature for 3 months. When the liposomes were placed in water bath at 50°C, 70°C or 80°C for 30 min, no visible change was observed and the entrapment efficiency of CUR was almost unaffected. It is demonstrated that the liposomes had excellent thermotolerance. Under the hydrochloric acid solution condition, tolerant maximum concentration of the CUR liposomes was 1 mol/L. And below the maximum concentration, no activity such as turbidity or aggregation was observed. Thus, thin-film ultrasonic dispersion can be used to prepare CUR liposomes that can tolerate acidic condition encountered after oral administration.
Thin-film hydration method for CUR
Briefly, an ethanol solution of CUR and chloroform solution of lipids were added in a round bottom flask. Then, the organic solvent was evaporated under reduced pressure using a rotary evaporator until a thin film formed. The round bottom flask in a vacuum oven was kept overnight to remove the residual solvent. Next, the thin film of lipids was hydrated using deionized water at 4°C overnight. Then, the solution was sonicated for 10 min at the same temperature.62 Pamunuwaa et al63 prepared CUR liposomes with positive and negative charge using thin-film hydration method. The formulation of CUR liposomes with positive hybrid charge (PHL) was 200 mg of egg yolk PC, 10 mg of CUR, 25 mg of CH, polysorbate 80 and stearylamine (SA), respectively, while CUR liposomes with negative hybrid charge (NHL) did not contain SA. The results showed that the encapsulation efficiencies and loading capacities of PHL and NHL were (54.5±2.2)% and (77.8±5.7)% and (1.9±0.1)% and (3.0±0.2)%, respectively. Besides, particle size and zeta potential of PHL and NHL were (265.3±3.3) nm and (225.7±5.0) nm and (+48.2±0.8) mV and (−48.7±1.7) mV, respectively. In vitro release experiment, NHL (21%) showed faster average release of CUR compared with PHL (9%) at 24 h, which demonstrated that the charge of liposomes has a significant effect on the release properties of liposomal CUR when incorporation of SA in the lipid bilayer may be a strategy to achieve slow release effect for CUR from liposomes. Moreover, the amount of skin deposition of NHL (8.6±1.4 μg/cm2) was higher than PHL (2.5±0.5 μg/cm2) using excised pig ear skin. Thus, CUR liposomes with positive hybrid charge showed favorable slow release behavior. In another study, CUR was incorporated in liposomes as formation of hydropropyl-β- or hydroxypropyl-γ-cyclodextrin (HPβCD or HPγCD) complexes by thin-film hydration method.64 The results showed that the mean diameters of the various CUR liposome formulations ranged between 96.8 nm and 130.3 nm and the zeta potential values measured for all CUR liposome types were slightly negative but close to zero. In terms of loading efficiency for CUR, HPγCD increased it by 2.02 times compared to unloaded CUR. Between the two CD types, HPγCD complex containing liposomes demonstrated 1.41–1.55 times higher loading efficiencies. Stability experiment showed that complex formation had a significant stabilizing effect only at the low CUR concentration conditions. Furthermore, HPβCD complex provides higher stabilization (compared to HPγCD). Thus, the CUR-in-CD-in-liposome hybrid formulations significantly enhanced solubility and stability for CUR. In another study, liposomes-loaded CUR with four lipid compositions including soy phosphatidylcholine (SPC), dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG) and a mixture of DPPC + DPPG (7:3, m/m) were prepared by thin-film hydration technique, respectively.65 Spherical shape and bilayer were observed in four CUR liposomes. All studied liposomes were found to be more stable in terms of size, polydispersity index (PDI), surface charge, thermal behavior, encapsulation efficiency and release behavior than free CUR. Besides, these liposomes demonstrated sustained release behavior. Thus, liposomes as effective drug delivery systems can achieve sustained and prolonged release of CUR.
Freeze–thawing method
This way involves two procedures: first, freezing unilamellar vesicles that contain drugs. Second, liposomes slowly melted to form a new stable kind of liposomes after a period of time. CUR liposomes were prepared by freeze–thawing method.66 It was found that as CH concentration increased, entrapment efficiency of the liposomes decreased and the highest entrapment efficiency was ~98.4% with 3.5 mg CUR and 10 mg CH. Scanning electron microscopy revealed round vesicles with an average size of 131±10 nm. Besides, 10% sucrose was selected as cryoprotectants to protect the liposomes from crystallization during freezing. This study demonstrated that liposomes containing CUR performed excellent entrapment efficiency using this approach.
Freeze-dried method
Liposome suspension is prone to aggregate and undergoes fusion, leakage, oxidation and hydrolysis during storage so that it is difficult to meet the requirements of the stability of pharmaceutical preparations. At present, the freeze-dried method has become one of the ways to improve long-term stability of the liposome preparation. It was reported that hyaluronan, phospholipid and Eudragit S100, which is an anionic copolymer able to form an effective and stable enteric coating with a fast dissolution in the upper bowel, were combined to immobilized liposomes for the intestinal delivery of CUR by freeze-dried method.67 Four different formulations were frozen at 80°C and freeze-dried for 24 h at 80°C and with 10 mg/mL of CUR, 90 mg/mL of phospholipid and at varying eudragit–hyaluronan ratios. The results showed that size ranged from 220 nm to 287 nm, spherical or oval shape and the entrapment efficiency was very similar for all the samples, ranging from 78% to 82%. Dispersibility was good in water, and a homogeneous suspension was formed upon hydration without drug aggregates or precipitate, while CUR liposomes without the polymers were large in size (≥700 nm) and not stable with drug precipitation, which indicated that the polymers contributed to the dissolution of CUR. Using two media to simulate stomach-to-colon transit under the condition of pH 2 and pH 7, it was found that the size of the vesicles did not vary significantly after 2 h while observed only a slight increase in zeta potential (less negative). It is suggested that the polymeric combination protects CUR from damage in gastrointestinal tract. In vivo biodistribution results showed that the amount of CUR found in liver and kidneys was negligible, while the local accumulation in the intestines was favored. In particular, after the administration of the liposomes, the amount of CUR in the jejunum reached 25% while in the colon was negligible with only 3%. It was demonstrated that the vesicles provided a higher CUR deposition in the intestine tracts. Thus, the vesicles showed promising properties for the intestinal delivery of CUR by protecting the polyphenol from the gastric environment.
Solvent injection methods
These include ethanol injection and ether injection methods. In the ether injection method, lipids and hydrophobic drugs are co-dissolved in organic solvent as oil phase. Then, the oil phase is rapidly injected into the water-soluble drugs with stirring. Liposomes are formed when the organic solvent is removed. However, ether injection method is less frequently used in the preparation of CUR liposomes because of the toxicity of ether. Ethanol injection is much more commonly used in liposome preparation. CUR liposomes were prepared using this method.68 A single-factor experiment results revealed that entrapment efficiency of the liposomes was the highest at 72.32% when the dosage of CUR was 1.0 mg, CH:lecithin was at 1:3 (v/v) and phosphate buffer (pH 6.5) volume used was 20.0 mL. The CUR liposomes were spheres or elliptic spheres and had an average particle size of ~830 nm. The abovementioned data suggested that ethanol injection method for the preparation of CUR liposomes was simple and practical. Zhao et al69 have developed propylene glycol liposomes (PGL) as CUR carriers for skin delivery using ethanol injection method. The mean particle size of CUR-PGL was 182.4±89.2 nm compared with CUR liposomes (632.9±184.1 nm). The CUR-PGL liposomes were spherical vesicles. No aggregation or fusion was observed under transmission electron microscopy (TEM), while traditional liposomes exhibited some vesicle aggregation over time. Compared with CUR liposomes, encapsulation efficiency of PGL was higher at 92.74%±3.44%. In terms of drug release behavior, CUR-PGL showed gradual release, with maximum values of cumulative release curve reaching 46%. In stability studies for 3 months, CUR-PGL did not exhibit significant changes in particle size and PDI at room temperature and the encapsulation efficiency remained stable (92.87%) in contrast to the CUR liposomes (64.6%). CUR-PGL prepared by ethanol injection method effectively improved encapsulation efficiency as well as reduced oral dose and side effects of CUR. In another study, Li et al70 prepared silica-coated ethosomes loaded with CUR (CU-SE) using ethanol injection method. The results showed that the mean diameter, PDI, zeta potential and entrapment efficiency of CU-SE were 478.5±80.3 nm, 0.285±0.042, −28.6±7.88 mV and 80.77%, respectively. In vitro release assays demonstrated that CU-SE was stable and showed nearly no release in 3 h and then gradually released CUR over time in 2% sodium dodecyl sulfate (SDS) artificial intestinal fluid. Besides, the bioavailability of CU-SE was 11.86-fold higher than that of CUR suspensions. Thus, the CU-SE not only significantly promoted the stability of CUR but also improved the bioavailability relative to CUR.
