Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Left Ventricular Remodeling Risk Predicted by Two-Dimensional Speckle Tracking Echocardiography in Acute Myocardial Infarction Patients with Midrange or Preserved Ejection Fraction in Western Romania
Authors Bordejevic DA , Pârvănescu T , Petrescu L, Mornoș C, Olariu I, Crișan S, Văcărescu C, Lazăr M, Morariu VI, Citu IM , Tomescu MC , Cozma D
Received 2 December 2020
Accepted for publication 16 February 2021
Published 23 March 2021 Volume 2021:17 Pages 249—258
DOI https://doi.org/10.2147/TCRM.S295251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Diana Aurora Bordejevic,1 Tudor Pârvănescu,2 Lucian Petrescu,1 Cristian Mornoș,1 Ioan Olariu,1 Simina Crișan,1 Cristina Văcărescu,1 Mihai Lazăr,1 Vlad Ioan Morariu,2 Ioana Mihaela Citu,2 Mirela Cleopatra Tomescu,2 Dragoș Cozma1
1Cardiology Department, Institute of Cardiovascular Diseases, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania; 2Cardiology Department, City Hospital, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
Correspondence: Tudor Pârvănescu; Ioana Mihaela Citu
Victor Babes University of Medicine and Pharmacy, 2nd Eftimie Murgu Square, Timișoara 300041, Romania
Tel +40 724 369729
; +40 723 280 623
Fax +40 256 220636
Email [email protected]; [email protected]
Background: Patients with acute myocardial infarction (AMI) are at high risk for left ventricular (LV) remodeling and heart failure. We aimed to study whether LV strains (S) and strain rates (SR) could predict cardiac remodeling in patients with AMI having a midrange or preserved LV ejection fraction (EF) following a percutaneous coronary intervention (PCI) within the first 12 hours from the onset of symptoms.
Patients and Methods: This is a case-control observational study including patients admitted for their first AMI, either with ST-segment elevation (STEMI) or without ST elevation (NSTEMI), with an LVEF > 40% after a successful PCI. Echocardiography was repeated after 6 months, and the patients were divided into two groups, according to whether LV remodeling was determined on echocardiography.
Results: Of the 253 AMI patients (mean 66 aged ± 13 years), including 185 males (73%), 61 (24%) presented signs of LV remodeling. In univariate logistic regression analysis, age, male sex, smoking history, hypertension, hypercholesterolemia, Killip class, renal function, peak creatine phosphokinase - MB level, 2- and 3-vessel coronary artery disease (CAD), and several echocardiographic parameters were significantly associated with LV remodeling (P< 0.05). In multivariate logistic regression analysis harmed (H) LS and SR, Killip class, 3-vessel CAD, and LV end-diastolic volume were outlined as independent predictors for LV remodeling. Receiver operating characteristic curve analyses showed that HLS and HLSR were the most powerful independent predictors for LV remodeling (P< 0.001), with an area under the curve (AUC) of 0.85 (sensitivity 83%; specificity 84%; p < 0.001) and 0.77 (sensitivity 93; specificity 61%; p < 0.001), respectively. The identified cut-off values for predictor variables were HLS< − 11%, and HLSR< − 0.65s− 1.
Conclusion: We concluded that 2D-STE was the best method to evaluate LV remodeling in patients with AMI and midrange or preserved LVEF following myocardial revascularization by a PCI.
Keywords: AMI, PCI, preserved LVEF, midrange LVEF, 2D speckle tracking echocardiography, LV remodeling
Introduction
Harmful left ventricular (LV) remodeling occurs in acute myocardial infarction (AMI) patients with (AMI) even after revascularization by a percutaneous coronary intervention (PCI), its incidence is around 30%.1 This is a complex process, that involves both the infarcted and the non-infarcted myocardium, changing the wall thickness, as well as the ventricular size, shape, and function.2 It is associated with LV dilatation and mural hypertrophy.3 Post AMI LV remodeling leads to heart failure and life threatening arrhythmias and thus increases cardiovascular morbidity and mortality.3 It is mandatory to detect it early and to take the required therapeutic measures in order to improve both life quality and survival in these high-risk patients. It is known that conventional echocardiography is the preferred imaging method in AMI patients. Left ventricular ejection fraction (LVEF) and the wall motion score index (WMSI) are known as valuable predictors for LV remodeling and prognosis.4 LV strain (S) and strain rate (SR) measured by the two-dimensional speckle tracking echocardiography (2D-STE) represent modern and more efficient methods to estimate myocardial performance, being able to highlight fine alterations in LV function, especially in patients with preserved or midrange LVEF.5–7
The aim of this study was to assess the clinical, biochemical, echocardiographic, and angiographic factors associated with LV remodeling following an AMI with midrange or preserved LVEF after successful myocardial revascularization by a PCI performed within the first 12 hours from the onset of symptoms, and to emphasize the most powerful independent predictors of LV remodeling.
