Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
Landscape on CT screening for lung cancer in Asia
Authors Triphuridet N , Henschke C
Received 28 April 2019
Accepted for publication 28 August 2019
Published 30 September 2019 Volume 2019:10 Pages 107—124
DOI https://doi.org/10.2147/LCTT.S192643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Antonio Araujo
Natthaya Triphuridet,1,2 Claudia Henschke2
1Faculty of Medicine and Public Health, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand; 2Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Correspondence: Claudia Henschke
Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, Box 1234, New York, NY 10029, USA
Tel +1 212 241 2821
Fax +1 212 241 9655
Email [email protected]
Abstract: Lung cancer remains the leading cause of cancer incidence and mortality worldwide. Approximately 60% of the world’s new cases of lung cancer and deaths from it are expected in Asia in 2018. Currently, lung cancer screening using low-dose computed tomography (LDCT) is recommended for heavy smokers in North America, Europe and some countries in Asia. Tobacco smoking being the major risk factor for lung cancer, but in Asia, lung cancer in never-smokers (LCINS) is also a concern. This paper reviews on lung cancer incidence, mortality, etiology, smoking in Asia, and systematic reviews on LDCT lung cancer screening studies, including ongoing projects and recommendation on lung cancer screening in Asia. Some of the earliest studies of LDCT lung cancer screening worldwide were in Asia. Many countries in Asia have developed LDCT screening studies in various high-risk participants. Currently, there are several ongoing large-scale lung cancer screening trials to evaluate the efficacy of LDCT screening for never-smokers and light smokers, as well as heavy smokers, and to evaluate the feasibility of population-based LDCT lung cancer screening.
Keywords: lung cancer, CT screening, LDCT, Asia, guidelines
Introduction
Lung cancer remains the leading cause of cancer incidence and mortality worldwide and these are increasing.1,2 So that approximately 60% of the world’s new lung cancer cases and death predicted occur in Asia in 2018, and over one-third of these will occur in China.3 Meanwhile, the lung cancer incidence of both sexes in the US and men in European countries has been decreasing over the past two decades.2,4,5 In China, the trend remains stable for men but is increasing in women between 2000 and 20116, and since the beginning of this century, lung cancer has the highest incidence and mortality of all types of cancer.7–9
Lung cancer screening using LDCT has been studied since the 1990s. Some of the earliest studies of LDCT screening for lung cancer were performed in Japan.10,11 Currently, LDCT has been recommended for lung cancer screening among heavy smokers in North America, Europe, and some countries in Asia.12–20 There are regional and racial/ethnic differences in lung cancer which impact the cancer susceptibility, incidence, and mortality. Moreover, the cost of LDCT screening and insurance coverage are also the important factors in lung cancer screening and mortality.
In this article, we initially compare lung cancer incidence, mortality, and current smoking in individual countries in Asia. Then, we review and summarize the published LDCT lung cancer screening trials including observational studies, randomized controlled trials, and other ongoing projects in Asia. Finally, we review and compare Asian guidelines for LDCT screening and lung nodule management in each country and provide the timeline of LDCT lung cancer screening studies and guidelines in Japan, China, Korea, and Israel. We provide the information by different regions of Asia, Eastern Asia (Japan, China, Korea, and Mongolia), South-Eastern Asia (Thailand, Indonesia, Viet Nam, Philippines, Myanmar, Malaysia, Singapore, Cambodia, Lao People’s Democratic Republic, Brunei, and Timor-Leste), South-Central Asia (India, Bangladesh, Pakistan, Iran, Kazakhstan, Nepal, Uzbekistan, Sri Lanka, Afghanistan, Kyrgyzstan, Turkmenistan, Tajikistan, Bhutan, and Maldives), and Western Asia (Turkey, Israel, Syrian Arab Republic, Iraq, Lebanon, Azerbaijan, Armenia, Georgia, Jordan, Saudi Arabia, Yemen, Cyprus, Gaza Strip and West Bank, United Arab Emirates, Kuwait, Oman, Bahrain, and Qatar).
Incidence and mortality rate of lung cancer in Asia
Lung cancer remains the leading cause of cancer incidence and mortality worldwide and is increasing, from 1.8 million new lung cancer cases and 1.6 million related deaths in 2012 to 2.1 million new cases (11.6%) and 1.8 million deaths in 2018 estimated by GLOBOCAN 2018.1,2 Most of the world’s new lung cancer cases (1.2 million, 58.5%) and lung cancer deaths (1.1 million, 60.7%) occur in Asia and over one-third of these occur in China with 774,323 and 690,567 cases, respectively.3
From recent data on global cancer statistics 2018, the highest age-standardized incidence rate per 100,000 population among Asian males are seen in Eastern Asia (47.2%), followed by Western Asia (38.8%), South-Eastern Asia (26.3%), and South-Central Asia (9.4%).2,3 Men in Turkey had the highest incidence rates (70.6), followed by Armenia (58.5%), North Korea (48.2%), and China (47.8%) (Table 1).3
The highest lung cancer incidence rates among Asian women are seen in Eastern Asia (21.9), South-Eastern Asia (9.6%), Western Asia (7.8%), and South-Central Asia (3.4%), respectively.2,3 Women in North Korea had the highest incidence rates (27.4), followed by Brunei (26.6%), China (22.8%), South Korea, and Singapore (17.2%).3
The mortality-to-incidence ratio ranged from 0.87 to 0.97 in men and from 0.78 to 0.94 in women. Lung cancer patients in Eastern Asia had the lowest ratio, particularly women (0.87, and 0.78 in men and women, respectively), followed by South-Eastern Asia (0.90, 0.88), South-Central Asia 0.94, 0.94), and highest in Western Asia (0.97 and 0.94).
Etiology
Tobacco smoking
Tobacco smoking is a major risk factor for lung cancer, particularly in men, and causes nearly 80% of male and 50% of female lung cancer deaths. China is the largest tobacco producer and consumer in the world with over 2.9 million tons of tobacco manufactured in 2016.12,13 According to the 2010 Global Adult Tobacco Survey, there were an estimated 301 million Chinese current smokers, aged 15 years, and older, nearly one-third of the world’s total21,22 with the prevalence of smoking was 52.9% among men and 2.4% among women.14,15 Although the percentage of smoking is decreasing to 48.4% and 2.0%, respectively, the rate remains high.23,24 In the United States, the percentage is 24.6% among men and 19.1% among women.24
From World Health Statistics on age-standardized prevalence of current tobacco smoking among persons aged 15 years and older in 2016.24 Among Asian men, Timor-Leste has the highest rate (78.1%), follow by Indonesia (76.1%), Georgia (55.5%) and Maldives (55%). Among Asian women, the highest smoking prevalence rate was observed in Lebanon (26.9%), Israel (15.4%), Turkey (14.1%) and Japan (11.2%) (Table 1).
