Back to Journals » Clinical Ophthalmology » Volume 14
Left-Right and Upper–Lower Light Sensitivity Asymmetry in Visual Field Defects Caused by Pituitary Adenoma: A Retrospective Observational Study
Authors Kotoda Y, Kotoda M , Ogiwara M, Kinouchi H , Iijima H
Received 18 October 2019
Accepted for publication 8 January 2020
Published 31 January 2020 Volume 2020:14 Pages 317—324
DOI https://doi.org/10.2147/OPTH.S234422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yumi Kotoda, 1 Masakazu Kotoda, 2 Masakazu Ogiwara, 3 Hiroyuki Kinouchi, 3 Hiroyuki Iijima 1
1Department of Ophthalmology, University of Yamanashi, Chuo, Yamanashi 409-3898, Japan; 2Department of Anesthesiology, University of Yamanashi, Chuo, Yamanashi 409-3898, Japan; 3Department of Neurosurgery, University of Yamanashi, Chuo, Yamanashi 409-3898, Japan
Correspondence: Yumi Kotoda 1110 Shimokato, Chuo, Yamanashi 409-3898, Japan
Tel +81-55-273-9657
Fax +81-55-273-6757
Email [email protected]
Purpose: The purpose of this study was to quantitatively investigate light sensitivity asymmetry both between left and right eyes and between upper and lower quadrant in the 30-degree visual field of patients with visual field defects caused by pituitary adenoma.
Patients and Methods: Preoperative Humphrey 30– 2 perimetry results were reviewed retrospectively using the charts of 28 pituitary adenoma patients who underwent surgery. Inter-eye light sensitivity comparisons of the temporal and nasal hemifields between the left and right eyes were conducted in each patient to study left-right asymmetry. Upper-lower asymmetry was investigated by comparing the frequency of severe scotoma (light sensitivity 5 dB or less) in the upper and lower visual field quadrants in the temporal and nasal hemifields.
Results: Left-right asymmetry was demonstrated in 61% of cases in the temporal hemifield and in 57% of cases in the nasal hemifield. Severe scotoma test points were investigated in the worse eye of each patient and were more frequent in the superotemporal quadrant of the visual field compared with the inferotemporal quadrant (P = 0.00029) and in the inferonasal quadrant compared to the superonasal quadrant (P = 0.00268).
Conclusion: Asymmetric visual field defects between left and right eyes are common in patients with pituitary adenoma. Severe scotoma is more frequent in the upper quadrant of the temporal hemifield and in the lower quadrant of the nasal hemifield.
Keywords: bitemporal hemianopsia, pituitary adenoma, asymmetry, Humphrey perimetry, light sensitivity
Introduction
Visual field defects observed in patients with pituitary adenoma are thought to result from compression of the optic chiasm.1 It has been demonstrated that chiasm compression from below generates significantly elevated tissue pressure in the central portion of the chiasm, where the nasal halves of the left and right optic nerve fibers cross to the contralateral optic tracts.2 The crossing nerve fibers corresponding to the temporal hemifield of vision in the left and right eyes are dominantly damaged.
While bitemporal visual field defects are characteristic in pituitary adenoma cases, it is rare to have complete bitemporal hemianopsia that is symmetrical in both eyes and has absolute scotoma throughout both temporal hemifields.3
Although magnetic resonance imaging (MRI) is a powerful diagnostic tool for pituitary adenomas, it is challenging to precisely assess and predict asymmetry in visual field defects based on MRI findings due to the complex anatomy and insufficient resolution of MRI.4
Unequal visual field defects in left and right eyes have also been described in the literature.5–8 Not only is inter-eye asymmetry present between left and right eyes, but intra-eye or upper-lower asymmetry may also be present. Visual field defects tend to show an upper-to-lower progression in the temporal hemifield and lower-to-upper progression in the nasal hemifield.9
Although several kinds of research have investigated asymmetric visual field defects in patients with pituitary adenoma,4,6,10 no precise investigation with statistical analysis regarding the inter-eye and intra-eye symmetry of visual field defects has yet been reported. In this study, we conducted a quantitative analysis to explore the asymmetric properties of visual field defects in pituitary adenoma patients.
Methods
Preoperative Humphrey 30–2 perimetry results were reviewed retrospectively using charts of pituitary adenoma patients who subsequently underwent surgery at the Department of Neurosurgery of the University of Yamanashi Hospital between June 2004 and July 2016. Patients had undergone a preoperative routine ophthalmic examination including visual acuity testing, slit-lamp examination, measurement of intraocular pressure, and funduscopic examination. Patients with glaucoma, retinal diseases, or optic nerve diseases causing visual field defects were excluded.