Patients and Methods
Patient Selection
This was a case-control observational study that included all consecutive patients admitted with a first, new-onset, AMI (STEMI/high-risk NSTEMI) from January 2019 to January 2020, that were successfully treated by a PCI within the first 12 hours of the onset of the symptoms, were in sinus rhythm and had a baseline LVEF >40%. High-risk NSTEMI patients were considered those with at least one of the following criteria: a Global Registry of Acute Coronary Events (GRACE)-score > 140, dynamic changes of the ST-segment/Twave, or a relative rise or fall in cardiac enzymes.8,9 High-risk NSTEMI patients in whom PCI could not be performed in the first 12 hours of the onset of symptoms were not enrolled in the study.
The study was performed in the Cardiology Clinic of the Timisoara Institute of Cardiovascular Disease and in the Cardiology Clinic of the Emergency Municipal Clinical Hospital Timisoara. Patients with a history of old myocardial infarction and those with severe valvular disease were excluded. Echocardiography was performed at baseline (immediately after the PCI) and after 6 months. LV remodeling was defined as an increase of ≥ 20% in the biplane LV end-diastolic volume from baseline to the 6-month follow-up.10,11 Harmed (infarct-related) segments were defined as those segments having longitudinal strains < −15%.12
The study was approved by the Ethics Committee of the “Victor Babes” University of Medicine and Pharmacy, Timisoara. All patients provided written informed consent for participation in the study, in accordance with the Human Rights Declaration of Helsinki.
Data Extraction
Baseline data were taken from hospital records and included age, gender, cardiovascular risk factors (smoking history, hypercholesterolemia, hypertension, smoking history, diabetes mellitus), the data of physical examination, laboratory data, 12 leads resting electrocardiogram, echocardiography, and coronary angiography.
Definition of Covariates
The diagnosis of STEMI was based on the presence of at least two of the following three criteria: 1) typical angina over 20 minutes; 2) ST-segment elevation ≥1 mV, lasting >0.08 seconds measured from point J, in a minimum of two contiguous leads; 3) transitory increase in cardiac enzymes to at least the double of the normal laboratory value.13 The diagnosis of NSTEMI was established in the presence of ischemic symptoms lasting over 20 minutes at rest and transitory augmentation in cardiac enzymes to at least twofold the normal laboratory value, without ST-segment elevation.9
Hypertension has been diagnosed when systemic BP ≥ 140/90 mmHg or the patient used antihypertensive agents.14 Hypercholesterolemia was diagnosed in the presence of total cholesterol level ≥ 190 mg/dL or the use of cholesterol-lowering medication.15 Diabetes mellitus was diagnosed when the fasting blood glucose was ≥ 126 mg/dL or the patient used antidiabetic medication.16 Chronic kidney disease was defined in the presence of an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 body surface, calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) formula.17 The AMI patients were graded into one of the Killip classes as follows: Class 1 – no clinical signs of heart failure; Class 2 – mild heart failure, limited rates in the lungs, a third heart sound present, and elevated jugular venous pressure; Class 3 – acute pulmonary edema; Class 4 – cardiogenic shock (SBP<90 mmHg, and signs of peripheral vasoconstriction: cyanosis, oliguria or sweating).18
Serum Biomarkers
Biochemical tests during hospitalization also included serum creatinine, N terminal- brain natriuretic peptide (NT-proBNP), and creatine phosphokinase MB isoenzymes (CK-MB).