 |
Table 1 Estimated age-standardized incidence and mortality rates per 100,000 person-years of lung cancer (LC), all ages, in 2018, and age-standardized prevalence of current tobacco smoking among persons aged 15 years and older in 20162,3,24 (sorted from high to low of male LC incidence) |
It is of note that the lung cancer incidence and smoking rates in Asian women are lower compared to women in Western countries with the similar lung cancer incidence rate, particularly in Brunei and China. In Brunei and China, women had lung cancer incidence rates of 26.6 and 22.8 per 100,000, respectively, with smoking prevalence of 2% and 1.9%, respectively. The corresponding rates for women in Poland and France were 24.5 and 22.5 with smoking rates of 23.3% and 30.1%, respectively).2,3,24
Nontobacco factors
It is estimated that 25% of the lung cancer patients are never-smokers. There are major clinical differences based on ethnicity, gender, and histology in lung cancer in never-smokers (LCINS), particularly in Asian women, which target the distal airways and flavor adenocarcinoma histology. It has been estimated that 15% of men and 53% of women with lung cancer worldwide are never-smokers.25 The proportion of lung cancers in women never-smokers is particularly high in women in Eastern (61%) and Southern Asia (83%), but in the United States, only 15% of all lung cancer found in women never-smokers.26
Environmental risk factors are reported to play a predominant role in LCINS, including second-hand smoke (SHS) exposure, environmental particulate matter, occupational exposures, indoor air pollution, and radon. It was estimated that more than half of the lung cancer deaths are attributable to ambient fine particles in China.7 These risk factors appear to be responsible for a significant proportion of lung cancer in Chinese never-smokers, but account for a smaller proportion of cases in Europe and North America. The combined population attributable fraction (PAF) for SHS exposure occurring at home and in the workplace was highest in Chinese women (24.11%), coal smoke (19.93%), and tuberculosis (12.67%). While the PAF for SHS was only 5.63%, and tuberculosis infection was 1.14% among North American women.27
Genetic factors also play an important role in lung cancer etiology of LCINS. There are differences in genetics and molecular changes between LCINS and lung cancer in smokers. Smoking-related cancers are associated with KRAS mutations, STK11, SMARCA4, and high numbers of other mutations, especially C:G>A:T transversions, while cancers in never-smokers are associated with EGFR mutations, ALK translocations, PTEN, PIK3CA, and low numbers of mutations targeting C:G>T:A transitions.26,28,29
The overall frequency of EGFR mutations in patients with NSCLC of adenocarcinoma histology in Asia was higher than in Europe (15%) and North America (22%). However, the EGFR rates vary in Asia, from being as high as 47% in Eastern and South-Eastern Asia) with the highest occurring rate of 64% in Vietnam, and lower rates of 26% in Indian subcontinent and 23% in Bangladesh.30 Moreover, a lower prevalence of KRAS mutations, which is associated with poorer prognosis in lung cancer patients with NSCLCs, is observed in Asians (3.8–8%) than in Caucasians (18–26%).31–33
There are more than 150 Genome-wide association studies (GWAS) that have been published in lung cancer. In 2008, three studies identified three potential susceptibility loci for lung cancer.34,35 Chromosomes 15q25 and 5p15.33, containing the telomerase reverse transcriptase (TERT) gene that have been confirmed, but the cancer-associated role of the locus on 6p21-6p22 remained more controversial.26,35 A multistage GWAS of lung cancers in never-smoking Asian women in six Asian countries (mainland China, South Korea, Japan, Singapore, Taiwan, and Hong Kong) identified associated loci; TERT at 5p15.33, TP63 at 3q28, VTI1A on chromosome 10 and ROS1-DCBLD1. Moreover, this study showed no evidence of association for lung cancer at 15q25, which the authors mentioned provided “strong evidence that this locus is not associated with lung cancer independent of smoking.36
LDCT screening for lung cancer in Asia
Japan
Japan initiated LDCT screening for lung cancer study in 1993 in one of the earliest studies of LDCT lung cancer screening worldwide and developed the mass screening with mobile CT in 1996.10,11,3740 As of 2009, the LDCT screening had spread throughout Japan, as 127,897 screenings in 61 institutions were performed. There are guidelines for Japanese LDCT lung cancer screening and management of pulmonary nodules detected by LDCT and also standardization of LDCT screening programs and certification of physicians and radiologic technologists.
In 1993, the Anti-Lung Cancer Association (ALCA), a for-profit organization to screen dues-paying participants for lung cancer, introduced LDCT at the initiative of the National Cancer Center Study Group as a screening modality.11,38,41 The ALCA screening project using LDCT, chest x-ray (CXR), and 3-day pooled sputum cytology with a 6-month interval in smokers aged 40–79 years, was supported by a Grant-in-Aid by the Ministry of Health and Welfare of Japan for Comprehensive 10-Year Strategy for Cancer Control. Kaneko et al11 reported the result of comparing LDCT with CXR from 1993 to 1995 in 1369 participants. This study demonstrated the superiority of LDCT to CXR in the screening and early detection of peripheral lung cancer (93% stage 1 lung cancer, 0.43% lung cancer detection rates) in high-risk individuals. Sobue et al in 200241 reported the 5-year survival rate for LDCT screen-detected lung cancer of the ALCA project, 76.2% and 64.9% for initial and repeated screening, respectively.
In 1996, Sone et al37,42,43 initiated mass screening for lung cancer with a mobile LDCT scanner for 5483 individuals from the general population of Matsumoto, aged 40–74 years, and provided baseline and two annual screening. This study was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare, Japan. The prevalence and overall lung-cancer detection rates with LDCT were 0.40% and 0.41%, respectively. Eighty-eight percent of lung cancer patients identified on screening and surgically confirmed were stage I. The 5- and 10-year survival from all-causes of death were 89.8%, and 83.1% and from lung cancer death were 91.5% and 86.2%, respectively. The excellent survival rate was observed in never-smokers, patients with BAC, and adenocarcinoma/mixed types with nonsolid nodule, associated with Noguchi’s type A or B and pathologic stage IA. In addition, Sone started a collaboration with the International Early Lung Cancer Action Program (I-ELCAP) in 2001.
Nawa et al38,44,46 reported a LDCT screening program during 1998–2006 in 25,385 screened individuals aged ≥50 years in Hitachi city. The baseline and overall lung cancer detected rate was 0.67% and 0.34%, respectively, with 91% and 85% stage I lung cancer, respectively. The 5-year survival rate was 90%. Comparison of all causes of death among patients with lung cancer detected on LDCT screening, current and former smokers (HR=4.7) had a poorer prognosis than never-smokers. When comparing on nodule consistency, patients with cancer in a solid nodule (HR=4.6) had a poorer prognosis than those with cancer in a nonsolid or part-solid nodules. The lung cancer mortality rate of Japanese aged 50–79 decreased by 24% in 2005–2009 compared with the national statistics during 1995–2004. The author suggested that a wide implementation of LDCT screening may decrease lung cancer in community 4–8 years after the introduction of the screening.44
The Japanese Society of CT Screening (JSCTS) was founded in 1994 as “The Society of Thoracic CT Screening”.38 The society has organized annual seminars to spread knowledge and improve skills of LDCT screening, produced and published “Manual for single-detector CT (SDCT) and MDCT imaging methods” in 2004, and revision in 2005,47 “guidelines for the management of pulmonary nodules detected by low-dose CT” for 3 versions, in 2005, 2009, and 2013, respectively,48 and preparing software for education (ALCA Project: The Simulation). In 2013, the LDCT screening for lung cancer recommendation was published in “Japanese Imaging Guidelines” by the Japan Radiological Society and the Japanese College of Radiology.19 (Details are in current guidelines in Asia section.)