The perimetry results consisted of light sensitivity measurements at test points 6 degrees apart from each other in the central visual field (30-degree radius). Results were obtained using a Humphrey Field Analyzer (Zeiss-Humphrey Systems, Dublin, CA, USA) with either the Swedish Interactive Thresholding Algorithm (SITA)-standard, SITA-fast, or Fastpac protocol. Unreliable results with fixation loss greater than 20%, false-positive error greater than 33%, or false-negative error greater than 33% were not included in the analysis.11 Eligible data were obtained from 28 patients (15 men and 13 women aged 28 to 78 years, median age = 58 years).
We evaluated light sensitivity values shown in the numeric display of Humphrey perimetry results at 74 locations in the visual field, excluding two points at 15 degrees temporal and 3 degrees superior or inferior, corresponding to Marriott’s blind spot. To investigate left-right asymmetry, we compared inter-eye light sensitivity at 36 test points in the temporal and at 38 test points in the nasal hemifields. We defined left-right asymmetry in the temporal or nasal hemifield as cases where light sensitivity in the left eye differed significantly from that in the corresponding hemifield of the right eye with a P-value <0.05 (Mann–Whitney U-test).
Upper-lower asymmetry was investigated in each patient’s worse eye, which had lower average light sensitivity at the 74 test points. In normal eyes light sensitivity differs in the upper and lower halves of the Humphrey 30–2 perimetry results,12 so we studied upper-lower asymmetry by comparing differences in the frequency of severe scotoma (light sensitivity of 5 dB or lower).
The preoperative MRI images of all patients included were reviewed, and the size of the tumor were analyzed.
Statistical analyses were conducted using StatFlex (Version 6.0; Artec, Osaka, Japan).
Data were analyzed using the Mann–Whitney U-test because the data did not appear to follow a normal distribution.
Results
Left-Right Asymmetry
A representative case displaying left-right asymmetry is shown in Figure 1A. Each light sensitivity value in the temporal hemifield of the left and right eyes (Figure 1A, red boxes) is plotted in Figure 1B, which indicates significantly lower sensitivity in the right eye (Mann–Whitney U-test, P < 0.0001). The left-right differences in the nasal hemifields (Figure 1A, blue boxes) shown in Figure 1C were also significant (P = 0.0001), although less prominent.
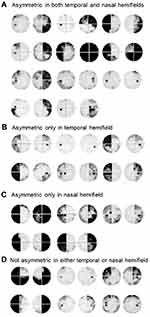
Left-right asymmetry was observed in 17 cases (61%) in the temporal hemifield and in 16 cases (57%) in the nasal hemifield. Only six cases (21%) showed no left-right asymmetry in either the temporal or nasal hemifield. We therefore classified the 28 cases into four categories: asymmetric in both temporal and nasal hemifields (11 cases, 39%) (Figure 2A); asymmetric only in the temporal hemifield (6 cases, 21%) (Figure 2B); asymmetric only in the nasal hemifield (5 cases, 18%) (Figure 2C); and not asymmetric in either hemifield (6 cases, 21%) (Figure 2D).
Upper–Lower Asymmetry
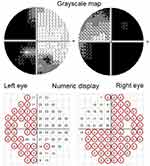
The test points for severe scotoma, defined as a light sensitivity less than or equal to 5 dB, are shown as red circles in the numeric display of Humphrey 30–2 perimetry results in the representative case in Figure 3. Average light sensitivity in the right eye (4.2 dB) was lower than in the left eye (12.0 dB), so in this case data from the right eye were analyzed to study upper-lower asymmetry.
The frequency of severe scotoma test points in the 28 cases is shown in Figure 4. The value at each test point shows the percentage of patients with less than or equal to 5-dB light sensitivity at the corresponding test point in their worse eye.
Test points that show severe scotoma at frequencies of 50% or higher are observed in ten out of 18 (56%) points in the superotemporal quadrant but only three out of 18 (17%) points in the inferotemporal quadrant. The frequency of severe scotoma at the 18 test points in the superotemporal quadrant was thus significantly higher than in the inferotemporal quadrant (P = 0.00029, Mann–Whitney U-test; Figure 5 left).
 |
Figure 5 Different frequencies of severe scotoma between the superior and inferior visual field quadrants in the temporal and nasal hemifields. |
Although upper-lower asymmetry was less prominent in the nasal hemifield, the upper-lower relationship in the nasal hemifield was the inverse of the relationship in the temporal hemifield, with the frequency of severe scotoma being higher in the inferonasal quadrant compared to the superonasal quadrant (P = 0.00268, Mann–Whitney U-test, Figure 5 right).
The maximum diameter of the pituitary adenoma in the patients of the present study measured on the preoperative MRI was 30.3 ± 8.8 mm (mean ± standard deviation; range, 12–50 mm). Representative Humphrey 30–2 perimetry results for asymmetric and symmetric visual field defect coupled with the corresponding MRI images are shown in Figure 6.