PCI
The PCI was done according to the standard procedures,19 as soon as possible after the confirmation of the AMI, without exceeding the interval of 12 hours from the onset of the symptoms for STEMI and high-risk NSTEMI patients. Significant coronary stenosis was defined in the presence of a stenosis > 75% in the anterior descending, circumferential, or right coronary artery and > 50% in the left coronary artery. Multivessel coronary artery disease was defined in the presence of significant stenosis in more than one coronary artery. The PCI was considered successful in the presence of a TIMI flow of the infarct-related artery of grade 3.
Echocardiography
Baseline echocardiography was performed at 1.3 ± 0.6 days after the PCI, and the follow-up echocardiography 6 months after the onset of the AMI, using a GE Vivid E7 echocardiographic system (GE Health Medical, Milwaukee, WI, USA).
Conventional Echocardiography
LV diameters, volumes, and ejection fraction were calculated according to the American Society of Echocardiography guidelines, using Simpson’s biplane method.20 Midrange LVEF was defined as an LVEF of 40–49%, while preserved LVEF as an LVEF≥ 50%.7 Regional wall motion was visually evaluated with a 17-segment model, each segment being graded as 1-normal, 2-hypokinesia, 3-akinesia, 4- dyskinesia, and 5- aneurysm. The WMSI was calculated as the average of the segmental scores. The diastolic function of the LV was analyzed by Doppler examination of the mitral valve, calculating the ratio between the maximal protodiastolic velocity of the transmitral flow (peak E) and the maximal telediastolic velocity (peak A), the E/A ratio.21
Two-dimensional Speckle-tracking echocardiography (2D STE) was used for measuring LV deformation performance.22,23 They were analyzed off-line using the EchoPAC system version 11.0.1 (GE Vingmed). Global strain and strain rate were measured in longitudinal (L), circumferential (C), and radial (R) directions. The LV circumferential and radial strains and strain rates (LVCS, LVCSR, LVRS, LVRSR) were calculated from the short-axis views at the apical, basal, and middle levels. The LV longitudinal strain and strain rates (LVLS, LVLSR) were measured from the 2-, 3-, and 4-chamber apical. The software automatically tracked the myocardium, but the endocardial border was manually corrected in order to improve the quality. Each apical and each short-axis view was divided into 6 segments, resulting in a total of 18 segments. The systolic strain rates were calculated in 3 directions: longitudinal (LSR), circumferential (CSR), and radial (RSR) and the average of the 18 segments resulted in the global peak longitudinal strain (GLS), global radial strain (GRS), and the global peak circumferential strain (GCS). Segments with longitudinal strains lower than – 15% were defined as harmed (infarct-related) segments.12 The average LS and LSR of the harmed segments were defined as the harmed longitudinal strain (HLS) and harmed longitudinal systolic strain rate (HLSR). The number of harmed segments was also recorded.
Follow-Up and Outcomes
All patients were followed-up for a period of 6 months. They were divided into two groups, according to the presence or absence of echocardiographically determined LV remodeling.
LV remodeling was established when at the 6-month follow-up the LV end-systolic volume (LVESV) increased by more than 15% from the baseline measurement.10,11
Statistical Analysis
Continuous variables were expressed as means ± 1 standard deviation and as median (25th, 75th percentile) when having a not normal distribution according to Kolmogorov–Smirnov tests. Categorical variables were presented as numbers and percentages. We used the MedCalc Statistical Software version 19.6 for statistical analyses (MedCalc Software, Ostend, Belgium).
The differences between the two patient groups were compared using the paired t-test for continuous and normal-distributed parameters, by the Mann–Whitney U-test for not normal-distributed parameters, and by the chi-squared test for categorical parameters. Odds ratio (OR) and confidence interval (CI) of 95% of various parameters, related to LV remodeling, were estimated by univariate analysis. Parameters statistically significant associated with LV remodeling in univariate analysis were included in the multivariate analysis using a forward stepwise logistic regression model. The independent predictors identified by multivariate logistical regression were analyzed using the receiver operating characteristic (ROC) to determine their optimal sensitivity and specificity. Cox regression analysis was used to investigate the effect of predictor variables on LV remodeling diagnosed at 6 months. Two-tailed values of P< 0.05 were considered statistically significant.