LDCT screening for lung cancer had spread widely in Japan, the JSCTS performed a nationwide survey of LDCT screening among member institutions and reported 34,181 screened participants in 20 institutions in 2000 had increased to 127,897 participants in 61 institutes in 2009 with the lung cancer detection rate of 0.33% and 0.15%, and 78.8% and 67.7%, of stage 1 lung cancer, respectively.38,49
To standardize LDCT screening, the Accreditation Council for Lung Cancer CT Screening was founded by a joint committee in April 2009.38,50 Physicians are certified on the basis of specialist qualifications and the completion of specified courses, and technologists are certified on the basis of the completion of specified courses and tests; the certifications are to be renewed every 5 years. As of 2015, there are 1347 certified physicians and 854 certified technologists.38,47
After many observational screening trials of asymptomatic adults in Japan, Sagawa et al51,52 started the JECS Study (The Japanese randomized trial for evaluating the Efficacy of low-dose thoracic CT Screening for lung cancer in non-smokers and smokers under 30 pack-years), a 10-year RCT compared LDCT once every 5 years with CXR in people aged 50–70 with a smoking history <30 pack-years, in 2010 (Figure 1). This study was supported by the Ministry of Health, Labour and Welfare, and the Japan Agency for Medical Research and Development. A sample size of 13,500 subjects for each arm was required to detect a 60% mortality reduction after 10 years. As of April 1, 2018, over 7500 participants have registered for the JECS Study.50
 |
Figure 1 Diagram for the Japanese randomized trial for evaluating the Efficacy of low-dose thoracic CT Screening for lung cancer in non-smokers and smokers under 30 pack-years (JECS) study protocol. |
China
The LDCT screening for lung cancer in China initiated in 1994.53 International collaborative programs were established in 2003 with I-ELCAP and later with US National Cancer Institute and with NELSON in Europe.53,57 LDCT screening was supported by the Chinese Central Government Public Health Special Subsidy in 2009. Large-scale screening programs have been developed with aims to evaluate the feasibility of conducting population-based LDCT lung cancer screening57,58 and determining the lung cancer–specific mortality benefit and effectiveness of LDCT screening with/without biomarkers in various high-risk participants.59
LDCT screening for lung cancer was initiated in 1994 at the 5th Affiliated Hospital of Sun Yat-Sen University in Zhuhai. In 2003, its collaboration with the I-ELCAP started.53 Prior to the collaboration with I-ELCAP, a single slice spiral CT was used for screening and CT interpretation was based on morphology and interval growth, and follow-up participants with benign nodules 12 months or later, and follow-up participants without nodule 2 years. After the collaboration, 16-slice MDCT and the I-ELCAP protocol were used60 with an interpretation based on size, nodule consistency, and presence of new nodule, and follow-up participant without nodule or with benign nodules 12 months. In 2011, Liu et al published the outcome difference of LDCT screening pre-collaboration (1994–2002) and post-collaboration (2003–2009) in 3348 and 3582 enrolled participants, respectively.53 The rate of surgery for benign disease, overall lung cancer detection, and stage 1 cancer were comparable, but during the later time, the positive rate increased (6.2% vs 9.8%, P<0.001), smaller cancers were diagnosed (18.6 mm vs 15.6 mm, P=0.04), and the interval between last routine screening and surgery decreased (213 days vs 96 days, P<0.001). All this led to an increase in lung cancer 5-year survival rate (75% vs 95%, P=0.032). The author concluded technology improvements along with a well-defined protocol improved outcomes of LDCT screening for lung cancer in Zhuhai.
In 2005, the Cancer Hospital & Institute, Chinese Academy of Medical Sciences (CICAMS) in Beijing collaborated with I-ELCAP and conducted a lung cancer screening study using the I-ELCAP protocol.8 Tang W. et al reported the result of LDCT screening in 4690 asymptomatic participants aged ≥40 years, between 2007 and 2012 with 0.55% detection rate of lung cancer and 76.9% stage I NSCLC, and showed that the lung cancer detection rate of female SHS was higher than in high-risk smokers and male SHS (1.4% vs 0.9%, 0.4%).8,54 Up to 2018, a total of 41,300 participants were enrolled with lung cancer detection rate of 0.4%.59
In 2009, LDCT lung cancer screening was included in the cancer early detection and treatment program supported by the Chinese Central Government Public Health Special Subsidy. A national demonstration program, Rural China Screening Programme (RuraCSP), was initiated to evaluate the feasibility of conducting population-based LDCT lung cancer screening in the Chinese setting.57,58,61 This program was a prospective multi-center observational study of annual LDCT screening among the high-risk population with different inclusion criteria at the different regions and centers. For example, tobacco smoking was the criteria at the Dagang Oilfield center, while indoor air pollution exposure was the criteria at the Xuanwei center.57 Up to July 2017, a total of 13,000 participants had. The lung cancer detection rate was 1% (0.3–2.9%) and 40% (25–73%) of early-stage lung cancers were identified in baseline round. For annual screening, the lung cancer detection rate was 0.4% (0.1–0.8%) and 56% (46–90%) were early-stage lung cancers. The highest prevalence (2.9%) and incidence (0.8%) were observed in Xuanwei center.58
In 2012, the Chinese government launched Cancer Screening Program in Urban China (CanSPUC), a 5-year annual LDCT screening for lung cancer in urban residents with high-risk criteria that is regional-dependent. This study aimed to promote the early detection and treatment of the five top cancers (lung, breast, colorectal, liver, and upper digestive tract cancers) in urban areas of China.8,58,61,62 Up to 2018, 20 provinces/municipalities have been included.61 The RuraCSP and CanSPUC programs involved other aspects including health promotion, technical training for doctors and technical personnel, smoking cessation, biomarker discovery, and validation. To sustain the development of a national screening program, the two programs have been included into the special program of medical insurance system reform in China to explore the possibility of incorporating LDCT lung cancer screening in the routine health insurance system in China.58
Following the national programs, several regional, national, and international programs have been initiated. During 2013–14, there were 2 major programs of science & technology commission on lung cancer screening project initiated in Shanghai (2013)63 and Beijing Municipality (2014), and a National Health Public Welfare Scientific Research Project to study the application of Biomarkers in Screening for Five Common Cancers (2014).59
In 2014, the CICAMS also collaborated with US NCI and initiated the China Cancer Screening Trial Feasibility Study, a multicenter RCT in 3 cities, funded by the CICAMS, and the National Health and Family Plan Committee of China56,61,64 to obtain information necessary to design a long-term RCT on lung and colorectal cancer screening, and to test colorectal cancer screening strategies in China. The study protocol is illustrated in Figure 2. As of March 2015, a total of 2696 participants were enrolled. There were 6.5% and 6.1% of participants who had baseline LDCT findings suspicious for lung cancer in arm 1 and arm 2, respectively. The positive colorectal cancer screening was 34.1% with colonoscopy screening, 8.0% with OC-FIT test, 4.5% with InsureFIT test, and 5.8% with Septin9 test. In addition, the CICAMS had a collaboration project with the MD Anderson Cancer on Lung Cancer Early Detection.59
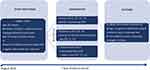 |
Figure 2 Diagram for the China Cancer Screening Trial Feasibility Study (CiCanSTri) protocol. |
In 2015, based on the protocol of LDCT lung cancer screening program in rural China, a China national lung cancer screening guideline was developed by lung cancer early detection and treatment expert group appointed by the National Health and Family Planning Commission and it was revised in 2018.18,58,65 The detail is in current guidelines in the Asia section.