Discussion
In the present study, we demonstrated that visual field defects in patients with pituitary adenoma were asymmetric between left and right eyes in about 80% of cases in the temporal or nasal hemifield or both.
The visual field defect in patients with pituitary adenoma has been investigated mostly via a qualitative approach. Although some recent studies aimed to quantitatively evaluate the visual field defect, precise investigation with statistical analysis regarding the inter-eye and intra-eye symmetry of visual field defects had not been reported.
Clinical studies using Humphrey 30–2 perimetry in patients with pituitary adenoma have been reported by several researchers, including Lee et al4 and Boland et al10. The former analyzed the Humphrey results qualitatively, classifying them as normal, unreliable, bitemporal, mixed (bitemporal and additional defects), monocular, homonymous, nonspecific, or other. The latter did not analyze light sensitivity itself but rather the sum of the total or temporal scores of the neurologic hemifield test, which was assessed by the original ranking scales for the probability of pattern deviation obtained from Humphrey 30–2 perimetry.
In the current study, we analyzed light sensitivity values instead of total deviation, the reduction in dB below the expected age-matched level, to directly and accurately compare the inter-eye light sensitivity. The use of total deviation would eliminate age and eccentricity effects, and therefore it is suitable to compare at different test points in the visual field in many subjects of different ages.13 However, since the aim of the present study is inter-eye comparison of light sensitivity at corresponding test points in the same patients, it seems reasonable to use the light sensitivity values instead of total deviation.
Left-right visual field defect asymmetry may be caused by the asymmetric enlargement of pituitary tumors, which is sometimes evidenced in the coronal section on MRI (Figure 6A and B).
However, it has been reported that the sensitivity of MRI in predicting the asymmetric compression of the chiasm is only 44%.4 Another recent study that investigated the possible relationship between tumor asymmetry on MRI and asymmetric visual field defects failed to achieve statistical significance.10 Considering the results of the earlier studies and the insufficient resolution of the current MRI, it seems difficult to precisely assess and predict visual field defects based on MRI findings.
Apart from detailing temporal hemifield defects, the present study revealed that visual field defects extend into the nasal hemifield, which also demonstrates left-right asymmetry in many cases. Advanced bitemporal visual field defects in pituitary adenoma patients are likely to be followed by visual field loss in the nasal hemifield.9 This could be attributed to damaged uncrossed nerve fibers, either because the growing pituitary tumor may successively damage directly the uncrossed fibers or because the growing tumor compresses the uncrossed fibers in the lateral part of the optic chiasm against the surrounding structures.
In addition to the left-right asymmetry, pituitary adenoma also causes upper-lower asymmetry. Although one earlier study demonstrated the trend toward greater total deviation of Humphrey 30–2 perimetry results in the superotemporal quadrant compared to the inferotemporal quadrant,14 the details of upper-lower asymmetry in patients with pituitary adenoma were poorly investigated in the literature. In the present study, by investigating the frequency of severe scotoma instead of total deviation, we were successfully able to demonstrate upper-lower asymmetry with upper-dominant deterioration in the temporal hemifield and lower-dominant deterioration in the nasal hemifield.
The pattern of asymmetry showing upper-dominant deterioration in the temporal hemifield and lower-dominant deterioration in the nasal hemifield may conform to Hedges’ report,9 in which visual field damage in the inferonasal quadrant precedes damage in the superonasal quadrant. The mechanism for higher frequencies of severe scotoma in the inferonasal compared to the superonasal quadrant of the visual field may implicate compression of the prechiasmal optic nerve against the falciform ligament, which has been reported recently in neurosurgical literature.15–18
The optic nerve is roofed by the falciform ligament, which forms the sharp edge of a dural fold where it enters the optic canal anterior to the chiasm. It has been reported that compression of the optic nerve against the falciform ligament caused by the ectatic internal carotid artery may result in visual field defects, which could be resolved with microvascular decompression surgery.16–18 Meningiomas that elevate the optic nerve from below and cause compression against the falciform ligament have also been reported to cause inferior altitudinal hemianopsia.15 When the optic chiasm is elevated by an inferiorly placed pituitary tumor, the superotemporal portion of the optic nerve fibers might become more damaged by the compression against the falciform ligament, leading to visual field damage in the inferonasal quadrant.
The limitations of the present study include its retrospective nature and a relatively small amount of patient data. The data in the study were obtained using three different types of Humphrey algorithms. However, we do not think that it would affect the comparison between right and left eyes, which were tested using the same algorithm. The leaning effect in Humphrey perimetry might have influenced the results of inter-eye asymmetry. We used only Humphrey perimetry for the visual test. Although the test is a well-established and commonly used quantitative method to evaluate visual field defects in the clinical practice, future studies using different tests would support our findings. Although the exploratory study using the anatomical approach to assess the relationship between vision defect and anatomy of the adenoma is beyond the scope of this study, future large-scale prospective studies undertaken with the cooperation of neuroradiologists may be expected to lead to a more precise understanding of visual field defect pathophysiology in patients with pituitary adenoma.
