Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Leptin Levels are Associated with Subclinical Cardiac Dysfunction in Obese Adolescents
Authors Imerbtham T , Thitiwuthikiat P , Jongjitwimol J, Nuamchit T, Yingchoncharoen T, Siriwittayawan D
Received 13 January 2020
Accepted for publication 11 March 2020
Published 27 March 2020 Volume 2020:13 Pages 925—933
DOI https://doi.org/10.2147/DMSO.S245048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Thamonwan Imerbtham,1 Piyanuch Thitiwuthikiat,1 Jirapas Jongjitwimol,2 Teonchit Nuamchit,1 Teerapat Yingchoncharoen,3 Duangduan Siriwittayawan1
1Department of Cardio-Thoracic Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok, Thailand; 2Department of Medical Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok, Thailand; 3Department of Internal Medicine, Faculty of Medicine, Mahidol University, Bangkok, Thailand
Correspondence: Duangduan Siriwittayawan
Department of Cardio-Thoracic Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok 65000, Thailand
Tel +66 55 966 417
Fax +66 55 966 234
Email [email protected]
Purpose: The purposes of this study were to use speckle tracking echocardiography to confirm the influence of obesity on cardiac functions and to assess their relationships with leptin and uric acid levels in obese adolescents.
Methods: Eighty-one participants aged 16– 19 years were recruited and classified as either non-obese (n = 30) or obese (n = 51). Global longitudinal strain (GLS), leptin and uric acid levels for each group were assessed and compared. The data from obese participants were then compared based on their leptin levels and analyzed for correlation using regression analysis.
Results: The obese group had significantly lower absolute GLS compared to the non-obese group (19.10 ± 0.30 versus 21.10 ± 0.30%, p < 0.001). In obese group, subclinical cardiac dysfunction was worse in the hyperleptinemic group than that of the normoleptinemic group (p = 0.03). Multivariate regression analysis showed that leptin and triglyceride levels were negatively associated with absolute GLS. Leptin could predict the absolute GLS with β = − 0.35 (p = 0.02).
Conclusion: Subclinical left ventricular systolic dysfunction was found in obese adolescents, while GLS was worse in the hyperleptinemic subjects. Leptin, but not uric acid, levels were associated with a worsening of GLS.
Keywords: leptin, global longitudinal strain, juvenile, speckle tracking echocardiography, uric acid
Introduction
Obesity in adolescence is associated with reduced longevity and risk of premature death and diseases, including cardiovascular diseases.1 Evidence shows that it affects cardiac function and structure either at rest or during exercise.2–4 However, a number of studies demonstrated normal systolic function in obese participants.5,6 Recently, we also found normal left ventricular ejection fraction (LVEF) and fractional shortening (FS) with altered cardiac morphologic indices in obese adolescents.7 This discrepancy is probably because the stages of cardiac dysfunction are differentially affected by the severity and duration of obesity. Additionally, the EF and FS may not be sensitive parameters for detecting early-stage cardiac alterations, especially in healthy young obese individuals.
It is well documented that body mass index (BMI) and insulin levels are associated with cardiac functions in obese subjects.8,9 However, the impact of potential metabolic biological markers, leptin, and uric acid (UA) has not been well established. Leptin is a hormone that is produced by adipocytes. It is involved in regulating body weight, appetite, and energy expenditure.10 Anywhere from 25 to 70% of obese people have hyperleptinemia.11,12 High leptin levels are associated with cardiovascular complications, including hypertension, stroke, and coronary heart disease.13 Investigations into the link between leptin and cardiovascular disorders highlight the direct effects of leptin on the heart.10 In in vitro studies, leptin induces hypertrophy in a concentration-dependent manner in neonatal rat ventricular myocytes.14,15 Cardiac functions in mice are altered due to leptin deficiency and exogenous leptin administration.16,17 However, there are limited data from human studies. One community-based study demonstrated that leptin levels are not related to LVFS.18 The impact of leptin levels on human cardiac functions in the obese population has not been assessed. Likewise, UA, the final product of purine metabolism, is now considered to be a marker for metabolic and hemodynamic disorders.19 An increased prevalence of hyperuricemia is found in patients with high blood pressure, insulin resistance, and obesity.20 Its role as a risk factor for cardiovascular disease has been extensively investigated in the past decades, and there is an apparent association between UA and cardiovascular mortality.21,22 The link between UA and cardiac functions was addressed in particular patient groups. Cicoira et al demonstrated that UA levels correlate with diastolic but not systolic dysfunction in ischemic cardiomyopathy patients, whereas other studies found a relationship between UA levels and LVEF in patients with ischemic heart disease and heart failure.23–26 However, the relationship between UA and cardiac functions in healthy obese people has not been studied.