Reversed-phase evaporation method
In this method, phospholipids are dissolved in chloroform, ether or another non-water miscible organic solvent. Then, the solution of drug to be encapsulated is added and a w/o emulsion is prepared by means of short-time ultrasonic. After removal of the solvent by rotary evaporation, the residual inversed emulsion is diluted in the buffer solution. Gel filtration chromatography or ultracentrifugation can be used to separate unentrapped drugs from liposomes. Zhao71 prepared CUR liposomes using reversed-phase evaporation method. Stable, homogeneous and semitransparent CUR liposomes were obtained by the optimum formula (lecithin:CH:CUR =60:15:1), pH of phosphate-buffered saline (PBS) was 6.5 and ultrasonic processing time was 5 min. The average entrapment efficiency for CUR was 95.06%. In his other study, vitamin A CUR liposomes were prepared by the same method.72 The results showed that the average entrapment efficiency for CUR was 89.3%±0.62% and VA binding rate was 61.3%±0.79%. In in vitro release, there was sustained release. These data revealed that it was reasonable, simple and feasible for CUR liposomes to be prepared by reversed-phase evaporation method. VA-CUR liposomes were reported by Meng et al.73 The results showed that the average entrapment efficiency for CUR was 89.67%±0.47% and VA binding rate was 62.35%±0.77%. In a stability study, the VA-CUR liposome was stored at 4°C and 25°C for 30 days. The results showed that the entrapment efficiency, peroxide value and VA binding rate of the liposome changed negligibly at 4°C. However, the entrapment efficiency and VA binding rate decreased and peroxide value of the liposome increased at 25°C. It demonstrated that liposomes should be stable at 4°C.
As stated earlier, traditional methods including thin-film dispersion method, thin-film ultrasonic dispersion method, thin-film hydration method, freeze–thawing method, freeze-dried method, injection method and reversed-phase evaporation method (Figure 5) can be used to prepare CUR liposomes with high entrapment efficiency and good stability.
CUR nanoliposomes
Nanoliposomes have many advantages such as sustained release, tumor targeting, low toxicity, high stability, high bioavailability and less oral dose of drugs.74 Drugs can be encapsulated or incorporated into the lipid bilayer.75 In terms of stability, absorption and distribution in the body, they have the special effects that can carry hydrophilic, hydrophobic and amphiphilic drugs and directly deliver them to the target tissues. For example, Sun et al76 chose CS that has been widely known in pharmaceutics for parenteral and oral carries as polymeric materials and tripolyphosphate (TPP) to develop CUR nanoparticle systems at the ratio of CUR:CS:TPP =3:24:8 (w/w). The trial showed that the average size, zeta potential and drug loading of the CUR nanoliposomes were 110.5±5.6 nm, 18.3±0.7 mV and 13.7%±0.12%, respectively. And encapsulation was up to 84.2%±2.50% by measuring the concentration of unencapsulated drug. Stability studies revealed that the CUR nanoliposomes were hermetically placed at 4°C, and there was no significant change after 10 months. Moreover, after rats were administrated the CUR nanoliposomes at an oral dose of 100 mg/kg and CUR suspension as the control group, HPLC analyzed plasma samples and found that Cmax and the area under the curve of the CUR nanoliposomes were greater than CUR suspension. Besides, the relative bioavailability reached 448% that significantly improved the bioavailability of CUR. It is probably because CS with positive charge prolonged contact time of drug with absorptive surface leading to better bioavailability. In another study, Shin et al77 prepared chitosan-coated curcumin nanoliposomes (CS-Cur-NLs) using the ethanol injection method (Figure 6). Encapsulation, particle size and zeta potential of CS-Cur-NLs with 0.1% CS coating were 54.70%, 101.42 nm and −14.10 mV, respectively. The stability of CS-Cur-NLs was evaluated by measuring the change in mean size at 4°C and 25°C for up to 40 days. And the particle size was measured at 1 day, 3 days, 5 days, 7 days, 15 days and 40 days. The results showed that the mean size was slightly (P<0.05) increased both at 4°C and at 25°C until 5 days and a little decreased further during storage, which demonstrated that the liposomes had satisfactory stability. Moreover, Hasan et al78 prepared CUR nanoliposomes, respectively, using salmon, soya and rapeseed as lecithin. Three liquids came from the same type of fatty acids but appeared different results. Compared with free liposomes, the average size of CUR nanoliposomes that was measured by dynamic light scattering was smaller (135.5±0.1 nm, 110.3±0.8 nm and 133.1±0.8 nm with respect to salmon, soya and rapeseed). The maximum solubility of salmon, soya and rapeseed was 0.28±0.03 mg/mL, 0.27±0.03 mg/mL and 0.25±0.05 mg/mL, respectively, in comparison to free CUR (8.33 μg/mL). And entrapment efficiency was 67.3%±1.1%, 55.0%±1.1% and 63.2%±0.7%, respectively. Thus, CUR, which is prepared as nanostructured liposomes preparation, not only can get rid of its own disadvantages such as weak oral stability, bad absorption, fast metabolism and low bioavailability but also preforms the properties of the nanoparticle materials so as to effectively improve drug transport. Moreover, CUR nanoliposomes were prepared by combining thin film and dynamic high-pressure microfluidization (DHPM).79 The results showed that particle size, PDI, zeta potential and entrapment efficiency of the nanoliposomes were 68.1±1.5 nm, 0.246, −3.16±0.34 mV and 57.1%±1.1%, respectively. TEM showed that the apparent morphology of the CUR nanoliposomes was spherical shape. And the solubility of CUR was 490.8 μg/mL. It indicated that water solubility of CUR was significantly improved by nanoliposome encapsulation. Fourier transform infrared spectoscopy (FT-IR) verified that CUR was successfully incorporated into the nanoliposomes because CUR’s main absorption peaks weakened and the peaks shifted when encapsulated in nanoliposomes. In vitro drug release, the nanoliposomes was stable for 24 h and possessed an integral structure under the in vitro release conditions. Besides, CUR nanoliposomes displayed a slow diffusion rate across the dialysis bag and only 19.8%±6.2%, 47.8%±6.5% and 63.1%±5.1% of CUR was released from the nanoliposomes at 2 h, 5 h and 24 h, respectively, whereas free CUR approximately released 93.4%±3.7% after 5 h. In stability of CUR against metal ions experiment, the stability of CUR in nanoliposomes was improved. There was no sediment in CUR nanoliposomes after mixing with Fe3+, Fe2+ and Cu2+ ions at the beginning of the reaction, while free CUR could chelate with metal ions and sediments with different colors were generated immediately. After 2 h of incubation, there was 70.3%±5.2%, 56.5%±2.1% and 76.5%±7.5% of the remaining CUR in nanoliposomes in Fe3+, Fe2+ and Cu2+ ions solution while in free CUR was 25.5%±1.2%, 18.8%±0.7% and 4.8%±0.7%, respectively. It is demonstrated that nanoliposomes encapsulation could improve the stability of CUR against metal ions. However, the nanoliposomes encapsulation cannot prevent CUR hydrolysis at pH 12.0 because high alkalinity caused the degradation of the nanoliposomes’ phospholipid layer resulting in the rapid leakage and hydrolysis of CUR. In addition, CUR nanoliposomes were separately stored at 4°C and 25°C for 90 days. The results showed that the size and zeta potential did not change obviously and the encapsulation efficiency slightly decreased at 4°C. However, when stored at 25°C, the average diameter increased rapidly from 68.1±1.5 nm to 847.9±38.7 nm, the encapsulation efficiency decreased by 16% and the zeta potential negligibly changed. It indicated that CUR nanoliposomes be suitable for storing at 4°C. Overall, nanoliposomes are a type of available method to improve the stability, sustained release and bioavailability of CUR. Nanoliposomes as a kind of drug carrier in some aspects such as preparation methods, technology and route of administration are gradually maturing and widely used. With the constant understanding in depth on normal body tissues, lesion groups and material science, nanoliposomes are gradually making progress.