In 2016, a collaborative project, Netherlands-China Big-3 screening,55 a multicenter study, was started to improve the early detection of 3 important diseases: lung cancer, COPD, and cardiovascular diseases, by using the newest ultra-LDCT techniques and identifying the biomarkers of the diseases at a very early stage. The result will demonstrate differences between Netherlands and China. This project was assessed and will be managed by the Royal Netherlands Academy of Arts and Sciences and co-financed by the Ministry of Education, Culture and Science. The Chinese Ministry of Science and Technology in Beijing is responsible for assessing and financing the Chinese component.56,64
In 2017, the National Key R&D Program of China launched the China National Cancer Early Screening (CHANCES) Trial: Lung and Colorectal Cancer, a multicenter nationwide RCT on 78,500 high-risk smokers and never-smokers (1) to perform the largest prospective RCT on lung cancer screening in China, (2) to evaluate the effectiveness of different screening frequency (no screening, 3 annual LDCTs and 2 biannual LDCTs), (3) to evaluate the effectiveness of LDCT with/without biomarkers, and (4) to determine whether screening with LDCT could reduce mortality from lung cancer in China. The study protocol is illustrated in Figure 3. The project also established data management system with several main functions as web-based data management platform, automated risk assessment, structured reporting, and management, automatically generating to-do list, and auto-notification.59
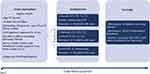 |
Figure 3 Diagram for the China National Cancer Early Screening (CHANCES) Trial: lung and colorectal cancer study protocol. |
Taiwan
Lung cancer screening studies, initiated in 2007, were first reported in Taiwan in 2012. Most of them demonstrated a high baseline incidence in non-NLST eligible criteria, particularly in women and individual with a family history of lung cancer in Taiwan.66,67 Currently, there is ongoing a National Lung Screening Program aimed to perform LDCT screening for lung cancer in never‐smokers and validate the risk single-nucleotide polymorphisms (SNPs) previously identified that associated with susceptibility to lung cancer in never‐smokers.
In 2007, Wang et al68 started a 3-annual LDCT screening in lung cancer families with the following criteria: (1) first-degree relatives had lung cancer (simplex family) or ≥2 relatives with lung cancer (multiplex family), and (2) age≥55 years. A total of 1125 participants from 559 families of lung cancer, 810 patients from 418 simplex families and 315 came from 141 multiplex families, were recruited between August 2007 and November 2009. The baseline lung cancer detection was 1.7% with 63% stage I. This study demonstrates evidence of lung cancer prevalence based on family risk.
Chen et al67 reported a retrospective study of LDCT screening in the setting of annual medical examinations in one hospital in 3339 individuals aged ≥18 years and no prior history of any cancer during January–December 2012. The overall cancer detection rate was 1.02% with 99% of stage 1 carcinoma. A very high detection rate (6.2%) in subgroup aged younger than 50 years with a positive family history of all types of cancers in first-degree relatives was demonstrated.
Wu et al66 showed retrospective data of 1763 asymptomatic healthy subjects aged 40–80 years who underwent LDCT during 2013–2014. The lung cancer detection rate in NLST eligible participants vs noneligible female and male participants were 0.7% vs 2.6% and 0.6%, respectively. The data showed that female sex (OR 6.367; P=0.003) and a family history of lung cancer (OR 3.017; P=0.016) were significant predictors of lung cancer in Taiwan. From this cohort with 2 months of extension period, Hsu et al69 analyzed the diagnostic accuracy of modification of the ACR Lung Imaging Reporting and Data System (LungRADS), from data of 1978 screened participants with 32 pathological proven adenocarcinoma (3AAH, 3 AIS, and 20 invasive adenocarcinoma). The data show using modified Lung-RADS category 2C (GGN: <20 mm) as cutoff, had the higher area under the curve (AUC) of 0.973 in predicting adenocarcinoma spectrum lesions (sensitivity of 100%, specificity of 89.3%), significantly higher than of LungRADS (0.815, P<0.001) and NLST criteria (0.906, P<0.001). Moreover, using category 3B (Part solid: ≥6 mm with solid component <6 mm) as cutoff had an AUC of 0.992 in predicting invasive adenocarcinoma (sensitivity of 95%, specificity of 97.8%). The author concluded that the modified Lung-RADS may substantially improve sensitivity while maintaining specificity for detection of adenocarcinoma spectrum lesions in an Asian population.
In 2014, Taiwan developed a National Lung Screening Program, Taiwan Lung Cancer Screening for Never‐smoker Trial (TALENT), sponsored by the Ministry of Health and Welfare. This prospective, nationwide, multicenter study, aimed to perform LDCT screening for lung cancer in never‐smokers and validate the risk SNPs previously identified that associated with susceptibility to lung cancer in never‐smokers.70,71 LDCT were performed annually for three consecutive years in participants aged between 55 and 75 years, never-smoker with one of the following risk: (i) family history of lung cancer within third-degree relatives, (ii) passive smoke exposure, (iii) history of pulmonary TB or COPD, (iv) cooking index ≥110, or (v) not using ventilator during cooking. SNP typing was studied in every enrolled subject and integrated into a risk score prediction model. Up to May 13, 2018, a total of 10,397 subjects were enrolled, approximately 75% were women and had environmental smoking exposure. The lung cancer detection rate was 2.34% with 95.1% stage I. Four SNPs (TERT, TP63, HLA-DRB9/HLA-DRB5, HLA-DRB1/HLA-DQA1) significantly associated with the risk of lung cancer. The lung cancer cases had the risk score point significantly higher than normal (59.4±28.23 vs 50.1±28.84, p<0.0001).
South Korea
LDCT lung cancer screening has been studied in Korea since 1999 and the Korean guideline for lung cancer screening was launched in 2015. Currently, there is a Korean-Lung Cancer Screening Project (K-LUCAS), a pilot project to assess the effectiveness and feasibility of lung cancer screening to implement national cancer screening program in Korea.
Chong et al72 reported a LDCT screening in 6406 asymptomatic Korean participants aged ≥45 years during 1999–2003 at Samsung Medical Center. Of the 6406, 52% were high-risk (≥20 pack-years) ever-smokers, 25% were non-high-risk ever-smokers, and 23% were never-smokers. The overall proportion of total lung cancer cases over the number of baseline participants was 0.36% with 62% being stage 1.
Yi C.A. et al73 evaluated the performance of LDCT screening comparing with CXR for lung cancer detection in 12,427 asymptomatic Korean participants with diverse risks for lung cancer in a nontrial setting during 2006–2008. In the non-high-risk group, LDCT had a higher lung cancer detection rate (adjusted OR, 5.07) and survival than of CXR group (adjusted HR, 0.08). Meanwhile, no difference in detection or survival of lung cancer was observed in the high-risk group. Lung cancers in the non-high-risk group were predominantly adenocarcinomas (96%), and more likely to be part-solid or nonsolid compared with those in the high-risk group (p=0.023).
In 2015, the Korean guideline for lung cancer screening was launched by a Korean multi-society collaborative committee.20 (Details are givens in current guidelines in Asia section.)
In 2017, a K-LUCAS Project, a 2-year multicenter lung cancer screening pilot project, was initiated to as evaluate the effectiveness and feasibility of lung cancer screening and validate new standards for the reporting form of LDCT and the quality of the screening by a web-based network system, and develop a risk prediction model for lung cancer.74,76 This project was sponsored by National Cancer Center and collaborated with Multidisciplinary Expert Committee. The LDCT screening was performed in high-risk smoker as a NLST criterion, (asymptomatic smokers aged 55−74 years with a smoking history ≥30 pack-years who had used tobacco within the last 15 years), in 14 regional tertiary hospitals. All LDCT images were analyzed by network-based computer-aided diagnosis in a screening center and reported and managed by using Lung-RADS version 1.0 categories. Until May 2018, 8234 participants underwent LDCT screening. Lung cancer detection rate was 0.53% with 52.8% Stage I.76
Israel
Results of LDCT screening for lung cancer study, started in Israel in 1998, were reported in 2006. In collaboration with ELCAP, Shaham et al77,78 initiated annual LDCT screening for lung cancer program during 1998–2000 among 571 adults aged 50 years and older with a history of at least 10 pack-years, and later with I-ELCAP, for smokers aged at least 40 years with 271 additional enrolled subjects during 2000–2004. Follow-up of 68 months showed that the baseline and overall lung cancer detection rates were 1.43% and 0.78%, respectively, with 86% being stage 1.