Results
Of 271 patients who were initially enrolled, 18 were dropped out and not evaluated for LV remodeling due to the following reasons: 10 had poor echocardiographic images, and 8 needed revascularization procedures during the 6-month follow-up (5- new coronary angioplasty; 3- coronary artery by-pass graft). Finally, the study group included 253 patients, aged 32 to 92 years (mean age 66 ±13 years), 185 (73%) being men. The AMI patients’ age distribution is presented in Figure 1.
 |
Figure 1 Distribution of acute myocardial patients by age groups. |
At the 6-month echocardiography, 61 (24%) were detected with LV remodeling and were included in group II, while the rest of 192 (66%) were included in the nonremodeling group (group I). The baseline demographic, clinical, biochemical, and angiographic characteristics of the two AMI patient groups are compared in Table 1. The patients with LV remodeling were older, more often hypertensive, with higher Killip functional classes, higher peak values of CK-MB isoenzymes, and lower values of eFGR. They also had more often multivessel coronary artery disease. The proportion of patients with STEMI was higher in group II than in group I, but this result was not statistically significant (94% vs 89%).
 |
Table 1 The Baseline Characteristics of Patients with AMI Categorized by Echocardiography-Determined LV Remodeling at 6-Month Follow-Up |
Regarding the echocardiographic findings (Table 2), we found that at baseline, LVEDV and LVESV were significantly lower in the remodeling group, while the differences between averaged LVEF and stoke volume, E/A ratios, and WMS indexes were not significant. At the 6-month follow-up, we found that in the remodeling group the LVESV and the WMSI became significantly higher, while the LVEF and stroke volume index became significantly lower than in group I.
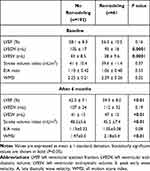 |
Table 2 Echocardiography Findings |
The odds ratio of developing LV remodeling was 1.81 when comparing the STEMI patients with the high-risk NSTEMI patients (95% CI:0.66–5.00, P=0.24).
The results of 2D STE at baseline and after 6 months are shown in Table 3. At baseline, only HLS and HLSR were significantly weaker in the remodeling group, while at the 6-month re-evaluation, the LV deformation performance was much more affected, as shown by the significantly lower values of the GLSR (P=0.02), the GRS (P<0.01), the GRSR (P=0.02), the HLS (P <0.01), and the HLSR (P<0.01), when compared to group I. An illustration of HLS is presented in Figure 2.
 |
Table 3 Two-Dimensional Speckle Tracking Results |
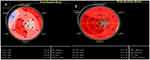 |
Figure 2 Two-dimensional speckle tracking imaging of left ventricular longitudinal strain. (A) Patient with left ventricular remodeling. (B) Patient without left ventricular remodeling. |
In univariate logistic regression, we found 15 predictors for LV remodeling (P<0.001) in AMI patients revascularized by PCI and having a midrange or preserved LVEF. These included age, systemic hypertension, hypercholesterolemia, smoking history, systolic and diastolic blood pressure at admission, the Killip class, the estimated glomerular filtration rate, the peak values of CK-MB isoenzymes, the 2- and 3 vessel CAD found at angiography, the LVEDV, and the LVESV, as well as HLS and the HLSR. The multivariate logistic regression selected 5 independent predictors for LV remodeling, and these were: the Killip class, the 3-vessel CAD, and the baseline LVESV, HLS, and HSR (Table 4).
 |
Table 4 Predictors for LV Remodeling |
For the independent predictors of LV remodeling, the ROC curves were analyzed and compared (Figure 3). The most powerful predictors of LV remodeling were the HLS (AUC=0.85, sensitivity 83%, specificity 84%, P<0.001), and the HLSR (AUC=0.77, sensitivity 93%, specificity 61%, P<0.001). The other independent predictors were the LVEDV (AUC=0.66, sensitivity 67%, specificity 58%, P<0.001), the 3-vessel CAD (AUC=0.62, sensitivity 39%, specificity 84%, P<0.001) and the Killip class (AUC=0.61, sensitivity 88%, specificity 34%, P<0.001). The AUC calculated for the HLS was significantly higher than the AUCs calculated for the other independent predictors (P<0.01). The ROC analysis revealed as cut-off values of the infarct-related LS and LSR predicting LV remodeling −11% and −0.65 s−1 respectively. At Cox-regression analysis, the coefficients for LV remodeling were 1.4 for baseline HSL< −11% (95% CI 0.66 to 0.78, P<0.0001, and 2.16 for baseline HLSR<-0.65s−1 (95% CI 0.10 to 0.33, P<0.001).