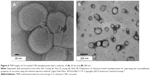  | Figure 6 TEM images of CS-coated CUR nanoliposomes with a scale bar of (A) 20 nm and (B) 200 nm. |
However, there are still some problems. For example, when nanoliposomes enter the systemic circulation, they are easily phagocytized by monocyte–macrophages.80 After the treatment, they easily concentrate on tissues of rich endothelial cells such as liver, spleen and kidney, which occupy so large range that targeting specificity is not strong and the effective dose on target areas will decrease. At present, the main way to solve the problem is using PEG or ligand modification.
Long-circulating CUR liposomes
Hydrophilic polymers such as PEG can be used to coat the surface of liposomes to form long-circulating liposomes. The polymer forms a steric protective layer that can prevent plasma protein from binding to liposomes and thereby reduce liposome clearance by the RES. Thus, the liposomes are able to exhibit prolonged blood circulation.81,82 Guo and Wang83 prepared long-circulating PEGylated CUR liposomes using thin-film dispersion method. The results showed that the average particle size, polydispersity coefficient, zeta potential and entrapment efficiency were 115±8 nm, 0.17±0.02, −4.8±0.4 mV and 76.1%, respectively. It was demonstrated that long-circulating liposomes were distributed uniformly without aggregation. In calf serum, the particle size of the long-circulating liposomes did not change after 12 h. Therefore, the long-circulating PEGylated CUR liposomes had better stability. You et al84 prepared CUR long-circulating liposomes (CUR-LCL) using ethanol injection method. The results showed that the average particle size, zeta potential, entrapment efficiency and drug loading of CUR-LCL with circular shape were 110±3.20 nm, −5.8±0.87 mV, 80.25%±1.61% and 2.06%±0.06%, respectively. The in vitro release study demonstrated that the CUR-LCL had sustained-release effect. After tail iv administration, half-life of CUR-LCL in rats was 13 times that of CUR and 1.8 times that of CUR liposomes. After CUR-LCL was stored at 4°C for 3 months, its entrapment had no significant change. In another study, a kind of long-circulating nanoliposomes of CUR were prepared by ethanol injection method.85 The results showed that the average diameter of the CUR liposomes was 136±18 nm and the encapsulation efficiency was 88.27%±2.16%. Besides, there was no change on encapsulation efficiency within 30 days. In another report, a cationic liposome-PEG-polyethylenimine (PEI) for the encapsulation of hydrophobic CUR was prepared (Figure 7).86 TEM analyzed the structure and revealed that the liposomes had a roughly spherical shape with hair-like projections on the surface. And the average size of the liposomes ranged from 258 nm to 269 nm, zeta potential was +40 mV and the encapsulation efficiency was 45%±0.2%. In vitro drug release experiment, only 10%–15% of the CUR was released from the liposomes at 4°C and 30% of the CUR was released at 25°C after 120 h.
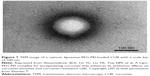  | Figure 7 TEM image of a cationic liposome-PEG-PEI-loaded CUR with a scale bar of 100 nm. |
Ligand-modified CUR liposomes
Ligands can be used to direct liposomes to specific receptors. Therefore, to improve the therapeutic effect, generate synergy, reduce side effects, decrease dosage, shorten period of treatment and produce specific targeting effects on the body with high selective drugs, researchers considered to combine CUR with ligands and incorporated them into liposomes. Meng et al73 prepared vitamin A-modified CUR liposomes (VA-CUR-L) using reverse evaporation method. The results under the condition of optimum prescription measured average encapsulation rate of 89.67%±0.47% and the average binding rate for VA was 62.35%±0.77%. The oxidative product of phospholipids contained 0.097 μg/mg that was lower than hemolytic dosage (0.1 μg/mg). This showed that PC had good stability in this prescription. Moreover, VA-CUR-L showed significant sustained-release effect in in vitro release experiments. In stability test, it was found that the liposomes were more stable when stored at 4°C than 25°C for 30 days. Folate receptors (FRs) are overexpressed in many tumor cells. Folate-modified liposomes are targeted to tumors via FR endocytosis, which could bypass multidrug resistance.87 Lu et al88 incorporated CUR and folic acid in liposomes (F-CUR-L) using a thin-film dispersion method. The size, zeta potential and drug-loading efficiency of the F-CUR-L were 182.3±13.5 nm, −26.1±4.3 mV and 67.3%±8.0%, respectively. The liposomes had satisfactory stability. Moreover, 8 μg/mL of free CUR and F-CUR-L were, respectively, diluted in PBS (pH 7.4) and incubated in a water bath at 37°C for 0–240 min. The formulations of F-CUR-L were well dispersed, while the free CUR in the equivalent quantity of F-CUR-L (666.7 μg/mL) could not be dissolved in the same amount of PBS buffer. This showed the enormous improvements of liposomal CUR in aqueous solubility. Thus, the F-CUR-L largely improved the stability and solubility of CUR. Hyaluronic acid-targeted curcumin liposome (HA-CUR-L) was prepared by reverse evaporation method.89 Particle size was distributed uniformly, and the average particle size was 160 nm. The average entrapment rate and the binding rate of HA-CUR-L were 88.75% and 71.69%±0.45%, respectively. The total accumulative in vitro release amount of HA-CUR-L was 34.12%, while CUR was as high as 90.12%. And after 36 h, the accumulative release amount of HA-CUR-L and CUR was 82.26% and 98.55%, respectively. The data demonstrated that HA-CUR-L had sustained release. In another study, nanoliposome-loaded CUR with 0.1% and 0.5% (w/v) sodium hyaluronate was prepared.90 Both formulations were measured by cryogenic electron transmission microscopy and found that the shape was spherical with a single membrane. When CUR was encapsulated in the sodium hyaluronate liposomes, mean diameters and zeta potential were smaller than free CUR liposomes. And entrapment efficiencies have effectively improved from ~66% to ~79%. In stability studies, both formulations were more stable at room temperature for 90 days in comparison with free CUR liposomes. The data demonstrated that sodium hyaluronate is promising for the delivery of CUR. Liposomes were first reported in 1965 for the first time. Since then, liposomes have been developing very fast from traditional liposomes. Nowadays, liposomes have offered huge potential for the development of CUR. For example, some liposomes were applied in CUR and revealed great performances in entrapment efficiency, stability, solubility and bioavailability. Besides, these CUR liposomes still have excellent cancer-fighting properties.