Thus, the purposes of this study were to use speckle tracking echocardiography (STE) to confirm the impact of obesity on cardiac functions and to assess the association between leptin, UA levels, and cardiac functions with obesity in adolescents.
Materials and Methods
Participants
Eighty-one adolescents aged 16 to 19 years were recruited from Naresuan University and nearby schools. The study was approved by the Ethical Review Board, Naresuan University, Phitsanulok, Thailand (IRB No. 796/58) and was conducted in accordance with the Declaration of Helsinki. Written consent was obtained from all participants and participants’ parents after being informed regarding the nature of the study. All data were collected between March and September 2016. The inclusion criteria were those aged between 16 to 19 years, without any history of illness or prescription medication, and who did not exercise regularly. The exclusion criteria were adolescents with structural cardiac abnormalities or underlying diseases, including hypertension, sleep apnea (screened using the Modified Berlin questionnaire), anemia, and diabetes. Obesity severity was classified according to WHO guidelines for the Asia-Pacific region.27 Specifically, healthy participants with a BMI of less than 25 kg/m2 are defined as non-obese, whereas those with a BMI of ≥ 25 kg/m2 are classified as obese. The obese group was then classified as normoleptinemic (obeseNL) or hyperleptinemic (obeseHL) according to their leptin levels. The cut-off value was 20 ng/mL in males and 22 ng/mL in females, according to the reference values cited by Dâmaso AR et.al.28
Experimental Design
Demographic data were obtained via questionnaire-based study. The anthropometric data were collected by a single well-trained cardiothoracic technologist throughout the study. Body weight and percentage of body fat were measured by using a body composition monitor (Omron HBF 375, Japan). After fasting for 12 h overnight, the participants’ resting heart rate and blood pressure were checked, plasma was drawn, and biochemical and hematologic parameters were measured. Following these procedures, the participants underwent two-dimensional conventional echocardiography as well as two-dimensional speckle tracking echocardiography. Data were analyzed with SPSS (SPSS Inc. Chicago, USA). Resting blood pressure and heart rate were measured three times (to obtain an average value) in a supine position after subjects rested for 5 min. All procedures were performed in a quiet environment in the faculty of Allied Health Sciences, Naresuan University.
Echocardiography
Echocardiographic Measurement
The echocardiographic procedure was performed by a well-trained cardiothoracic technologist throughout the study. Intra- and inter-observer variability tests were performed using interclass correlation to assess the agreement between echocardiogram findings of the cardiothoracic technologist and an expert cardiologist. Intra- and inter-observer reliabilities were analyzed with intraclass correlation coefficients (ICCs) with 95% confidence intervals using SPSS.
Two-Dimensional echocardiography
An echocardiographic evaluation was performed in the left lateral position, using a Vivid S6 commercial ultrasound scanner (GE Vingmed Ultrasound AS, Horten, Norway) with an active matrix single-crystal phased-array transducer (GEM5S-D; GE Vingmed Ultrasound AS). The grayscale recordings were optimized at a mean frame rate of ≥ 50 frames per second, and the images were obtained using standard tomographic LV views. Mitral inflow velocities of early diastolic filling (E) and late diastolic filling (A), E/A ratio, and E deceleration time (DecT) were measured using a pulsed-wave Doppler. The LV diameter and wall thickness were assessed according to the criteria of the American Society of Echocardiography. LV end-diastolic and end-systolic volumes and the LVEF at rest were evaluated using a modified Simpson’s biplane method. A pulsed-wave tissue Doppler of the septal and lateral annulus was used to measure early peak diastolic mitral annular velocity (E’ septal and E’ lateral), and each representative value was the average of three measurements.
