Back to Journals » Clinical Ophthalmology » Volume 13
Lamellar macular holes: monitoring and management strategies
Authors Coassin M, Mori T, Di Zazzo A, Sgrulletta R, Varacalli G, Bonini S
Received 11 April 2019
Accepted for publication 17 June 2019
Published 9 July 2019 Volume 2019:13 Pages 1173—1182
DOI https://doi.org/10.2147/OPTH.S180454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Marco Coassin, Tommaso Mori, Antonio Di Zazzo, Roberto Sgrulletta, Giuseppe Varacalli, Stefano Bonini
Department of Ophthalmology, University Campus Bio-Medico of Rome, Rome, Italy
Abstract: Lamellar macular holes are a vitreoretinal condition characterized by abnormalities in foveal contour with splitting of the neuroepithelium and often an intact photoreceptor layer. Recent developments in high-resolution imaging have increased our ability to study the details of the vitreoretinal interface and to distinguish between different forms of lamellar holes. A new classification is needed to help clinicians in the management of lamellar macular holes. Some clinicians prefer to observe these clinical entities, especially when visual acuity is maintained or alterations of the photoreceptor layer are present. Nevertheless, lamellar holes may sometimes progress, and visual acuity can deteriorate. On the other hand, surgical treatment may lead to positive anatomical and functional outcomes, but not without risks. This review provides a critical overview of the available data on lamellar macular holes, focusing on diagnosis and managing options.
Keywords: degenerative lamellar hole, tractional lamellar hole, lamellar hole-associated epiretinal proliferation, epiretinal membrane, vitrectomy, macular surgery
Introduction
The first description of a lamellar macular hole (LMH) was reported by Gass in 1975. He described an LMH as an oval reddish macular lesion on biomicroscopy in a patient affected by cystoid macular edema.1 Nowadays, with the aid of the high-resolution optical coherence tomography (OCT), many more morphological features of the LMH have been identified and described.2 A definition of LMH has been established by the International Vitreomacular Traction Study. LMH is morphologically described as a retinal condition characterized by a partial-thickness defect of the fovea, separation between outer and inner retinal layers, irregular foveal profile, and persistence of the foveal photoreceptor layer.3 Although this description allows easy differentiation of this condition from a full-thickness MH (FTMH), other parameters should be considered. LMHs might present different grades of photoreceptor disruption, with interruption of the ellipsoid layer. LMHs may also be accompanied by an epiretinal membrane (ERM), and in this case a potential role in LMH pathogenesis should be considered.4,5
The involvement of photoreceptors at the ellipsoid zone leads to worse visual acuity (VA) compared to those with an intact photoreceptor layer.5 This pattern can be assessed by OCT, and is a predictor of poor vision in patients with LMHs.6 Two subtypes of ERM have been identified according to OCT features: ERM with high reflectivity, and ERM with homogeneous medium reflectivity, named lamellar hole–associated epiretinal proliferation (LHEP).7 Although OCT helps in defining the features that characterize a lamellar hole, a deeper understanding of the pathophysiology of LMH is still missing.
Currently, the scientific community agrees upon the recognition of two subtypes of LMH: tractional and degenerative. Tractional LMH presents conventional ERM with an intact ellipsoid layer (Figure 1), while the degenerative form displays a schisis-like configuration, LHEP and often ellipsoid-layer disruption (Figure 2).7–9 Most patients affected by LMH remain stable over years. Few develop more severe visual loss with signs of progression on OCT and eventually a FTMH.10 Not only does the morphological heterogeneity of LMHs cause diagnostic difficulties, but the poor understanding of the causes and natural history of lamellar holes causes incomplete consensus in terms of managing and approaching this challenging retinal condition.