Discussion
Our case-control observational study is the first study in Romania addressing the predictive value of 2D-STE for LV remodeling in AMI patients with a midrange or preserved LVEF after successful reperfusion by PCI.
Heart failure with midrange ejection fraction (40–49%) is a recently described category of heart failure, situated in the interval between HF with reduced ejection fraction (< 40%) and HF with preserved ejection fraction (≥ 50%). This new category of HF seems to have transitional features, as well as transitional outcomes in comparison with the older HF categories, being closer to those of HF with preserved LVEF. However, the data regarding long-term follow-up and best therapeutic procedures are still unclear.7
As known, the acute injury of the myocardium in AMI induces a sudden over-loading of the heart work initiating the process of ventricular remodeling, that involves both the infarcted region and the distant non-infarcted myocardium.24 LV remodeling after AMI occurs as the result of the interaction between several factors, such as loss of contractile myocardium, activation of circulating neurohormones, patency of the infarct-related artery, infarction size, and LV size to reduce the wall stress.25,26
Early reperfusion therapy is essential for reducing infarct expansion and LV dilatation, it improves survival by limiting the infarct size and thus preserving LV function. But, several studies have shown that LV remodeling may occur even after successful reperfusion therapy with persistent patency of the infarct-related coronary artery.1,27 For that reason, it is important to identify patients at risk of LV remodeling, among those having a preserved LVEF after an AMI successfully reperfused by PCI.
The LVEF is a major predictor of prognosis in AMI, either STEMI or NSTEMI. Echocardiography is one of the preferred methods to evaluate the LVEF, as it can detect a number of abnormalities associated with a poor outcome, such as LV diastolic dysfunction, involvement of the right ventricle, a high wall motion score index, mitral regurgitation, and an increased left atrial volume. The ability of STI to assess regional LV function has been demonstrated by previous studies. It has been demonstrated that in HF patients with impaired systolic function, a reduced GLS increases the prognostic value.28 The role of STI also expands in the evaluation of LV diastolic function. GLS longitudinal strain is a precise, non-invasive predictor of hemodynamic worsening in AMI patients. It proved to be superior to LVEF and WMIS especially in AMI patients with an LVEF >40%.29 Ersbøll demonstrated that a reduced GLS was significantly associated with augmented neuro-hormonal activation, early hemodynamic aggravation, and prediction of a poor outcome in AMI with preserved LVEF. Early measurement of GLS in this population could be used as a risk stratification tool for added monitoring and clinical trials.30 In a recent study including 153 AMI patients with an LVEF ≥55% at 2.5 days after a successfully PCI, the GLS was independently associated with the occurrence of in-hospital clinical heart failure (P=0.003). The authors proposed the adding GLS into a screening model may increase the prediction of clinical heart failure after AMI.31
Our AMI cohort patients with successful PCI performed within the first 12 hours from the onset of symptoms included 253 patients with a mean age of 66 years. Compared to our studies, the proportion of STEMI was very high (96%), as other studies reported a proportion of about 60–80% for STEMI.32,33 This particularity is due to the small number of centers that can perform emergency PCI in Romania (23, for a population of 20 million inhabitants), and the fact that not all high-risk NSTEMI patients had access to an early (<12 hours) PCI. We used the peak value of CK-MB for evaluation of the AMI and not the troponin–I, because troponin-I is not serially determined in our hospital and so we didn’t have a peak-value for it.