Analysis of Myocardial Deformation
Two-dimensional strain data were stored in a digital format and analyzed offline using the EchoPAC workstation, PC 2008 (GE, Horten, Norway). Cardiac motion was determined from a user-defined tracing along the endocardial-myocardial border, and after proper manual tracing, the software automatically (speckle tracking) determined six segments. Longitudinal systolic deformation was characterized as shortening, which was obtained from apical, mid, and basal segments in the septum and LV lateral wall from apical three-, four-, and two- chamber views. Data were derived when the values for all six segments were considered acceptable by the software. All parameters shown in this paper were obtained from averages of three measurements. Strain parameter, the global longitudinal strain (GLS) was used to assess LV function.
Blood Test
Standard blood analyses were performed for all subjects, including complete blood count (CBC), lipid profile, and UA and leptin levels, after 12-h overnight fasting. CBC was analyzed using an autoanalyzer, and the UA and lipid profiles were analyzed using a commercially available Enzymatic Colorimetric Test kit (CHOD-PAP-Method, GPO-PAP-Method, PAP-Method), according to the manufacturer’s instructions. Leptin levels were measured using a commercially available enzyme-linked immunosorbent assay kit (EZHL 80SK, Sigma Aldrich).
Statistical Analysis
The data are presented as mean ± standard error of the mean (SEM) for normally distributed data and as median [25th-75th percentile] for non-normally distributed variables. Normally distributed data were compared with a paired t-test whereas the non-normally distribution was analyzed with non-parametric test. Chi-square test was used to compare categorical variables. Correlation between parameters was analyzed using univariate and multivariate regression. P < 0.05 indicated a statistical significance.
Results
Baseline Characteristics
Eighty-nine participants were recruited, but 8 participants were excluded during the test due to a poor-quality echocardiogram. Baseline characteristics and clinical parameters of the participants are reported in Table 1.
 |
Table 1 Baseline Characteristics and Clinical Parameters of All Participants |
Speckle Tracking Echocardiographic Findings
For GLS measurement, both the intra- and inter-observer reliabilities were very good (ICCs were 0.92–0.99 for intra-observer and 0.8–0.99 for inter-observer). However, circumferential and radial strain ICCs were poor, and so we only performed GLS in this study. There was a significant reduction in absolute GLS in the obese compared to the control group (19.18 ± 0.30 versus 21.07 ± 0.31%, respectively; p < 0.001). Further regression analysis showed that BMI was an independent predictor of the GLS (β = −0.28, p = 0.04).
Leptin Levels and Cardiac Functions in Obese Subjects
To test the impact of leptin on cardiac functions in obese subjects, the 51 obese participants were categorized based on their leptin levels as normoleptinemic (obeseNL) or hyperleptinemic (obeseHL). Fifty-one per cent of the participants exhibited hyperleptinemia. The data showed that only BMI, percent body fat, and hemoglobin levels were significantly different between the obeseNL and obeseHL groups (Table 2). Structural cardiac indices, EF and FS did not significantly differ between groups, whereas the absolute GLS in obeseNL was higher compared to obeseHL (19.80 ± 0.40 versus 18.50 ± 0.30%, respectively; p = 0.03) (Table 3).
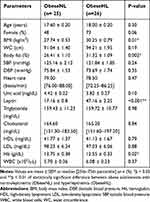 |
Table 2 Characteristics and Clinical Parameters of Obese Adolescents According to Leptin Levels |
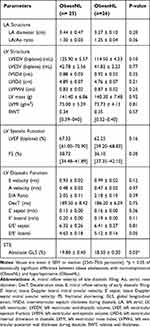 |
Table 3 Echocardiographic Parameters in Obese According to Leptin Levels |
Correlation Between Leptin and Potential Laboratory Parameters and Cardiac Functions in Obese Adolescents
The relationship between obesity and other independent risk factors for metabolic cardiomyopathy was assessed. It was found that triglyceride, uric acid and leptin levels were associated significantly with BMI (r = 0.35, 0.31 and 0.31 for triglyceride, uric acid and leptin levels, respectively) whereas there was no correlation between BMI and Hb, HDL and LDL.