Application of CUR liposomes on cancer
CUR liposomes give us an important tool that improves pharmacokinetic properties and therapeutic value of CUR. Table 1 summarizes liposome-based delivery systems of CUR used for the treatment of cancers including lung, cervical, prostate, breast, osteosarcoma (OS) and liver cancers. Numerous studies have indicated that anticancer properties of CUR are related to enzymes such as COX-2, AMPK, MMPs, NADPH and LOX; transcription factors such as NF-κB, AP-1, β-catenin and STAT-3 and protein kinases and growth factors such as MAPK, AKT, JAK, VEGF, ERK, PKA and Bcl-2 (Figure 8).13,91–94 More details are discussed later.
  | Table 1 A list of liposome-based delivery systems of CUR on cancer |
Lung cancer
Lung cancer is one of the most common primary malignant tumors.95 Over the past 2 decades, morbidity of lung cancer has increased 11% in China, and 80% of first clinical confirmed cases have been advanced stage. Lung cancer patients will reach 1 million in China, which will have the largest number of patients in the world in 2025.96 Common therapeutic methods of lung tumor include surgical resection, radiotherapy and chemotherapy. Even so, 5-year survival rate is only 16%, though new drugs of anti-lung cancer such as angiogenesis-targeted drugs, epidermal growth factor receptor (EGFR)-targeted drugs, protein peptides of anti-lung cancer and lung cancer antibody drugs were developed.97–102 Recently, a study reported that nanoliposomes using sodium hyaluronate and trimethyl CS form polymer-glycerosomes that can effectively deliver CUR to lung so as to improve the therapeutic index of CUR.103 Recently, many studies reported that CUR liposomes have anticancer activity. Wang et al104 prepared CUR liposomes using ethanol injection method. Lewis lung carcinoma in mice model was established and used to evaluate the antitumor effect of CUR liposomes by MTT method. Right-hind legs of 12 female mice at 6 weeks were inoculated with 5×105 LL/2 cells. Experimental group was injected CUR-liposomes 200 μL/day iv for 2 weeks, while the control group was injected saline. The results showed that with increase of drug concentration from 5 μg/mL to 40 μg/mL, the cell survival rate gradually decreased. And cells distribution percentage of 40 μg/mL of CUR-liposomes (43.6%±1.3%) was less than the control group (51.3%±2.1%) in S phase, while cells distribution percentage of the liposomes (19.1%±0.4%) was higher than control group (10.3%±0.87%) in G2/M phase. It was demonstrated that the CUR liposomes made LL/2 cells stagnate in G2/M phase. Besides, it was observed that the liposomes inhibited angiogenesis of tumor. Thus, the CUR liposomes significantly inhibited the LL/2 tumor cells’ growth and had stronger anti-lung cancer effect. In another study, Lin et al86 prepared a cationic liposome-PEG-PEI-encapsulated CUR and tested on CUR-sensitive A549 cells. IC50 of the liposomes and free CUR was 1.4±0.1 μM and 30.0±9.5 μM, respectively, which demonstrated that the liposomes were more potent than free CUR. CUR-PEG-PEI liposomes were shown to rapidly accumulate in the cytosol over 2.5-h incubation, while free CUR was undetectable for 4 h. Furthermore, CUR-PEG-PEI liposomes showed that 1.5 μg of CUR accumulated in cells within the 2.5 h and continued to gradually increase. From these results, it is revealed that the liposomes enhanced the delivery of CUR into A549 cells that increased the cytotoxic efficacy of the drug. To increase solubility and anticancer property of CUR, β-cyclodextrin–CUR (βCD-C) inclusion complexes were prepared and encapsulated into liposomes by Rahman et al. βCD-C liposomes efficiently inhibited proliferation of lung cancer A549 cells.105 The median effective dose (ED50) of βCD-C liposomes and free CUR was 2.9 μM and 1.5 μM, respectively. Apiratikul et al106 synthesized CH-based cationic liposomes. ED50 of CUR and liposomal CUR was 10 μM and 50 μM, respectively, which demonstrated that the cytotoxicity on A549 cells of liposome-encapsulated CUR had five times higher activity than that of free CUR. Besides, it was found that the liposomes were not toxic to the normal cells even when the IC50 value was up to 600 μM, which suggested that the CUR liposomes had quite low adverse effects on normal cells.
Cervical cancer
Cervical cancer is the second most common type of malignant tumors of women all over the world. Human papilloma virus (HPV) infection has been identified as the main cause of cervical cancer, especially the type of HPV16 and HPV18. According to World Health Organization (WHO) statistics, there are 50 new cases and 274,000 deaths every year. Among them, >83% of cases are from developing countries.107–110 It was reported that cationic liposomes could selectively accumulate in tumorous vascular endothelial cells so that cationic liposomes were made from antitumor carriers that would target tumorous vascular to reduce the toxicity of antitumor drugs.111,112 CUR-loaded cationic liposomes were prepared by Saengkrit et al113 using conventional thin-film hydration method and investigated the inhibitory activity against HPV18- and HPV16-positive cells. The results revealed that half maximal inhibitory concentration of Hela and SiHa cells was 21 μM and 16 μM, respectively. The reason why CUR-loaded cationic liposomes exhibited more cytotoxicity than free liposomes might be charge-induced cell death. Apoptosis assay showed that the more liposomal surface charge was, the more antitumor effect of CUR had. At the same concentration of CUR, CUR-loaded cationic liposomes showed a gradual increasing apoptosis, while there was no sign of cell damage in the free liposomes. Huang et al114 used carboxymethyl dextran (CMD) to modify CUR-loaded liposomes for improving the anticancer efficacy of CUR on Hela cells. It was found that CMD-Cur-Lip was stable for at least 72 h upon incubation with calf serum. In HeLa cells, liposomal encapsulation enhanced the cytotoxicity of CUR. With increase of CUR concentration, all groups showed higher cytotoxicity, among which CMD-Cur-Lip exhibited the strongest cytotoxicity. IC50 of CMD-Cur-Lip was 6.6 μM in comparison with free CUR (24.8 μM). Therefore, the CUR liposomes were more effective.
Prostate cancer
Prostate cancer (PCa) is one of the most common malignant tumors in elderly men. Its incidence ranks first in the United States in all male malignant tumors.115 In the last few years, it has significantly increased in China. Endocrine therapy is the primary method to treat PCa. But after treatment for 14–30 months, almost all patients will gradually develop androgen-independent prostate cancer (AIPC).116–119 Radiotherapy, chemotherapy and biological treatment are of limited effectiveness. Finally, the median survival period is <20 months. Abnormal activation of androgen receptor (AR) signal in AIPC plays an important role. However, CUR can cut down transcription activity and inhibit expression of AR.120 Tian et al121 investigated antitumor efficacy and the biochemical mechanisms triggered of CUR liposomes in PC-3 human PCa cells. MTT assay showed that compared with free CUR, the survival rate of PC-3 cells treated by CUR liposomes was lower in a concentration- and time-dependent manner. Using fluorescence microscope and HPLC to investigate the uptake of CUR liposomes on PC-3 cells, it was found that liposomes could promote CUR uptake into cell, and cellular fluorescence intensity duration was stronger and longer lasting than the control group. Reverse transcription polymerase chain reaction (RT-PCR) and Western blot methods, respectively, were used to detect the expression of MMP-2-messenger RNA (mRNA) and protein levels. Then, results showed that with the increase of concentration of CUR liposomes, MMP-2-mRNA and its protein expression level were gradually reduced. Therefore, the trial implied that the CUR liposomes promoted PC-3 cells ingesting drug-containing liposomes so as to enhance the cytotoxicity of intracellular drugs. Simultaneously, PC-3 cells were inhibited by downregulation of MMP-2 levels. CUR liposomes have a long-lasting activity. Thangapazham et al122 prepared CUR nanoliposomes containing phospholipids:CH:CUR at a ratio of 90:10:10 (w/w). PCa LNCaP and C4-2B cells were used to examine the efficacy of various concentrations of CUR-loaded nanoliposomes and free CUR by MTT at 570 nm. The results showed that LNCaP cells were more sensitive to nanoliposomal CUR than C4-2B cells. After 48 h incubation, nanoliposomal CUR resulted in death of 70%–80% cells while free CUR needed 10-fold higher doses to reach similar inhibition. Thus, the trial showed that nanoliposomes improved the bioavailability of CUR. In another study, Narayanan et al123 encapsulated CUR and resveratrol in liposomes and examined their chemopreventive effect on PCa in male B6C3F1/J mice. The liposomes significantly increased CUR level in serum and prostate tissues (P<0.001). In vitro study revealed that CUR plus resveratrol effectively inhibited cells growth and induced apoptosis. In vivo studies showed that the liposomes significantly decreased growth of prostatic adenocarcinoma (P<0.001). Molecular targets of CUR in prostate cancer include Akt, cyclin D1 and AR.