Conclusions
Visual field defects observed in pituitary adenoma patients frequently exhibit inter-eye asymmetry in both temporal and nasal hemifields. Upper-lower asymmetry is also present, in which the upper quadrant in the temporal hemifield and the lower quadrant in the nasal hemifield are more severely damaged.
Abbreviations
MRI, magnetic resonance imaging; SITA, Swedish Interactive Thresholding Algorithm.
Ethics Approval and Informed Consent
This study was approved by the University of Yamanashi Hospital ethics committee (registration number: 1600). An opt-out consent option was provided for all patients.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
YK, conceptualization, design, data acquisition, data analysis, data interpretation, revision for important intellectual content; MK, data acquisition, data analysis, data interpretation, revision for important intellectual content; MO and HK, data analysis, data interpretation, revision for important intellectual content; HI, conceptualization, design, data acquisition, data analysis, data interpretation, drafting of the manuscript. All authors have approved the final manuscript to be published and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ikeda H, Yoshimoto T. Visual disturbances in patients with pituitary adenoma. Acta Neurol Scand. 1995;92:157–160. doi:10.1111/j.1600-0404.1995.tb01031.x
2. Kosmorsky GS, Dupps WJ
3. Schiefer U, Isbert M, Mikolaschek E, et al. Distribution of scotoma pattern related to chiasmal lesions with special reference to anterior junction syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242:468–477. doi:10.1007/s00417-004-0863-5
4. Lee IH, Miller NR, Zan E, et al. Visual defects in patients with pituitary adenomas: the myth of bitemporal hemianopsia. AJR Am J Roentgenol. 2015;205:W512–W518. doi:10.2214/AJR.15.14527
5. Miller NR. Walsh and Hoyt’s Clinical Neuro-ophthalmology.
6. Poon A, McNeill P, Harper A, et al. Patterns of visual loss associated with pituitary macroadenomas. Aust N Z J Ophthalmol. 1995;23:107–115. doi:10.1111/j.1442-9071.1995.tb00138.x
7. Glaser JS. Topical diagnosis: the optic chiasm. In: Glaser JS, editor. Neuro-Ophthalmology.
8. Rivoal O, Brezin AP, Feldman-Billard S, et al. Goldmann perimetry in acromegaly: a survey of 307 cases from 1951 through 1996. Ophthalmology. 2000;107:991–997. doi:10.1016/s0161-6420(00)00060-9
9. Hedges TR. Preservation of the upper nasal field in the chiasmal syndrome: an anatomic explanation. Trans Am Ophthalmol Soc. 1969;67:131–141.
10. Boland MV, Lee IH, Zan E, et al. Quantitative analysis of the displacement of the anterior visual pathway by pituitary lesions and the associated visual field loss. Invest Ophthalmol Vis Sci. 2016;57:3576–3580. doi:10.1167/iovs.16-19410
11. Birt CM, Shin DH, Samudrala V, et al. Analysis of reliability indices from Humphrey visual field tests in an urban glaucoma population. Ophthalmology. 1997;104:1126–1130. doi:10.1016/S0161-6420(97)30173-0
12. Li Y, Wu Z, Jiang Y. Quantitative analysis of light sensitivities in central 30-degree visual field of normal Chinese and glaucoma patients. Chin Med J (Engl). 1997;110:129–133.
13. Iijima H. Reduced light sensitivity due to impaired retinal perfusion in branch retinal vein occlusion. Jpn J Ophthalmol. 2018;62:151–157. doi:10.1007/s10384-017-0546-5
14. Kerrison JB, Lynn MJ, Baer CA, et al. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol. 2000;130:813–820. doi:10.1016/S0002-9394(00)00539-0
15. Colapinto EV, Cabeen MA, Johnson LN. Optic nerve compression by a dolichoectatic internal carotid artery: case report. Neurosurgery. 1996;39:604–606. doi:10.1097/00006123-199609000-00035
16. Shapey J, Danesh-Meyer HV, Kaye AH. Suprasellar meningioma presenting with an altitudinal field defect. J Clin Neurosci. 2012;19:155–158. doi:10.1016/j.jocn.2011.06.005
17. Fargen KM, Blackburn S. Surgical decompression for optic neuropathy from carotid artery ectasia: case report with technical considerations. World Neurosurg. 2014;82(239):e239–e212. doi:10.1016/j.wneu.2013.06.023
18. Woodall MN, Alleyne CH
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.