Further univariate regression analysis showed that BMI, DBP, triglyceride, hemoglobin, and leptin (but not UA) levels were associated with absolute GLS. After adjustment for confounding variables including age, sex, BMI, DBP, triglyceride and hemoglobin, multivariate regression analysis demonstrated that leptin levels were independently associated with absolute GLS. In this model, leptin levels could predict absolute GLS with β = −0.35 (p = 0.02). The results of univariate and multivariate analysis between absolute GLS and clinical parameters are shown in Tables 4 and 5.
 |
Table 4 Univariate Correlation Analysis Between Absolute GLS and Clinical Parameters |
 |
Table 5 Multivariate Stepwise Regression Analysis Between Absolute GLS and Clinical Parameters |
It was noted that leptin was also associated with some morphological indices. It could predict LA diameter (β = - 0.39, p = 0.01), LVEDV (β = - 0.28, p = 0.03), and LVIDd (β = - 0.37, p = 0.01).
Discussion
To the best of our knowledge, our study is the first to demonstrate that leptin, but not UA, influences cardiac strain in disease-free obese adolescents. Leptin and serum triglyceride levels were independently correlated with GLS. Subclinical cardiac dysfunction was also found in obese participants, data that confirm the impact of obesity on cardiac function in the young.
Obesity and Cardiac Functions of Obese Adolescents
The association between obesity and cardiac functions and geometry has been well documented in obese adults, but it is still poorly understood in adolescents. Recently, we showed the influence of obesity on cardiac structure in adolescents by using conventional echocardiography.7 In our present study, we found that even though obese participants had a normal EF, their absolute GLS significantly differed from non-obese subjects. Mangner et al also found reduced cardiac strain with normal EF and FS and altered cardiac geometry in obese children and adolescents, while Ingul et al detected impaired systolic functions, represented by reduced EF, FS, GLS, and LV stroke volume in obese children aged 13–16 years.4,29 However, some studies demonstrated normal or increased EF and FS in disease-free participants with slight and moderate obesity.2,30 These contradictory results may be due to the differences in the stages of cardiac dysfunction and/or related to the severity and duration of obesity.2,30
The mechanism whereby obesity affects cardiac function is likely complicated and may involve hemodynamic, neurohormonal, and/or metabolic factors as well as lipotoxic effects.31 It is unclear whether the cardiac structural change is a precursor or a consequence of cardiac dysfunction. Our results imply that cardiac morphology alterations in obesity can be detected even in people with early stage cardiac dysfunction. The higher blood pressure in obese participants suggests that pressure overload may be involved in impaired contractility, given the changes in leptin or lipid profiles.
Leptin and Cardiac Function
Based on previous studies, there is an association between serum leptin levels and compromised cardiac function in animal models. In humans, a large prospective study showed that leptin is an independent risk factor for coronary artery disease.32 However, the association between leptin and cardiac functions in obesity has not been assessed. Our study is the first to show that leptin levels are independently associated with GLS. Furthermore, the negative relationships between leptin levels and LA diameter, LVEDV, and LVIDd in young obese subjects are novel. These data suggest that leptin is involved in both structural and functional heart changes in obesity. The role of leptin in morphological changes has been extensively investigated in the past decades. Our results supported the findings of a community-based study, which found the inverse relationship between leptin and LVMI and LA diameter as well as the MESA study.18,33 In contrast, some evidence shows that leptin levels are positively associated with ventricular mass in hypertensive patients and in morbid obesity.34,35 The dissimilar results in these clinical studies are probably due to the differences in comorbidity, duration of being obese, and/or serum leptin levels of the participants. However, the contradictory trophic effects of leptin were also found in animal model and in vitro study. The antihypertrophic effects of leptin, independent of body mass, were addressed by Barouch et al.36 In contrast, some studies presented the hypertrophy effects of leptin on cardiomyocytes.14,15 This issue requires further investigation.