Breast cancer
Breast cancer is a common malignant tumor and a serious threat to the lives of women. The morbidity and mortality data of breast cancer are gradually increasing. Concurrently, the patients seem to be getting younger. In addition to surgery and radiation therapy, the primary treatment is chemotherapy. But adverse reactions to chemotherapy greatly reduce the patient’s quality of life.124,125 CUR has been demonstrated that it has inhibitory effect on the breast cancer cells.126,127 Hasan et al78 prepared nanoliposomal CUR and evaluated its antitumor activity on MCF-7 cells. Nanoliposomal CUR showed significant inhibitory effect on breast cancer MCF-7 cells with dose-dependence. In this context, CUR might cause cell cycle arrest and induce apoptosis. Thus, nanoliposomes enhanced bioavailability of CUR that will help to enhance its effect on cell proliferation. In another study, 2-hydroxypropyl-γ-cyclodex-trin/CUR liposome complex showed promising anticancer potential both in vitro and in vivo against MCF-7 breast cancer cell line.128 The IC50 of CUR-loaded-γ-CD liposomes was 11.5±1.1 μg/mL in comparison with free CUR (20±1.8 μg/mL). Besides, the study showed the CUR liposomes had no adverse effects even at the highest concentration of 28 μg/mL. Moreover, CUR in combination with paclitaxel (PTX), which were encapsulated in liposomes, significantly inhibited the growth of MCF-7 cells compared with CUR or PTX alone. It was demonstrated that the liposomes could effectively deliver both CUR and PTX to tumor cells.129
Osteosarcoma
OS is a primary bone cancer that commonly occurs in the longer bones of children and adolescents, particularly distal femur and proximal tibia.130,131 Ceramides are sphingolipids and play an important role in cell differentiation, cell cycle arrest, apoptosis, growth inhibition and senescence.132 CUR has shown potent anticancer activity against all stages of cancer because of its action on NF-κB, TNF-α, VEGF, cyclooxygenase, MMP and many other signal transduction molecules. Hence, providing exogenous ceramide along with CUR could be a useful combination to treat cancer cells. Dhule et al133 combined CUR with C6 ceramide in liposomal nanoparticles to examine the antitumor potential against OS cell lines. Three formulations of liposomes were prepared: CUR liposomes, C6 liposomes and C6-CU liposomes. Results showed that compared with CUR liposomes, cytotoxic effect was improved 1.5 times by CU-C6 liposomes in the case of MG-63 and KHOS cell lines. Importantly, C6-CU liposomes were found to be less toxic. In addition, cell cycle assays on a KHOS cell line revealed that C6-CU liposomes induced G2/M arrest by upregulation of cyclin B1 and induced G1 arrest by downregulation of cyclin D1, while CUR liposomes only induced G2/M arrest and C6 liposomes only induced G1 arrest. Therefore, C6-CU liposomes enhanced the cytotoxic effect and validated the potential of combined drug therapy. In another study, CUR-loaded-γ-CD liposomes were synthesized and evaluated its anticancer effect on KHOS cells.128 Results showed that KHOS cells have a strong sensitivity to the liposomes, with IC50 of 6.4±0.7 μg/mL. And cytotoxic effects of the liposomes were three to four times stronger than non-liposomal formulations (IC50: 22.8±1.9 μg/mL). The data demonstrated that the liposomes formulation promoted CUR uptake in the KHOS cells and were more effective than free CUR.
Liver cancer
Liver cancer is a common cancer and a leading cause of cancer deaths in China. In cancer registration areas in 2009, the incidence of liver cancer was 28.71/100,000, making it the fourth most common cancer in China. The mortality of liver cancer was 26.04/100,000 making it the second leading cause of cancer death in China.134 The incidence and mortality of liver cancer in recent years remained high worldwide. In 2010, the incidence and mortality of liver cancer were 27.29/100,000 and 23.76/100,000, respectively, while in 2012, the values were 22.3/100,000 and 21.4/100,000, respectively.135 These data confirmed that liver cancer was a common and fatal cancer in China. It was reported that CUR had anti-hepatoma effect. CUR induced apoptosis of liver cancer cells mainly through 3 mechanisms: 1) regulate apoptosis-related proteins including the Bcl-xl and the Bcl-xs. 2) Control cytochrome C and ROS release. 3) Adjust the cyclin and induce cell cycle arrest including caspase-3 and caspase-8 pathways. Besides, CUR can lead to mitochondrial and nuclear DNA damage in liver cancer cells, especially its mitochondrial DNA.136–138 A study by Li et al139 investigated the inhibitory effect of CUR liposomes on Bel-7402 cells after storage for up to 12 months. With the extension of time, the inhibitory effects of Bel-7402 cells’ proliferation and apoptosis inducement showed no significant decrease. At 12th month, the inhibitory effects of drug (0.25 μg/mL, 5 μg/mL and 10 μg/mL) were very weak but still had certain effect. The apoptosis rate of CUR liposomes was 63.7%±7.2% at 12th month while that of CUR solution was 9.2%±3.5%. Thus, liposomes significantly improved the effect of CUR on inhibiting the Bel-7402 multiplication and inducing apoptosis. In another study, CH-based cationic liposomes as drug delivery vehicles for CUR were synthesized.106 Free and liposome-encapsulated CUR cytotoxicity against HepG2 cell line was assessed. The results showed that ED50 values of the liposomes and free CUR were 4 μM and 30 μM, respectively. It is suggested that the cationic liposomes exhibited 7.5 times higher cytotoxicity than free CUR. Such effect was in part mediated by downregulation of bcl-2 expression and upregulation of bax. Another study reported that CUR could inhibit growth and angiogenesis of tumor and significantly reduce expression of COX-2 and VEGF in HepG2 liver cancer tissues.140,141 CUR was able to inhibit the occurrence of the capillaries and formation of related heterogeneous network. Thus, neovascularization was suppressed to some extent.
Conclusion
CUR has exerted therapeutic effects in many types of cancer including lung, cervical, prostate, breast, OS and liver cancers. However, in vivo activities of CUR are limited due to its poor solubility and low bioavailability. Liposomes provide a type of effective drug delivery system for CUR. As we discussed in this review, liposomes could enhance antitumor and pharmacological activities of CUR by improving pharmacokinetics and pharmacodynamics and reduce the dosage required for targeting tumor. Especially, CUR was incorporated in liposomes with different supports such as CS, vitamin A, folic acid, hyaluronic acid, β-CD, CMD, silica and PEG conjugates. In addition, drug combination encapsulated in liposomal nanoparticles could also sensitize cancer cells, such as CUR and C6 ceramide in OS cell line. Therefore, the combination of CUR and liposomes may be an ideal strategy in clinical practice to treat cancers. With the constant development of liposomes, CUR liposomes will be more optimized. Meanwhile, clinical application of CUR will be extended.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (81341124, 81101678), Science and Technology Support Project of Sichuan Province (2017JQ0013), Sichuan Provincial Human Resource and Social Security Department (2016-183), the Joint Fund of Sichuan Province, Luzhou City, and Southwest Medical University (14JC0134, 14ZC0026, 14ZC0066) and the Joint Fund of Luzhou City and Southwest Medical University (2015LZCYD-S09 (4/8)).