India
Raghava et al79 reported a result from a large collaborative lung cancer screening using low-dose CT study of 28,351 asymptomatic persons at risk from 2004 to 2005 through 2014. The overall lung cancer detection rate was 1.7% with 85% having clinical stage 1 lung cancer. The estimated 10-year lung-cancer-specific survival rate among participants with biopsy-proven clinical stage I lung cancer was 87% and 91% for who underwent surgical resection within 1 month after diagnosis.
Thailand
Triphuridet et al80,82 reported a high lung cancer detection rate (1.4%) of a prospective LDCT screening in endemic areas of tuberculosis (TB) started in 2012 among 634 heavy smokers aged 50–70 years without a history of active TB. Baseline lung cancer detection rate was 1.4% with 56% lung cancer being stage I. And the 1st- and 2nd-year incidence of lung cancer were 0.67% and 0.70%, respectively, and of active pulmonary TB were 0.50% and 0.52%, respectively.81 From this cohort, the comparable diagnostic value on lung cancer detection of findings suspicious for lung cancer detected by LDCT and digital tomosynthesis (DT) was demonstrated (positive predictive value 34.8% vs 40%, respectively, with comparable sensitivity (80%) and specificity (98%)).80
Saeteng et al.83 reported an initial result of LDCT screening in the first relative of lung cancer patients, aged 20–65 years during January 2013 to May 2013. Nodules or other suspicious findings were classified as positive results. Nearly half of them (45.2%) had positive nodule with an average number and size of nodules was 2.1 nodules and 0.4 cm in diameter, respectively.
Summary of LDCT screening for lung cancer trials in Asia
The LDCT screening for lung cancer studies published in all languages before 21 February 2019 was identified by a literature search from the PubMed, Medline, Embase, and Scopus databases. There are 25 studies with published results in abstracts and manuscripts, 19 prospective cohorts, 5 retrospective cohorts, and 1 RCT study, with many differences between the studies such as study design, eligible subjects, screening regimens, and protocol for nodule management. Most of the study published data screened in asymptomatic adults, with annual follow-up, only one study had a 6-month interval41 and three studies using 24-month interval.53,84,85 The positive screening criteria varied from any pulmonary nodule including calcified nodule to only solid and part-solid nodule diameter 10 mm or larger. The studies were summarized and categorized according to the lung cancer risks, 16 had general participants,3 had with smoking history, 1 with family history of lung cancer, 4 with different lung cancer risks, and unknown risk factor (in Table 2).
 |
Table 2 Summary of LDCT screening for lung cancer trials in Asia (sorted by start year and screening criteria) |
Asymptomatic adults
There are 16 studies on LDCT screening in general subjects, 6 from Japan and 4 from China, 4 Korea, and 2 Taiwan, with baseline and overall lung cancer detection rates with range of 0.17–1.36% and 0.20–0.78%, respectively. The baseline and overall stage I lung cancers were 27.27–100% and 59.6–100%, respectively, with 75–95% 5-year lung cancer–specific survival rate. However, the low detection rates were observed more frequent in the early trials using LDCT with thicker slice protocol than the trials using the thinner slice. Moreover, in the trials with high lung cancer or stage 1 cancer detection rate, there were reported rates of AIS (formerly Bronchiolo-alveolar cell carcinoma (BAC)) as high as 40–70% of the total lung cancers diagnosed.42,67,86,87 The overall lung cancer detection rate and stage 1 lung cancer in the 6-month interval screening11,41 were 0.38% and 80.56%, respectively, while the corresponding rates in the annual studies42,43,53,54,72,73,87,89 were 0.21–0.78% and 60.87–93.87% and biennial studies53,84 were 0.45–0.76% and 66.67–100%, respectively.
Smokers
There are three prospective studies on annual LDCT screening in smokers from Israel,78 Thailand,81,82 and Korea76 Two studies screened among heavy smoker with the history of tobacco smoking pack at least 30 pack-year aged 50–55 years or older76,81,82 and one study screened among younger and lighter smoker as minimum aged of 40 years with 10 pack-year smoking history.78 The baseline lung cancer detection rates ranged from 0.53% to 1.43% with 53.0–85.71% stage I lung cancer. As noted, the studies of heavy smokers reported the lower prevalence of lung cancer and stage 1 lung cancer using the higher baseline cutoff positive criteria were used for solid and PSN, and subsolid nodules were not defined as positive.76,81,82
Different lung cancer risks
Three Chinese58,85 and one Taiwanese70,71 studies on the participant who had different lung cancer risks such as active and passive smoking history, lung cancer family history, history of kitchen fume or dust exposure, occupational exposure, and history of pulmonary TB or COPD. The studies demonstrated a wide range of baseline lung cancer detection rates ranging from 0.2% to 2.9%, the highest (2.9%) was observed in participants with indoor pollution, with 25–95% stage I lung cancer. Although the high overall lung cancer (2.3%) and early detection rate (95%) observed in the screening trial among never-smokers,70,71 the high frequency of invasive procedures, benign nodule underwent invasive procedure (false positive biopsy rate) and frequency of adenocarcinoma in situ (AIS) diagnosed also observed 3.16%, 26.14%, and 17.28%, respectively.
Family history of lung cancer and others
A prospective study on LDCT screening in participants with a family history of lung cancer from Taiwan68 showed a high baseline lung cancer detection rates as 1.69% with 63% stage I lung cancer. However, 3.11% of study subjects underwent invasive procedures with 46% of them benign and 26% of lung cancer diagnosed as formerly BAC. An Indian study unknown lung cancer risk of participant showed an overall lung cancer detection rate 0.89% with 85% stage I lung cancer.
The LDCT screening for lung cancer could yield high lung cancer and early-stage detection rates not only in the high-risk smoking and also never-smoker with other risk factors, and it seems also beneficial in countries with previously reported low and moderate lung cancer incidence and smoking prevalence as India, Thailand, and Israel. However, there were many factors that affect the lung cancer detection rate such as lung cancer risks of study participants, study protocol; LDCT slice thickness, screening interval, positive criteria, and nodule management. Identifying high risk and optimizing the study protocol and nodule management are important parts of effective LDCT screening.