The main finding of our study was that higher leptin levels were associated with reduced cardiac functions. In animal models, leptin deficiency, hyperleptinemia, or exogenous administration of leptin can reduce EF an FS in mice under physiologic conditions.16,17,37 In humans, there are few studies that demonstrate the correlation between cardiac functions and leptin levels. A number of studies found that high leptin levels are associated with diastolic dysfunction, but not EF, in the general population and participants with coronary artery disease.38,39 Likewise, the Framingham Heart study found no relationship between FS and leptin levels.18 Nonetheless, our study showed a significant relationship between leptin levels and GLS in healthy obese participants. The mechanism whereby high leptin levels alter cardiac function remains unclear, but it is hypothesized that leptin may be involved in cardiac myocyte hypertrophy, sympathoactivation, increased total peripheral resistance, oxidative stress, and/or alterations in fatty acid metabolism.10 The findings that leptin levels are negatively related to LV size and leptin-induced alterations in cardiac performance are independent of the pro-hypertrophic system40–42 may indicate possibilities other than the hypertrophic effects of leptin on cardiac dysfunction. Furthermore, although sympathetic nervous system activation and an increased vascular resistance are thought to plays a role in leptin-related cardiac dysfunction, we did not observe any significant changes of associated parameters, heart rate and blood pressure, in the obeseHL group. The effect of leptin on cardiac fatty acid metabolism is one potential explanation. Experimental studies in animal models showed that leptin administration increases fatty acid oxidation and fatty acid uptake of cardiomyocytes and consequently leads to cardiac dysfunction.43,44
Uric Acid
A number of studies indicated a significant association between high UA levels and cardiac functions in patients with cardiovascular disease.23–25 However, our study showed that UA levels did not correlate with morphology, LVEF, or GLS in disease-free obese adolescents, even though higher levels were observed in obese subjects. These results support the Framingham Heart Study findings demonstrated that UA does not correlate with the risk of cardiovascular disease, at least in men.45 This discrepancy may result from differences in the participants’ background, especially, comorbidities. For example, diuretic use is suggested as a confounder for analyzing the association between LVEF and UA level in heart failure.45,46 The lack of an association between UA and cardiac functions in obese adolescents may be due to the dual role of UA, a contributor of low-grade inflammation and a scavenger of free radicals. The harmful UA effects may be counterbalanced by its protective effects.47
Other Relevant Variables
The major confounder identified in this study was triglyceride levels. It was shown that triglyceride levels were negatively correlated with absolute GLS. This evidence suggests that triglyceride levels should be concerned when assessing cardiac functions in obesity.
Clinical Perspectives
In the past decades, leptin has been of interest as an important metabolic regulator. The finding that leptin is an independent predictor of cardiac functions in young obese individuals will be fundamental for the proper prevention and management of obesity-induced cardiomyopathy. Evaluation of leptin levels, independent of BMI, may be useful when monitoring cardiac functions in obese adolescents. However, further study of actual effects and mechanism of leptin on heart functions using in vitro along with multi-center or large clinical studies is needed. Leptin may be a potential therapeutic target in obesity-related cardiac dysfunction.
Limitations
There were some limitations in this study. First, we studied a particularly small group of disease-free adolescents. The lager sample size is required especially when using multivariate regression analysis with many confounders. Furthermore, we studied in Thai participants, which is doubtful whether the results are generalizable to other groups. Secondly, we did not evaluate circumferential and radial strains in this experiment due to the poor reliability of the measurements. Evaluation of those parameters should provide insightful results. Finally, the fasting blood sugar and insulin levels, factors that can determine cardiac functions, were not measured. However, all participants in our study were checked for diabetes mellitus via an annual physical examination and participants with the history of this disease were excluded.
Conclusions
Obese adolescents demonstrated significantly worse GLS than non-obese individuals. Leptin was independently associated with worse GLS in the obese group, whereas UA levels were not correlated with cardiac function indices.
Acknowledgments
The authors would like to thank Naresuan University for research funding support under the Naresuan University Research Grant number R2560C129. We appreciate Dr. Piyameth Dilokthornsakul for his assistance in data analysis.
Disclosure
The authors report no conflicts of interest in this work. A part of this paper (the demographic data, Table 1) was presented at the 7th National and International Graduate Study Conference 2017 as a conference talk with interim findings. The abstract of this paper was also presented at the American College of Cardiology (ACC) 2018 Annual Scientific Session and published in “Poster Abstracts” in Journal of the American College of Cardiology: http://dx.doi.org/10.1016/S0735-1097(18)32066-7.
References
1. Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond). 2009;33:S60. doi:10.1038/ijo.2009.20
2. Chinali M, de Simone G, Roman MJ, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47(11):2267–2273. doi:10.1016/j.jacc.2006.03.004
3. Schuster I, Karpoff L, Perez-Martin A, et al. Cardiac function during exercise in obese prepubertal boys: effect of degree of obesity. Obesity. 2009;17(10):1878–1883. doi:10.1038/oby.2009.197
4. Ingul CB, Tjonna AE, Stolen TO, Stoylen A, Wisloff U. Impaired cardiac function among obese adolescents: effect of aerobic interval training. Arch Pediatr Adolesc Med. 2010;164(9):852–859. doi:10.1001/archpediatrics.2010.158
5. Patil S, Mandade A, Shelke A, Kawade R, Sanyal KA. Study to assess the effects of obesity on ventricular function by 2D echocardiography. Int J Contemp Med Res. 2017;4(9):1892–1897.