Disclosure
The authors report no conflicts of interest in this work.
References
Erez A, Shchelochkov OA, Plon SE, Scaglia F, Lee B. Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism. Am J Hum Genet. 2011;88(4):402–421. | ||
Prasanna A, Ahmed MM, Mohiuddin M, Coleman CN. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. J Thorac Dis. 2014;6(4):287–302. | ||
Giacosa A, Morazzoni P, Bombardelli E, Riva A, Bianchi Porro G, Rondanelli M. Can nausea and vomiting be treated with ginger extract? Eur Rev Med Pharmacol Sci. 2015;19(7):1291–1296. | ||
Lang JY. The concept and prospect of combined for tumor. Cancer Control Treat. 2008;21(2):116–118. | ||
Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):951–955. | ||
Lu CS, Shieh GS, Wang CT, et al. Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget. 2017;8(19):30844–30858. | ||
Ji P, Zhang Y, Wang SJ, et al. CD44hiCD24lo mammosphere-forming cells from primary breast cancer display resistance to multiple chemotherapeutic drugs. Oncol Rep. 2016;35(6):3293–3302. | ||
Venkata M, Sripathy R, Anjana D, et al. In silico, in vitro and in vivo assessment of safety and anti-inflammatory activity of curcumin. Am J Infect Dis. 2012;8(1):26. | ||
Ren J, Xu Y, Huang Q, et al. Chabamide induces cell cycle arrest and apoptosis by the Akt/MAPK pathway and inhibition of P-glycoprotein in K562/ADR cells. Anticancer Drugs. 2015;26(5):498–507. | ||
Tham CL, Hazeera HH, Wai LK, et al. The synthetic curcuminoid BHMC restores endotoxin-stimulated HUVEC dysfunction: specific disruption on enzymatic activity of p38 MAPK. Eur J Pharmacol. 2015;749:1–11. | ||
Akkoc Y, Berrak Ö, Arisan ED, et al. Inhibition of P13k signaling triggered apoptotic potential of curcumin which is hindered by Bcl-2 through activation of autophagy in MCF-7 cells. Biomed Pharmacother. 2015;71:161–171. | ||
Tuorkey MJ. Curcumin a potent cancer preventive agent: mechanisms of cancer cell killing. Interv Med Appl Sci. 2014;6(4):139–146. | ||
Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm. 2010;343(9):489–499. | ||
Troselj KG, Kujundzic RN. Curcumin in combined cancer therapy. Curr Pharm Des. 2014;20(42):6682–6696. | ||
Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C. Potential anticancerproperties and mechanisms of action of curcumin. Anticancer Res. 2015;35(2):645–651. | ||
Hu A, Huang JJ, Jin XJ, et al. Curcumin suppresses invasiveness and vasculogenic mimicry of squamos cell carcinoma of the larynx through the inhibition of JAK-2/STAT-3 signaling pathway. Am J Cancer Res. 2014;5(1):278–288. | ||
Zhang C, Li B, Zhang X, et al. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: potential role for STAT-3 and NF-κB signaling. J Invest Dermatol. 2010;130(8):2110–2119. | ||
Shehzad A, Ha T, Subhan F, Lee YS. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr. 2011;50(3):151–161. | ||
Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, Surarit R, Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through STAT3 and PI3K/AKT inhibition. Anticancer Res. 2014;34(4):1857–1864. | ||
Jung KT, Lim KJ. Curcumin, COX-2 and protein p300/CBP. Korean J Pain. 2014;27(4):365–366. | ||
Shishodia S. Molecular mechanisms of curcumin action: gene expression. Biofactors. 2013;39(1):37–55. | ||
Zheng J, Zhou Y, Li Y, et al. Spices for prevention and treatment of cancers. Nutrients. 2016;8(8):495. | ||
Zeinab KH, Maha HD. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(7):3259–3264. | ||
Heath DD, Pruitt MA, Brenner DE, Rock CL. Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783(1): 287–295. | ||
Song IS, Cha JS, Choi MK. Characterization, in vivo and in vitro evaluation of solid dispersion of curcumin containing d-α-Tocopheryl polyethylene glycol 1000 succinate and mannitol. Molecules. 2016;21(10):1386. | ||
Singh SP, Sharma M, Gupta PK. Cytotoxicity of curcumin silica nanoparticle complexes conjugated with hyaluronic acid on colon cancer cells. Int J Biol Macromol. 2015;74:162–170. | ||
Shinde RL, Devarajan PV. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017;24(1):152. | ||
Wei X, Senanayake TH, Bohling A, Vinogradov SV. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: synthesis, pharmacokinetics, and tumor growth inhibition. Mol Pharm. 2014;11(9):3112–3122. | ||
Jyoti K, Bhatia RK, Martis EAF, et al. Soluble curcumin amalgamated chitosan microspheres augmented drug delivery and cytotoxicity in colon cancer cells: in vitro, and in vivo, study. Colloids Surf B Biointerfaces. 2016;148:674–683. | ||
Kim S, Diab R, Joubert O, Canilho N, Pasc A. Core-shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf B Biointerfaces. 2016;140:161–168. | ||
Kotcherlakota R, Barui AK, Prashar S, et al. Curcumin loaded mesoporous silica: an effective drug delivery system for cancer treatment. Biomater Sci. 2016;4(3):448. | ||
Bollu VS, Barui AK, Mondal SK, et al. Curcumin-loaded silica-based mesoporous materials: synthesis, characterization and cytotoxic properties against cancer cells. Mater Sci Eng C Mater Biol Appl. 2016;63:393–410. | ||
Manatunga DC, de Silva RM, Nalin KM, et al. pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur J Pharm Biopharm. 1–39. Epub 2017 Mar 18. | ||
Chopra M, Jain R, Dewangan AK, et al. Design of curcumin loaded polymeric nanoparticles-optimization, formulation and characterization. J Nanosci Nanotechnol. 2016;16(9):9432–9442. | ||
Zhang W, Jiang P, Chen Y, et al. Supressing the cytotoxicity of CuO nanoparticles by uptake of curcumin/BSA particles. Nanoscale. 2016;8(18):9572–9582. | ||
Johnson SM, Bangham AD, Hill MW, et al. Single bilayer liposomes. Biochim Biophys Acta. 1971;233(233):820–826. | ||
Bingham RJ, Olmsted PD, Smye SW. Undulation instability in a bilayer lipid membrane due to electric field interaction with lipid dipoles. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81(5 pt 1):703–708. | ||
Wang XY, Ishida T, Ichihara M, Kiwada H. Influence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in rats. J Control Release. 2005;104(1):91–102. | ||
Liliemark E, Liliemark J, Kållberg N, Björkholm M, Sjöström B, Peterson C. Studies of the organ distribution in mice of teniposide liposomes designed for treatment of diseases in the mononuclear phagocytic system. Pediatr Res. 1995;38(1):7–10. | ||
Patil Y, Amitay Y, Ohana P, Shmeeda H, Gabizon A. Targeting of pegylated liposomal mitomycin-C prodrug to the folate receptor of cancer cells: intracellular activation and enhanced cytotoxicity. J Control Release. 2016;225:87–95. | ||
Lohade AA, Jain RR, Iyer K, et al. A novel folate-targeted nanoliposomal system of doxorubicin for cancer targeting. AAPS PharmSciTech. 2016;17(6):1298–1311. | ||
Liang MT, Davies NM, Toth I. Encapsulation of lipopeptides within liposomes: effect of number of lipid chains, chain length and method of liposome preparation. Int J Pharm. 2005;301(1–2):247–254. | ||
Heger M, van Golen RF, Broekgaarden M, Michel MC. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev. 2013;66(1):222–307. | ||
Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–1876. | ||
Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamtics of curcumin. Thromb Haemost. 1999;82(6):121–123. | ||
Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. | ||
Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the antiinflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. | ||
Rajitha B, Belalcazar A, Nagaraju GP, et al. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulatesthymidylate synthase in colorectal cancer. Cancer Lett. 2016;373(2):227–233. | ||
Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or premalignant lesions. Anticancer Res. 2001;21:2895–2900. | ||
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. | ||
Schiborr C, Eckert GP, Rimbach G, et al. Avalidated method for the quantification of curcumin in plasma and brain tissue by fast narrow bore high performance liquid chromatography with fluorescence detection. Anal Bioanal Chem. 2010;397(5):1917–1925. | ||
Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–494. | ||
Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064. | ||
Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1411–1417. | ||
Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. | ||
Sharma RA, Ireson CR, Verschoyle RD, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. 2001;7(5):1452–1458. | ||
Kaminaga Y, Nagatsu A, Akiyama T, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of catharanthus roseus. FEBS Lett. 2003;555(2):311. | ||
Lin QP, Guo RP, Xu XY. Preparation and quality evaluation of curcumin liposomes for injection. Chin J Nat Med. 2007;5(3):207–210. | ||
Chen H, Wu J, Sun M, et al. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J Liposome Res. 2012;22(22):100–109. | ||
Gu JJ, Deng YJ. Preparation of curcumin liposomes and its oral pharmacokinetics in rats. J Chengdu Med Coll. 2010;5(2):97–100. | ||
Sun J, Han M. Preparation of novel curcumin liposomes and associated preliminary stability study. World Sci Technol. 2008;10(4):66–72. | ||
Aditya NP, Chimote G, Gunalan K, Banerjee R, Patankar S, Madhusudhan B. Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei, infection in mice. Exp Parasitol. 2012;131(3):292–299. | ||
Pamunuwaa KMGK, Karunaratnea V, Karunaratnea DN. Effect of lipid composition on in vitro release and skin deposition of curcumin encapsulated liposomes. J Nanomater. 2016;9:1–9. | ||
Matloob AH, Mourtas S, Klepetsanis P, Antimisiaris SG. Increasing the stability of curcumin in serum with liposomes or hybrid drug-in-cyclodextrin-in-liposome systems: a comparative study. Int J Pharm. 2014;476(1–2):108–115. | ||
Roy B, Guha P, Bhattarai R, et al. Influence of lipid composition, pH, and temperature on physicochemical properties of liposomes with curcumin as model drug. J Oleo Sci. 2016;65(5):399–411. | ||
Jangle RD, Thorat BN. Effect of freeze-thawing study on curcumin liposomes for obtaining better freeze-dried product. Drying Technol. 2013;31(9):966–974. | ||
Catalan LA, Ravaghi M, Manca ML, et al. Freeze-dried eudragit-hyaluronan multicompartment liposomes to improve the intestinal bioavailability of curcumin. Eur J Pharm Biopharm. 2016;107: 49–55. | ||
Ou CF, Liang YL, Shen SW, et al. Preparation of liposomal in curcumin using ethanol injection method. J South Agric. 2011;42(10):1259–1264. | ||
Zhao YZ, Lu CT, Zhang Y, et al. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int J Pharm. 2013;454(1):302–309. | ||
Li C, Deng L, Zhang Y, et al. Silica-coated ethosome as a novel oral delivery system for enhanced oral bioavailability of curcumin. Yao Xue Xue Bao. 2012;47(11):1541–1547. | ||
Zhao Y, Meng LH, Pan LJ, et al. Preparation of curcumin liposomes and preliminary study on its stability. J Chongqing Med Univ. 2010;35(4): 582–585. | ||
Zang G, Zhao Y, Pan L, et al. Preparation and quality evaluation of curcumin liposomes modified with vitamin A. Chin J Pharm. 2011;42(6):431–434. | ||
Meng LH, Zhao Y, Pan LJ, et al. Study on preparation of vitamin A-curcumin liposome and its cytotoxicity. Chin Pharm J. 2011;46(14):1104–1107. | ||
Mozafari MR. Nanoliposomes: preparation and analysis. Methods Mol Biol. 2010;605(605):29–50. | ||
Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. | ||
Sun WT, Zou Y, Guo YP, et al. Construction and characterization of curcumin nanoparticles system. J Nanopart Res. 2014;16(3):1723–1728. | ||
Shin GH, Chung SK, Kim JT, Joung HJ, Park HJ. Preparation of chitosan-coated nanoliposomes for improving the mucoadhesive property of curcumin using the ethanol injection method. J Agric Food Chem. 2013;61(46):11119. | ||
Hasan M, Belhaj N, Benachour H, et al. Liposomes encapsulation of curcumin: physicochemical characterizations and effects on MCF7 cancer cell proliferation. Int J Pharm. 2014;461(1–2):519–528. | ||
Chen X, Zou LQ, Niu J, Liu W, Peng SF, Liu CM. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules. 2015;20(8):14293–14311. | ||
Brayner R. The toxicological impact of nanoparticles. Nano Today. 2008;3(8):48–55. | ||
Zalipsky S, Brandeis E, Newman MS, Woodle MC. Long circulating, cationic liposomes containing amino-PEG-phosphatidylethanolamine. FEBS Lett. 1994;353(1):71–74. | ||
Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253(1–2):121–132. | ||
Guo HY, Wang XY. Preparation and characterization of long-circulating PEGy-lated curcumin liposomes. Guangzhou Chem Ind. 2013;41(13):131–133. | ||
You J, Dai DB, He WJ, et al. Preparation of curcumin-loaded long-circulating liposomes and its pharmacokinetics in rats. Zhongguo Zhong Yao Za Zhi. 2014;39(7):1238–1242. | ||
Hong W, Chen DW, Zhao XL, et al. Preparation and study in vitro of long-circulating nanoliposomes of curcumin. Zhongguo Zhong Yao Za Zhi. 2008;33(8):889–892. | ||
Lin YL, Liu YK, Tsai NM, et al. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine. 2012;8(3):318–327. | ||
Gupta A, Kaur CD, Saraf S, Saraf S. Targeting of herbal bioactives through folate receptors: a novel concept to enhance intracellular drug delivery in cancer therapy. J Recept Signal Transduct Res. 2017;37(3):314–323. | ||
Lu Y, Ding N, Yang C, Huang L, Liu J, Xiang G. Preparation and in vitro evaluation of a folate-linked liposomal curcumin formulation. J Liposomes Res. 2012;22(2):110–119. | ||
Zhao Y, Meng LH, Wang C. Study on preparation of hyaluronic acid-targeted curcumin liposome and its quality evaluation. J Mod Med Health. 2013;29(2):169–174. | ||
Manca ML, Castangia I, Zaru M, et al. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials. 2015;71:100. | ||
Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors. 2013;39(1):69–77. | ||
Shehzad A, Lee YS. Curcumin: multiple molecular targets mediate multiple pharmacological actions: a review. Drugs Future. 2010;35(2):113–119. | ||
Marchiani A, Rozzo C, Fadda A, Delogu G, Ruzza P. Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem. 2013;21(2):204–222. | ||
Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin’s effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. J Interferon Cytokine Res. 2011;31(2):219–226. | ||