In our study, LV remodeling was found in 24% of the AMI patients at the 6-month follow-up echocardiography. This rate is lower than reported by other studies (about 30%).33,34 A possible explanation for this finding could be the fact that we included only AMI patients with a baseline LVEF>40% after the successfully myocardial reperfusion using the PCI, this population having a lower risk to develop LV remodeling.35
Comparing the two groups of AMI patients with PCI done within the first 12 hours from the onset of symptoms, we found no differences among the 2 groups regarding the proportion of STEMI, male sex, obesity, hypercholesterolemia, smoking history, the NT-proBNP levels, the infarct-related arteries, or the medication prescribed at discharge.
The STEMI patients had a 1.57 higher risk to develop LV remodeling when compared to high-risk NSTEMI patients (P=0.27, 95% CI: 0.69–3.53).
At the baseline 2D-STE, we did not find any significant differences among the two groups regarding the longitudinal, circumferential, and radial strains and strain rates. Using the cut-off level of < −15% for the longitudinal strain,12 we identified the harmed (infarct-related) segments. The number of harmed segments was slightly higher in the remodeling group but the difference was not significant. However, the differences between longitudinal strains and strain rates at the level of the harmed segments were highly significant (Table 3). We did not find in the literature any cut-off levels for the harmed circumferential or radial strains, but these are relatively preserved in patients with LVEF> 40%.
The multivariate logistic regression selected five independent predictors for LV remodeling. Among these, the HLS was highlighted as being the most powerful, followed by the HLSR. These results are emphasizing the increasing value of 2D-STE in assessing the LV function in AMI patients and are concordant with those of other recently published studies.29,35 The identified cut-off levels predicting LV remodeling at baseline echocardiographic examination were −11% for HLS and −0.65 s−1 for HLSR. A baseline HLS < - 11% increased the hazard ratio for LV remodeling by a factor of 1.4, while the AMI patients with a baseline HLSR< −0.65s−1 were 2.16 more likely to develop LV remodeling (P<0.001).
The study has a number of limitations. We included only AMI patients at sinus rhythm and having a midrange or preserved LVEF following revascularization, presenting probably small extent infarctions. Echocardiography was performed as soon as possible after the PCI (1.3 ± 0.6 days), not immediately, although it is known that myocardial contractility may recover within 2 days after revascularization. However, these results encourage the use of 2D-STE in the risk stratification of AMI patients.
Conclusion
LV remodeling was diagnosed in 24% of AMI patients with a midrange or preserved EF after successfully PCI. The 2D-STE proved to be an efficient, practical, and reliable noninvasive technique to predict LV remodeling in this category of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106(18):2351–2357. doi:10.1161/01.CIR.0000036014.90197.FA
2. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling: concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi:10.1016/S0735-1097(99)00630-0
3. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi:10.1161/01.CIR.81.4.1161
4. Moller JE, Hillis GS, Oh JK, et al. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. 2006;151(2):419–425. doi:10.1016/j.ahj.2005.03.042
5. Bochenek T, Wita K, Tabor Z, et al. Value of speckle-tracking echocardiography for prediction of left ventricular remodeling in patients with ST-elevation myocardial infarction treated by primary percutaneous intervention. J Am Soc Echocardiogr. 2011;24:1342–1348. doi:10.1016/j.echo.2011.09.003
6. D’Andrea A, Cocchia R, Caso P, et al. Global longitudinal speckle tracking strain is predictive of left ventricular remodeling after coronary angioplasty in patients with recent non-ST elevation myocardial infarction. Int J Cardiol. 2011;153:185–191. doi:10.1016/j.ijcard.2010.08.025
7. Ponikowski P, Voors AA, Anker SD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(27):2129–2200.
8. Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333:1091. doi:10.1136/bmj.38985.646481.55
9. Roffi M, Patrono C, Collet JP, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;2016(37):267–315.
10. Cokkinos DV, Belogianneas C. Left Ventricular Remodelling: a Problem in Search of Solutions. Eur Cardiol. 2016;11(1):29–35. doi:10.15420/ecr.2015:9:3
11. Galli A, Lombardi F. Postinfarct Left Ventricular Remodeling: a Prevailing Cause of Heart Failure. Cardiol Res Pract. 2016;2016:1–12. doi:10.1155/2016/2579832
12. Tsai WC, Liu YW, Huang YY, Lin CC, Lee CH, Tsai LM. Diagnostic value of segmental longitudinal strain by automated function imaging in coronary artery disease without left ventricular dysfunction. J Am Soc Echocardiogr. 2010;23(11):1183–1189. doi:10.1016/j.echo.2010.08.011
13. Ibanez B, James S, Agewall S, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;2018(39):119–177.
14. Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;2018(39):3021–3104.
15. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias.The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41:111–188.
16. International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Available from: https://www.idf.Org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf.
17. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
18. Kourosh S, Diana S, Hassan J, et al. Prediction of survival after myocardial infarction using Killip class. Int J Clin Med. 2012;3:563–568. doi:10.4236/ijcm.2012.37102
19. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
20. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
21. Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–184. doi:10.1067/mje.2002.120202
22. Tsai WC, Liu YW, Huang YY, et al. Diagnostic value of segmental longitudinal strain by automated function imaging in coronary artery disease without left ventricular dysfunction. J Am Soc Echocardiogr. 2010;23(11):1183–1189.
23. Ingul CB, Malm S, Refsdal E, et al. Recovery of function after acute myocardial infarction evaluated by tissue Doppler strain and strain rate. J Am Soc Echocardiogr. 2010;23(4):432. doi:10.1016/j.echo.2010.01.018
24. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi:10.1161/01.CIR.101.25.2981
25. Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction: potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi:10.1161/01.CIR.87.3.755
26. Giannuzzi P, Temporelli PL, Bosimini E, et al. Heterogeneity of left ventricular remodeling after myocardial infarction: results of the Gruppo Italiano per lo Studio della Supravvivenza nell Infarcto Miocardico-3 Echo Substudy. Am Heart J. 2001;141:131–138. doi:10.1067/mhj.2001.111260
27. Cerisano G, Bolognese L, Buonamici P, et al. Prognostic implications of restrictive left ventricular filling in reperfused anterior acute myocardial infarction. J Am Coll Cardiol. 2001;37(3):793–799. doi:10.1016/S0735-1097(00)01203-1
28. Motoki H, Borowski AG, Shrestha K, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients wit chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–2088. doi:10.1016/j.jacc.2012.07.047
29. Ebeid H, El Hady RA, El Khashab K, Husein M. Left ventricular global longitudinal strain in acute myocardial infarction and incidence of in hospital heart failure. EurEcho. 2019;60(8):B4697.
30. Ersbøll MK. Left ventricular global longitudinal strain in acute myocardial infarction–with special reference to neurohormonal activation, in-hospital heart failure and prognosis. Dan Med J. 2013;60(8):B4697.
31. Luvsansuren B, Chimed S. Significance of left ventricular global longitudinal strain assessment in patients with preserved ejection fraction after acute myocardial infarction. Eur Heart J. 2020;41(Supplement_2). doi:10.1093/ehjci/ehaa946.1212
32. Altiok E, Tiemann S, Becker M, et al. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography for prediction of global and segmental functional changes after acute myocardial infarction: a comparison with late gadolinium enhancement cardiac magnetic resonance. J Am Soc Echocardiogr. 2014;27(3):249–257. doi:10.1016/j.echo.2013.11.014
33. Lacalzada J, de la Rosa A, Izquierdo MM, et al. Left ventricular global longitudinal systolic strain predicts adverse remodeling and subsequent cardiac events in patients with acute myocardial infarction treated with primary percutaneous coronary intervention. Int J Cardiovasc Imaging. 2015;31:575–584. doi:10.1007/s10554-015-0593-2
34. Zaliaduonyte-Peksiene D, Vaskelyte JJ, Mizariene V, Jurkevicius R, Zaliunas R. Does longitudinal strain predict left ventricular remodeling after myocardial infarction? Echocardiography. 2012;29(4):419–427. doi:10.1111/j.1540-8175.2011.01597.x
35. Scharrenbroich J, Hamada S, Keszei A, et al. Use of two-dimensional speckle tracking echocardiography to predict cardiac events: comparison of patients with acute myocardial infarction and chronic coronary artery disease. Clin Cardiol. 2018;41(1):111–118. doi:10.1002/clc.22860
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