Current guidelines in Asia
There are LDCT lung cancer screening guidelines published by profession societies from Japan, China, and Korea that are different based on their own national studies results reflecting different influences of medical subspecialties from each country. There are nodule management guidelines for lung cancer screening in Japan and China which are different in size and follow-up time of actionable nodules as summarized in Table 3.
 |
Table 3 Summary of national nodule management guidelines for lung cancer screening in Asia |
Japan
The Japanese “guidelines for the management of pulmonary nodules detected by low-dose CT” have been launched for 3 versions, at 2005, 2009, and 2013, respectively.48 The key points of the third revision are 1) nodule size on screening CT image is obtained by calculating the average of the maximal diameter and the perpendicular diameter, 2) follow-up examinations for solid nodules depend on whether the patient is a smoker or nonsmoker, 3) lumped pure ground-glass nodules (GGNs) and mixed GGOs (or part-solid nodules) into a single category (after new international classification of adenocarcinoma of the lung in 2011) and proposed a decision tree based on the maximal diameter of the pulmonary nodule (≥15 mm or 5 mm), and 4) proposed a more detailed follow-up examination of pulmonary nodules that have been newly detected by repeat LDCT screening. The detail of the recommendation is summarized in Table 3. The JSCTS have not introduced VDT measurements on a workstation or PET examinations into the decision tree in their guidelines as in Fleischner Society guidelines, the NELSON study, or the Danish Lung Cancer Screening Trial, because it is not easy to perform VDT measurements on workstations or PET studies in ordinary clinical settings in Japan.48
In 2013, the Japanese Imaging Guideline was published by the Japan Radiological Society and the Japanese College of Radiology.19 The guideline recommended LDCT screening for lung cancer for persons aged 50 or over with a Brinkman index ≥600 (comparable with ≥30 pack-years of smoking) as grade C1, and the guidelines state that while scientific evidence is insufficient, LDCT screening may be considered as a measure for population-based screening. For the non-high-risk group, LDCT screening is not recommended as a means for population-based screening because of the lack of scientific evidence, grade C2. For individual screening, it may be performed after appropriate explanation about its unclear effects and disadvantages such as overdiagnosis and exposure.
China
The China national lung cancer screening guideline was developed in 2015 and was revised in 2018.18,58,65 The guidelines recommended annual lung cancer screening with LDCT for high-risk individuals aged 50–74 years who have ≥20 pack-year smoking history and who currently smoke or have quit within the past 5 years.
Korea
In 2015, the Korean guideline for lung cancer screening was launched by a Korean multisociety collaborative committee. The guidelines recommended that the annual LDCT screening should be recommended to healthy subjects aged 55–74 years, current smokers, and ex-smokers (if less than 15 years have elapsed after smoking cessation) with ≥30 pack-years of smoking history, as the NLST criteria, with grade B quality of the evidence.20
Saudi Arabia
In 2018, a lung cancer prevention and screening guidelines were launched by a multidisciplinary team of experts in lung cancer representing different health care sectors and in coordination with the Saudi Lung Cancer Association of Saudi Thoracic Society.91 The guidelines address the primary and secondary prevention approaches in lung cancer, including tobacco control and detection at earlier presentation. However, the team does not recommend that a national screening program be mandated or implemented for lung cancer at this stage until more data and studies provide stronger evidence to justify adopting a national program.
 |
Figure 4 Timeline of LDCT lung cancer screening studies and guidelines in Japan, China, Korea, and Israel. |
Conclusion
Several countries in Asia have developed studies on LDCT lung cancer screening since 1990s and later since 2000s developed national lung cancer screening guidelines and nodule management protocol for their high-risk citizen (Figure 4). The screening studies enrolled various participants ranging from heavy smoker to never-smokers with other high-risk exposure and asymptomatic adults. The baseline lung cancer detection rates ranged from 0.2% to 2.9%, depending on risk characteristics of the participants. Higher rates (>1%) were observed in individuals with indoor air pollution exposure (RuraCSP - Xuanwei center, 2.9%),8,58 lung cancer family (1.7%),68 and smokers (1.4%).78,81
Currently, there are several ongoing large-scale trials in Eastern Asia to evaluate the efficacy of LDCT screening for LCINS and light smokers (JECS, CHANCES, and TALENT) and risk score prediction model by using SNPs in never-smoker (TALENT), and to evaluate the feasibility of conducting population-based LDCT lung cancer screening (RuraCSP, CanSPUC, and K-LUCAS) and develop a web-based network system, and risk prediction model for lung cancer (CHANCES, and K-LUCAS).
LDCT screening in developing countries in Asia is challenging as high-risk individuals are at high risk due to tobacco smoking and environmental risk exposure, but there are insufficient resources and health care infrastructure. Additionally, there is no good strategy to screen for lung cancer in the never-smoking population who will develop lung cancer without any easily quantifiable risk factors. However, only the three highly developed countries, China, Japan, and South Korea, have published national-wide screening guidelines for lung cancer in heavy smokers. Many other countries have high lung cancer rates with a large population who still actively smoke, such as Turkey, Armenia, Georgia, Vietnam, and Philippines. There are no national lung cancer screening guidelines indicating the difficulty of finding resources to implement a large-scale endeavor that requires investment for equipment, technology, and skilled personals to detect a low incidence of lung cancer. For LDCT lung cancer screening to be successful and cost-effective, better screening strategies or biomarkers considering specific high-risk population for both smokers and nonsmokers, and carefully implemented in countries that have local factor as granulomatous disease prevalence, region by region or country by country need to be developed.
To implement lung cancer screening in Asia to maximize the screening benefit and minimize harms when there are insufficient resources and health care infrastructure, it is important to identify high-risk individuals by using simplified risk prediction model and/or affordable biomarkers, optimize screening regimens, and develop screening networks of local or primary care and screening centers which have multidisciplinary teams to provide additional management. However, tobacco control and smoking cessation policy remains the most important national priority for reducing the burden of lung cancer and other smoking-related diseases, together with raising awareness of smoking and environment risk.
Disclosure
Dr. Claudia Henschke is a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest which are owned by Cornell Research Foundation (CRF). Since 2009, Dr. Henschke does not accept any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF. Dr. Henschke is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation, she does not receive compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases, recipients include, I-ELCAP, among others. The funding comes from a variety of sources including philanthropic donations, grants and contracts with agencies (federal and non-federal), imaging and pharmaceutical companies relating to image processing assessments. The various sources of funding exclude any funding from tobacco companies or tobacco-related sources. The authors report no other conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. World Health Organization, International Agency for Research on Cancer, The Global cancer Observatory, Cancer today; 2018. Available from: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=population&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=15&type=0&statistic=5&prevalence=0&population_group=4&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&group_cancer=1&include_nmsc=1&include_nmsc_other=1.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
5. Lortet-Tieulent J, Renteria E, Sharp L, et al. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988–2010. Eur J Cancer. 2015;51(9):1144–1163. doi:10.1016/j.ejca.2013.10.014
6. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
7. Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26(1):48–58. doi:10.3978/j.issn.1000-9604.2014.01.08
8. Zhao S-J, Wu N. Early detection of lung cancer: low-dose computed tomography screening in China. Thorac Cancer. 2015;6(4):385–389. doi:10.1111/1759-7714.12253
9. Chang S, Dai M, Ren JS, Chen YH, Guo LW. [Estimates and prediction on incidence, mortality and prevalence of lung cancer in China in 2008]. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33(4):391–394.
10. Pinsky PF. Lung cancer screening with low-dose CT: a world-wide view. Transl Lung Cancer Res. 2018;7(3):234–242. doi:10.21037/tlcr.2018.05.12
11. Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology. 1996;201(3):798–802.
12. Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33–38. doi:10.1016/j.jtcvs.2012.05.060
13. Moyer VA. Screening for lung cancer: U.S. Preventive Services task force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi:10.7326/M13-2771
14. Roberts H, Walker-Dilks C, Sivjee K, et al. Screening high-risk populations for lung cancer: guideline recommendations. J Thorac Oncol. 2013;8(10):1232–1237. doi:10.1097/JTO.0b013e31829fd3d5
15. Wender R, Fontham ET, Barrera E
16. Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5Suppl):e78S–e92S. doi:10.1378/chest.12-2350
17. Couraud S, Cortot AB, Greillier L, et al. From randomized trials to the clinic: is it time to implement individual lung-cancer screening in clinical practice? A multidisciplinary statement from French experts on behalf of the French intergroup (IFCT) and the groupe d’Oncologie de langue francaise (GOLF). Ann Oncol. 2013;24(3):586–597. doi:10.1093/annonc/mds476
18. Zhou Q, Fan Y, Wang Y, et al. [China National Lung Cancer screening guideline with low-dose computed tomography (2018 version)]. Zhongguo Fei Ai Za Zhi. 2018;21(2):67–75. doi:10.3779/j.issn.1009-3419.2018.02.01
19. The Japanese Imaging Guideline; 2013. Available from: http://www.radiology.jp/content/files/diagnostic_imaging_guidelines_2013_e.pdf.
20. Jang SH, Sheen S, Kim HY, et al. The Korean guideline for lung cancer screening. J Korean Med Assoc. 2015;58(4):291–301. doi:10.5124/jkma.2015.58.4.291
21. World Health Organization: Tobacco in China. Available from: http://www.wpro.who.int/china/mediacentre/factsheets/tobacco/en/.
22. WHO report on the global tobacco epidemic, 2017.
23. Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364(25):2469–2470. doi:10.1056/NEJMc1102459
24. World Health Organization. World Health Statistics Data Visualizations Dashboar, Tobacco Control; 2018. Available from:http://apps.who.int/gho/data/view.sdg.3-a-data-ctry?lang=en. Accessed August 28, 2019.
25. Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31(2):139–148.
26. Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers–a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi:10.1038/nrc2190
27. Sisti J, Boffetta P. What proportion of lung cancer in never-smokers can be attributed to known risk factors? Int J Cancer. 2012;131(2):265–275. doi:10.1002/ijc.27477
28. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi:10.1093/jnci/dji055
29. An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One. 2012;7(6):e40109. doi:10.1371/journal.pone.0040109
30. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911.
31. El-Telbany A, Ma PC. Cancer genes in lung cancer: racial disparities: are there any? Genes Cancer. 2012;3(7–8):467–480. doi:10.1177/1947601912465177
32. Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14(18):5731–5734. doi:10.1158/1078-0432.CCR-08-0646
33. Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28(30):4616–4620. doi:10.1200/JCO.2010.29.6038
34. Chanock SJ, Hunter DJ. When the smoke clears. Nature. 2008;452:537. doi:10.1038/452537a
35. Timofeeva MN, Hung RJ, Rafnar T, et al. Influence of common genetic variation on lung cancer risk: meta-analysis of 14 900 cases and 29 485 controls. Hum Mol Genet. 2012;21(22):4980–4995. doi:10.1093/hmg/dds334
36. Lan Q, Hsiung CA, Matsuo K, et al. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nat Genet. 2012;44(12):1330–1335. doi:10.1038/ng.2456
37. Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351(9111):1242–1245. doi:10.1016/S0140-6736(97)08229-9
38. Nawa T, Nakagawa T, Mizoue T, Endo K. Low-dose computed tomography screening in Japan. J Thorac Imaging. 2015;30(2):108–114. doi:10.1097/RTI.0000000000000138
39. Eguchi K, Henschke C. Meeting summary of the 12th international conference on screening for lung cancer: Nara, Japan, April 2005. J Thorac Oncol. 2006;1(2):190–197. doi:10.1097/01243894-200602000-00018
40. Henschke CI, Shaham D, Yankelevitz DF, Altorki NK. CT screening for lung cancer: past and ongoing studies. Semin Thorac Cardiovasc Surg. 2005;17(2):99–106. doi:10.1053/j.semtcvs.2005.05.002
41. Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol. 2002;20(4):911–920. doi:10.1200/JCO.2002.20.4.911
42. Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84(1):25–32. doi:10.1054/bjoc.2000.1531
43. Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996–1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer. 2007;58(3):329–341. doi:10.1016/j.lungcan.2007.06.022
44. Nawa T, Nakagawa T, Mizoue T, et al. A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer. 2012;78(3):225–228. doi:10.1016/j.lungcan.2012.09.012
45. Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest. 2002;122(1):15–20. doi:10.1378/chest.122.1.15
46. Nawa T, Nakagawa T, Mizoue T, et al. Long-term prognosis of patients with lung cancer detected on low-dose chest computed tomography screening. Lung Cancer. 2012;75(2):197–202. doi:10.1016/j.lungcan.2011.07.002
47. Wada S. Producing a Manual for the Multidetector-Row Computerized Tomography (MDCT) Imaging Method for Lung Cancer Screening. 2006. Available from: https://www.jscts.org/pdf/guideline/mdct-english.pdfAccessed August 28, 2019.
48. The Japanese Society of CT Screening.Guidelines for the Management of Pulmonary Nodules Detected by Low-dose CT Lung Cancer Screening Version 3. Available from: https://www.jscts.org/pdf/guideline/gls3rd_english130621.pdf. Accessed August 28, 2019.
49. Nakayama T. Annual survey of CT screening in Japan. JSCTS. 2011;18:127–128.
50. Accreditation Council for Lung Cancer CT Screening. Available at: http://www.ct-kensin-nintei.jp/index.html.
51. Sagawa M, Nakayama T, Tanaka M, Sakuma T, Sobue T. A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of <30 pack-years aged 50-64 years (JECS study): research design. Jpn J Clin Oncol. 2012;42(12):1219–1221. doi:10.1093/jjco/hys157
52. Sagawa M. Japanese CT screening trial: JECS Study. IASLC CT screening workshop, The IASLC 19th World Conference on Lung Cancer. Toronto, Canada; 2018.
53. Liu X, Liang M, Wang Y, et al. The outcome differences of CT screening for lung cancer pre and post following an algorithm in Zhuhai, China. Lung Cancer. 2011;73(2):230–236. doi:10.1016/j.lungcan.2010.11.012
54. Tang W, Wu N, Huang Y, et al. [Results of low-dose computed tomography (LDCT) screening for early lung cancer: prevalence in 4 690 asymptomatic participants]. Zhonghua Zhong Liu Za Zhi. 2014;36(7):549–554.
55. Important grant for collaboration in research on early detection of lung cancer, COPD and cardiovascular diseases; 2016. Available from: https://www.rug.nl/news/2016/11/grote-subsidie-voor-samenwerking-in-onderzoek-vroege-opsporing-longkanker_-copd-en-hart--en-vaatziekten?lang=en.
56. Dai M, Hu P, Shi J-F, et al. The China cancer screening trial feasibility study. Lancet. 2015;386:S35. doi:10.1016/S0140-6736(15)00616-9
57. Zhou Q, Fan Y, Wu N, et al. Demonstration program of population-based lung cancer screening in China: rationale and study design. Thorac Cancer. 2014;5(3):197–203. doi:10.1111/1759-7714.12078
58. Zhou Q. MS16.02 NELCIN B3 screening program in China. J Thorac Oncol. 2018;13(10):S272–S273. doi:10.1016/j.jtho.2018.08.154
59. Wu N Lung cancer screening in Asia.
60. The International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. 2006;355(17):1763–1771. doi:10.1056/NEJMoa060476
61. Davies MPA, Cheng YI, Field JK, Liu D, Li W. Implementation planning for lung cancer screening in China. Precis Clin Med. 2019;2(1):13–44. doi:10.1093/pcmedi/pbz002
62. Published 2016. Available from: http://www.jmjtzyy.com/index.php/Index/newsContent/id/1237.
63. Liu Shiyuan ZC, Fan L Study on key problems of early screening and early diagnosis of peripheral lung cancer based on multimodal image; 2018. Available from: http://www.shcz.com/front/newOne.aspx?id=1637.