Zhang YP, Li YQ, Lv YT, Wang JM. Effect of curcumin on the proliferation, apoptosis, migration, and invasion of human melanoma A375 cells. Genet Mol Res. 2015;14(1):1056–1067. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. | ||
Dunlap JR, Binzel ML. The evaluation of exercise test as a predictor of operative morbidity of lung cancer patients. Int J Pharm Biol Sci. 2014;5(3):379–384. | ||
Molina JR, Adjei AA, Jett JR. Advances in chemotherapy of non-small cell lung cancer. Chest. 2006;130(4):1211–1219. | ||
Meador CB, Jin H, De SE, et al. Optimizing the sequence of anti-EGFR targeted therapy in EGFR-mutant lung cancer. Mol Cancer Ther. 2015;14(2):542–552. | ||
Chen DM, Mao KY, Yang L, Jiang HB. Status of anti-lung cancer drug patents applications in China from 2003 to 2012. Recent Pat Anticancer Drug Discov. 2014;9(2):221–240. | ||
Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–22512. | ||
Yonesaka K, Hirotani K, Kawakami H, et al. Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene. 2015;35(7):878–886. | ||
Manca ML, Peris JE, Melis V, et al. Nanoincorporation of curcumin in polymer-glycerosomes and evaluation of their in vitro-in vivo suitability as pulmonary delivery systems. RSC Adv. 2015;5(127):105149–105159. | ||
Wang LQ, Shi HS, Wang YS. Liposomal curcumin inhibits tumor growth and angiogenesis in lewis lung cancer. J Sichuan Univ Med Sci Edn. 2013;44(1):46–48. | ||
Rahman S, Cao S, Steadman KJ, et al. Native and β-cyclodextrin-enclosed curcumin: entrapment within liposomes and their cytotoxicity in lung and colon cancer. Drug Deliv. 2012;19(7):346–353. | ||
Apiratikul N, Penglong T, Suksen K, Svasti S, Chairoungdua A, Yingyongnarongkula B. In vitro delivery of curcumin with cholesterol-based cationic liposomes. Russ J Bioorganic Chem. 2013;39(4):444–450. | ||
Wang P, Liu S, Cheng B. Expression of cyclin D1 in cervical intraepithelial neoplasia and squamous cell carcinoma and its relationship with HPV16 E7 gene. Zhonghua Bing Li Xue Za Zhi. 2015;44(12):884–888. | ||
Waggoner SE. Cervical cancer. Lancet. 2003;361(9376):2217–2225. | ||
Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. | ||
Tewari KS, Sill MW, Long HR, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. | ||
Awada A, Bondarenko IN, Bonneterre J, et al; CT4002 study group. A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationiclipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann Oncol. 2014;25(4):824–831. | ||
Dicheva BM, Hagen TL, Schipper D, et al. Targeted and heat-triggered dixorubicin delivery to tumor by dual targeted cationic thermosensitive liposomes. J Control Release. 2014;195:37–48. | ||
Saengkrit N, Saesoo S, Srinuanchai W, et al. Influence of curcumin-loaded cationic liposomes on anticancer activity for cervical cancer therapy. Colloids Surf B Biointerfaces. 2014;114(10):349–356. | ||
Huang Q, Zhang L, Sun X, et al. Coating of carboxymethyl dextran on liposomal curcumin to improve the anticancer activity. RSC Adv. 2014;4(103):59211–59217. | ||
Tao ZQ, Shi AM, Wang KX, Zhang WD. Epidemiology of prostate cancer: current status. Eur Rev Med Pharmacol Sci. 2015;19(5):805–812. | ||
Jácome-Pita F, Sánchez-Salas R, Barret E, Amaruch N, Gonzalez-Enguita C, Cathelineau X. Focal therapy in prostate cancer: the current situation. Ecancermedicalscience. 2014;8:435. | ||
Van Poznak CH. Bone health in adults treated with endocrine therapy for early breast or prostate cancer. Am Soc Clin Oncol Educ Book. 2015;35:567–574. | ||
Linke B, Liebl P, Marten D, et al. Information concerning endocrine therapy and adherence of patients with breast and prostate cancer. Clin Oncol (R Coll Radiol). 2015;27(8):482–483. | ||
Tsuda M, Ishiguro H, Saji S. Mechanism and side effect of endocrine therapy. Nihon Rinsho. 2015;73(2):291–297. | ||
Nakamura K, Yesunaga Y, Segawa T, et al. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21(4):825–830. | ||
Tian YD, Guan YB, Zhang YQ, et al. Inhibitory effect of curcumin liposomes on PC-3 human prostate cancer cells. Chin J Exp Surg. 2014;31(5):1075–1078. | ||
Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32(5):1119–1123. | ||
Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. | ||
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. | ||
Miles RC, Gullerud RE, Lohse CM, Jakub JW, Degnim AC, Boughey JC. Local recurrence after breast-conserving surgery: multivariable analysis of risk factors and the impact of young age. Ann Surg Oncol. 2012;19(4):1153–1159. | ||
Ye LU, Pan XT, Yang YS, et al. Expression of erythropoietin-receptor and the relationship between ER, PR and HER-2 in breast cancer. China Oncol. 2010;20(3):187–191. | ||
Li J, Chang Q. Study on correlation between expressions of ER, PR, KI67, HER-2 and the effect of neoadjuvant chemotherapy in breast cancer. Chin J Clin Oncol Rehabil. 2011;18(5):407–410. | ||
Dhule SS, Penfornis P, Frazier T, et al. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8(4):440–451. | ||
Moku G, Gulla SK, Nimmu NV, Khalid S, Chaudhuri A. Delivering anti-cancer drugs with endosomal pH-sensitive anti-cancer liposomes. Biomater Sci. 2016;4(4):627. | ||
Farhangi H, Farzadnia M, Alamdaran A. Childhood facial osteosarcoma: a case report. Arch Bone Joint Surg. 2015;3(2):141–143. | ||
Rogozhin DV, Bulycheva IV, Konovalov DM, et al. Classical osteosarcoma in children and adolescent. Arkh Patol. 2015;77(5):68–74. | ||
Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–3474. | ||
Dhule SS, Penfornis P, He J, et al. The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm. 2014;11(2):417–427. | ||
Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China, 2009. Chin J Cancer. 2013;32(4):162–169. | ||
Wei KR, Yu X, Zheng RS, et al. Incidence and mortality of liver cancer in China, 2010. Chin J Cancer. 2014;33(8):388–394. | ||
Fan H, Tian W, Ma X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase. Target Oncol. 2014;9(3):279–286. | ||
Li PM, Li YL, Liu B, Wang WJ, Wang YZ, Li Z. Curcumin inhibits MHCC97H liver cancer cells by activating ROS/TLR-4/caspase signaling pathway. Asian Pac J Cancer Prev. 2014;15(5): 2329–2334. | ||
Notarbartolo M, Poma P, Perri D, et al. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells: analysis of their possible relationship to changes in NF-κB activation levels and in IAP gene expression. Cancer Lett. 2005;224(1):53–65. | ||
Li HM, Yan XS, Ming AP, et al. Stability of anti-liver cancer efficacy by liposomes-curcumin in water solution. Chin Tradit Herb Drugs. 2006;37(4):286–291. | ||
Axelsson H, Lönnroth C, Andersson M, Wang W, Lundholm K. Global tumor RNA expression in early establishment of experimental tumor growth and related angiogenesis following cox-Inhibition evaluated by microarray analysis. Cancer Inform. 2007;3(3):125–139. | ||
He CJ, Sun XP, Qiao H, et al. Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma. Cancer Sci. 2011;103(3):528–534. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.