6. Ghandi Y, Sharifi M, Habibi D, Dorreh F, Hashemi M. Evaluation of left ventricular function in obese children without hypertension by a tissue Doppler imaging study. Ann Pediatr Cardiol. 2018;11(1):28–33. doi:10.4103/apc.APC_75_17
7. Imerbtham T, Yingchonchareon T, Nuamchit T, Siriwittayawan D. Cardiac functions assessed by 2D-echocardiography in older obese adolescents.
8. Patel VG, Gupta DK, Terry JG, et al. Left ventricular function across the spectrum of body mass index in African Americans: the Jackson Heart Study. JACC Heart Fail. 2017;5(3):182–190. doi:10.1016/j.jchf.2016.12.020
9. Cauwenberghs N, Knez J, Thijs L, et al. Relation of insulin resistance to longitudinal changes in left ventricular structure and function in a general population. J Am Heart Assoc. 2018;7(7):e008315. doi:10.1161/JAHA.117.008315
10. Hou N, Luo J-D. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38(12):905–913. doi:10.1111/j.1440-1681.2011.05619.x
11. Foschini D, Dos Santos RV, Prado WL, et al. Platelet and leptin in obese adolescents. J Pediatr. 2008;84(6):516–521. doi:10.1590/S0021-75572008000700008
12. Yamborisut U, Riabroy N, Phonrat B, Tungtrongchitr R. Serum leptin levels and body composition in obese Thai children. Southeast Asian J Trop Med Public Health. 2009;40(3):544–552.
13. Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin. 2018;39(7):1176–1188. doi:10.1038/aps.2018.40
14. Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA, Karmazyn M. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006;41(2):265–274. doi:10.1016/j.yjmcc.2006.05.001
15. Xu F-P, Chen M-S, Wang Y-Z, et al. Leptin induces hypertrophy via endothelin-1–reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004;110(10):1269–1275. doi:10.1161/01.CIR.0000140766.52771.6D
16. Ren J, Ma H. Impaired cardiac function in leptin-deficient mice. Curr Hypertens Rep. 2008;10(6):448–453. doi:10.1007/s11906-008-0084-0
17. Samuelsson A-M, Clark J, Rudyk O, et al. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension. 2013;62(3):627–633. doi:10.1161/HYPERTENSIONAHA.111.00691
18. von Jeinsen B, Short MI, Xanthakis V, et al. Association of circulating adipokines with echocardiographic measures of cardiac structure and function in a community-based cohort. J Am Heart Assoc. 2018;7(13):e008997. doi:10.1161/JAHA.118.008997
19. Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014;2014:852954. doi:10.1155/2014/852954
20. Kuwabara M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse. 2015;3(3–4):242–252. doi:10.1159/000443769
21. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971–1992. JAMA. 2000;283(18):2404–2410. doi:10.1001/jama.283.18.2404
22. Strasak A, Ruttmann E, Brant L, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83 683 Austrian men. Clin Chem. 2008;54(2):273–284. doi:10.1373/clinchem.2007.094425
23. Cicoira M, Zanolla L, Rossi A, et al. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am Heart J. 2002;143(6):1107–1111. doi:10.1067/mhj.2002.122122
24. Tanaka Y, Nagoshi T, Kawai M, et al. Close linkage between serum uric acid and cardiac dysfunction in patients with ischemic heart disease according to covariance structure analysis. Sci Rep. 2017;7(1):2519. doi:10.1038/s41598-017-02707-y
25. Oki Y, Kawai M, Minai K, et al. High serum uric acid is highly associated with a reduced left ventricular ejection fraction rather than increased plasma B-type natriuretic peptide in patients with cardiovascular diseases. Sci Rep. 2019;9(1):682. doi:10.1038/s41598-018-37053-0
26. Pinelli M, Bindi M, Filardo F, Moroni F, Castiglioni M. Serum uric acid levels correlate with left ventricular ejection fraction and systolic pulmonary artery pressure in patients with heart failure. Recenti Prog Med. 2007;98(12):619–623.