64. Hu P, Dai M, Shi J, et al. Abstract 1795: the feasibility study of a randomized cancer screening trial in China. Cancer Res. 2016;76(14 Supplement):1795. doi:10.1158/0008-5472.CAN-16-0584
65. Zhou QH, Fan YG, Bu H, et al. China national lung cancer screening guideline with low-dose computed tomography (2015 version). Thorac Cancer. 2015;6(6):812–818. doi:10.1111/1759-7714.12287
66. Wu FZ, Huang YL, Wu CC, et al. Assessment of selection criteria for low-dose lung screening CT among Asian ethnic groups in Taiwan: from mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer. 2016;17(5):e45–e56. doi:10.1016/j.cllc.2016.03.004
67. Chen CY, Chen CH, Shen TC, et al. Lung cancer screening with low-dose computed tomography: experiences from a tertiary hospital in Taiwan. J Formos Med Assoc. 2016;115(3):163–170. doi:10.1016/j.jfma.2015.11.007
68. Wang C, Tsai Y, Cheung Y, et al. Baseline results of lung cancer screening program for family lung cancer risk with low-dose spiral CT in Taiwan. J Clin Oncol. 2010;28(15_suppl):7098. doi:10.1200/jco.2010.28.15_suppl.7098
69. Hsu H-T, Tang E-K, Wu M-T, et al. Modified lung-RADS improves performance of screening LDCT in a population with high prevalence of non–smoking-related lung cancer. Acad Radiol. 2018;25(10):1240–1251. doi:10.1016/j.acra.2018.01.012
70. Yang P. Taiwan lung cancer screening program for never smokers. Respirology. 2018;23(S2):69. doi:10.1111/resp.13419_168
71. Yang P. MS16.04 National lung screening program in Taiwan. J Thorac Oncol. 2018;13(10):S274–S275. doi:10.1016/j.jtho.2018.08.156
72. Chong S, Lee KS, Chung MJ, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci. 2005;20(3):402–408. doi:10.3346/jkms.2005.20.3.402
73. Yi CA, Lee KS, Shin M-H, et al. Low-dose CT screening in an Asian population with diverse risk for lung cancer: a retrospective cohort study. Eur Radiol. 2015;25(8):2335–2345. doi:10.1007/s00330-015-3620-8
74. Lee JW, Kim HY, Goo JM, et al. Radiological report of pilot study for the Korean Lung Cancer Screening (K-LUCAS) Project: feasibility of implementing lung imaging reporting and data system. Korean J Radiol. 2018;19(4):803–808. doi:10.3348/kjr.2018.19.4.803
75. Lee J, Lim J, Kim Y, et al. Development of protocol for Korean Lung Cancer Screening Project (K-LUCAS) to evaluate effectiveness and feasibility to implement National cancer screening program. Cancer Res Treat. 2019. doi:10.4143/crt.2018.464
76. Lee C-T, Kim Y. Korean-lung Cancer Screening Project (K-LUCAS).
77. Shaham D, Breuer R, Copel L, et al. Computed tomography screening for lung cancer: applicability of an international protocol in a single-institution environment. Clin Lung Cancer. 2006;7(4):262–267. doi:10.3816/CLC.2006.n.004
78. Shaham D, Goitein O, Yankelevitz DF, Vazquez M, Reeves AP, Henschke CI. Screening for lung cancer using low-radiation dose computed tomography. Imaging Decisions MRI. 2002;6(4):4–13.
79. Raghava S, Siddque S. PUB075 survival of patients with stage I lung cancer detected on CT screening in South Indian population. J Thorac Oncol. 2017;12(1):S1491–S1492. doi:10.1016/j.jtho.2016.11.2045
80. Triphuridet N, Singharuksa S, Sricharunrat T. Screening of Lung Cancer by Low-Dose ct (LDCT), Digital Tomosynthesis (DT) and Chest Radiography (CR) in a high risk population: a comparison of detection methods. Journal of Thoracic Oncology. 2013;8:S148–S149.
81. Triphuridet N, Singharuksa S, Vidhyakorn S. P1.03-043 practical difficulty of low dose computerized tomography as a lung cancer screening tool in an endemic area of tuberculosis: topic: screening. J Thorac Oncol. 2017;12(1):S568–S569. doi:10.1016/j.jtho.2016.11.714
82. Triphuridet N, Vidhyarkorn S, Worakitsitisatorn A, et al. Screening values of carcinoembryonic antigen and cytokeratin 19 fragment for lung cancer in combination with low-dose computed tomography in high-risk populations: initial and 2-year screening outcomes. Lung Cancer. 2018;122:243–248. doi:10.1016/j.lungcan.2018.05.012
83. Saeteng S, Tantraworasin A, Euathrongchit J, Wannasopha Y, Lertprasertsuke N. Pilot study of low dose computed tomography screening in first relative of lung cancer patient (preliminary report).
84. Fujikawa A, Takiguchi Y, Mizuno S, et al. Lung cancer screening—comparison of computed tomography and X-ray. Lung Cancer. 2008;61(2):195–201. doi:10.1016/j.lungcan.2007.12.010
85. Yang W, Qian F, Teng J, et al. Community-based lung cancer screening with low-dose CT in China: results of the baseline screening. Lung Cancer. 2018;117:20–26. doi:10.1016/j.lungcan.2018.01.003
86. Kakinuma R, Kusumoto M, Asamura H, et al. Lung cancers detected using low-dose ct screening: results of an eight-year observational study. Journal of Thoracic Oncology. 2013;2:S684.
87. Fan L, Wang Y, Zhou Y, et al. Lung cancer screening with low-dose CT: baseline screening results in Shanghai. Acad Radiol. 2018. doi:10.1016/j.acra.2018.12.002
88. Nawa T, Chonan T, Morikawa S, et al. Five-year experience with lung cancer screening using low-dose computed tomography in the Hitachi area. JSCTS. 2008;15:63–69.
89. Kashiwabara. The present State of Helical CT screening of lung cancer, and future trends in Kyushu. Haigan. 2002;42(7):851–858. doi:10.2482/haigan.42.851
90. Luo X, Zheng S, Liu Q, et al. Should nonsmokers be excluded from early lung cancer screening with low-dose spiral computed tomography? Community-based practice in Shanghai. Transl Oncol. 2017;10(4):485–490. doi:10.1016/j.tranon.2017.02.002
91. Jazieh A, AlGhamdi M, AlGhanem S, et al. Saudi lung cancer prevention and screening guidelines. Annals of Thoracic Medicine. 2018;13(4):198–204. doi:10.4103/atm.ATM_147_18
92. Nie Y, Cai Z, Zhao S. Early lung cancer baseline screening: preliminary study with low-dose spiral CT. Chin J Radiol. 2002;36(3):230–234.
93. Kang H-R, Cho JY, Lee SH, et al. Role of low-dose computerized tomography in lung cancer screening among never-smokers. J Thorac Oncol. 2019;14(3):436–444. doi:10.1016/j.jtho.2018.11.002
94. Ju SM, Park HB, Kang H, et al. Prevalence of non‐calcified pulmonary nodules in screening chest computed tomography. Thoracic Cancer. 2013;4(4):405–409.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