27. World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia; 2000.
28. Dâmaso AR, de Piano A, Sanches PL, et al. Hyperleptinemia in obese adolescents deregulates neuropeptides during weight loss. Peptides. 2011;32(7):1384–1391. doi:10.1016/j.peptides.2011.04.025
29. Mangner N, Scheuermann K, Winzer E, et al. Childhood obesity: impact on cardiac geometry and function. JACC Cardiovasc Imaging. 2014;7(12):1198–1205. doi:10.1016/j.jcmg.2014.08.006
30. Pascual M, Pascual D, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89(10):1152–1156. doi:10.1136/heart.89.10.1152
31. Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:12. doi:10.1155/2018/3407306
32. Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001;104(25):3052–3056. doi:10.1161/hc5001.101061
33. Allison MA, Bluemke DA, McClelland R, et al. Relation of leptin to left ventricular hypertrophy (from the multi-ethnic study of atherosclerosis). Am J Cardiol. 2013;112(5):726–730. doi:10.1016/j.amjcard.2013.04.053
34. Perego L, Pizzocri P, Corradi D, et al. Circulating leptin correlates with left ventricular mass in morbid (grade iii) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90(7):4087–4093. doi:10.1210/jc.2004-1963
35. Paolisso G, Tagliamonte MR, Galderisi M, et al. Plasma leptin concentration, insulin sensitivity, and 24-hour ambulatory blood pressure and left ventricular geometry. Am J Hypertens. 2001;14(2):114–120. doi:10.1016/S0895-7061(00)01241-3
36. Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108(6):754–759. doi:10.1161/01.CIR.0000083716.82622.FD
37. Gómez-Hurtado N, Domínguez-Rodríguez A, Mateo P, et al. Beneficial effects of leptin treatment in a setting of cardiac dysfunction induced by transverse aortic constriction in mouse. J Physiol. 2017;595(13):4227–4243. doi:10.1113/tjp.2017.595.issue-13
38. Puurunen VP, Lepojarvi ES, Piira OP, et al. High plasma leptin levels are associated with impaired diastolic function in patients with coronary artery disease. Peptides. 2016;84:17–21. doi:10.1016/j.peptides.2016.08.002
39. Fontes-Carvalho R, Pimenta J, Bettencourt P, Leite-Moreira A, Azevedo A. Association between plasma leptin and adiponectin levels and diastolic function in the general population. Expert Opin Ther Targets. 2015;19(10):1283–1291. doi:10.1517/14728222.2015.1019468
40. Berzabá-Evoli E, Zazueta C, Cruz Hernández JH, et al. Leptin modifies the rat heart performance associated with mitochondrial dysfunction independently of its prohypertrophic effects. Int J Endocrinol. 2018;2018:6081415. doi:10.1155/2018/6081415
41. Martinez-Abundis E, Rajapurohitam V, Haist JV, Gan XT, Karmazyn M. The obesity-related peptide leptin sensitizes cardiac mitochondria to calcium-induced permeability transition pore opening and apoptosis. PLoS One. 2012;7(7):e41612. doi:10.1371/journal.pone.0041612
42. Minhas KM, Khan SA, Raju SV, et al. Leptin repletion restores depressed {beta}-adrenergic contractility in ob/ob mice independently of cardiac hypertrophy. J Physiol. 2005;565(Pt 2):463–474. doi:10.1113/jphysiol.2005.084566
43. Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem. 2002;277(33):29424–29430. doi:10.1074/jbc.M203813200
44. Sharma V, Mustafa S, Patel N, Wambolt R, Allard MF, McNeill JH. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide–p38 MAPK-dependent mechanism. Eur J Pharmacol. 2009;617(1–3):113–117. doi:10.1016/j.ejphar.2009.06.037
45. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi:10.7326/0003-4819-131-1-199907060-00003
46. Yamauchi Y, Fujita S-I, Shibata K, et al. Is serum uric acid independently associated with left ventricular mass index, ejection fraction, and B-type natriuretic peptide among female and male cardiac patients? Int Heart J. 2017;58(4):562–569. doi:10.1536/ihj.16-359
47. Muiesan ML, Agabiti-Rosei C, Paini A, Salvetti M. Uric acid and cardiovascular disease: an update. Eur Cardiol. 2016;11(1):54–59. doi:10.15420/ecr.2016:4:2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