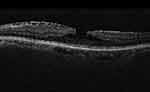 |
Figure 1 Optical coherence tomography of a tractional lamellar macular hole, characterized by epiretinal membrane with surface wrinkling, sharp intraretinal split, and “schisis-like” appearance. |
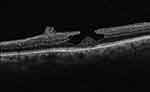 |
Figure 2 Degenerative lamellar macular hole identified by round-edged intraretinal cavitation, “foveal bump”, and epiretinal proliferation of medium reflectivity. |
Diagnostic armamentarium
OCT has become the gold standard in the diagnosis of LMH.11 OCT is a noninvasive, high-resolution imaging technique clinically available for evaluation of retinal morphology. It allows assessment of vitreoretinal interface disorders by visualizing details of the relationship of the posterior hyaloid to the retina.12 OCT represents a more accurate tool for the diagnosis of LMH than biomicroscopic examination. Different studies have displayed how only a limited percentage of LMHs (27%–36%) were correctly diagnosed on biomicroscopy.2,13
From its first description in 1991 by Huang et al, OCT has become an essential medical tool in ophthalmology to visualize the posterior pole.14 Time domain (TD) OCT uses a light source and a moving reference mirror, comparing the time delay of light waves scattered from the retina to generate the images.15 The success of TD-OCT has been doubled with the advent of spectral domain (SD) OCT, which offers the advantages of Fourier transformation. Compared to TD-OCT, SD-OCT permits increased speed of image acquisition and axial resolution, resulting in higher sensitivity to detect details of the vitreoretinal interface.16 Rao et al demonstrated the superiority of SD-OCT in detecting the presence of ERM preoperatively, with sensitivity of 79% versus only 21% for TD-OCT.17 The latest addition to OCT technology is angiography (OCT-A): this new technique built on the OCT platform provides high-resolution images of blood flow in the retina and choroid.18
Analysis and understanding of LMHs have benefited greatly from OCT technology and its ability to show details of the posterior pole. OCT is not only essential in the description and workup of LMHs but also plays a role in differentiating LMHs from FTMHs and other similar conditions, such as macular pseudoholes.2 SD-OCT can measure multiple different parameters in a standardized way, which becomes helpful in monitoring morphological changes in MHs. Among these parameters, several authors have focused on central foveal thickness (CFT) and diameter of the hole.2,11,19 In this contest, Haochine et al reported that LMHs present with a significantly thinner retina at the macular center when compared to pseudoholes. Also, both horizontal and vertical diameters of the macular aperture appear to be greater in LMHs than in pseudoholes.2
It is debated if CFT may be a correct parameter to detect signs of anatomic evolution of LMHs over time and/or deterioration of VA.10,19 In a report of 41 LMHs followed for a mean period of 37 months, the diameter of LMHs increased by 14%, and this enlargement was associated with the presence of ERM. During the same time frame, mean FT and mean best-corrected VA (BCVA) decreased by 10% and 6.4%, respectively. BCVA deteriorated in eleven eyes, and eight patients lost more than five ETDRS letters.19 In contrast, a study where 34 LMHs with ERM were followed for a mean period of 18 months reported no significant changes in BCVA or FT. Two eyes (5.8%) developed FTMHs after 6 and 15 months.10
With the improvements in OCT, a new stage of ultrahigh-resolution visualization of retinal anatomy has been reached. It is now possible to take two-dimensional cross-sectional images of the retina in vivo, which has shed light on the pathology of MHs.16 The definition of LMH has thus been revisited, with four basic criteria defined according to OCT images and based on the integrity of the external retinal layers.3
Assessing the presence of an ERM associated with an LMH has emerged as an important step in the clinical evaluation of LMHs. A case series of 125 eyes included all types of non-FTMH, and were analyzed with SD-OCT technology.5 All cases enrolled in the study showed a hyperreflective linear structure on the inner retina, suggesting that ERM may be a factor in the development of non-FTMHs. ERM has been reported in 89% of patients with LMHs, and it can be distinguished in two subtypes based on OCT: a thin, highly reflective line separated from the retinal nerve–fiber layer (RNFL), and an unusual aspect of a moderately reflective substance between the inner border of the ERM and the RNFL.13
Recently, authors described an advantage of SD-OCT technology: the en face scan. En face SD-OCT technology allows the clinician to view specific retinal layer length and width, including the ellipsoid layer. Those are important parameters for VA outcome in patients with MHs and ERM.6 En face OCT can also permit visualization of the presence of a contractive epicenter of ERM. Several studies have reconsidered the distribution of ERM and retinal folds, displaying how an intraretinal separation in the LMH can be due to a different pattern of tractional force on the fovea. Among these, different class of tangential traction associated with ERM have been defined: unidirectional, pluridirectional, and concentric patterns of traction.20,21
Another helpful technique in the diagnostic flow of MHs is fundus autofluorescence (FAF). FAF represents a fast approach in evaluating the retinal pigment epithelium by working on the intrinsic light emitted by retinal cells after stimulation by excitation energy.22 In normal conditions, autofluorescence of the retinal pigment epithelium beneath the fovea is low, since the overlying macular pigments (lutein and zeaxanthin) absorb the exciting light. When an LMH occurs, it creates a defect in retinal layers, and without the masking effect of the macular pigments, foveal autofluorescence is increased.22 Recently, it was suggested that this fluorescence gain seen in MHs may be due to the absence of the outer plexiform layer, where pigments are mostly concentrated.23 Data from other studies indicate that images acquired through FAF examination are a rapid way for differential diagnosis and follow-up of MHs, whereas OCT remains the gold standard. Additionally, some authors have suggested that FAF may be used to predict visual outcomes in patient candidates for surgery.11,24
Recently, an additional method has been proposed for evaluation of MHs: swept-source OCT-A. OCT-A technology uses light reflectance of moving red blood cells to represent vessels precisely on different retinal areas. Studies have reported how choriocapillaris vascular density is a relevant parameter in differential diagnosis between FTMH and LMH. They also suggested that choriocapillaris vascular density and choriocapillary flow were involved in MH development.25,26 Anyway, swept-source OCT-A may have different pitfalls in the evaluation of vitreoretinal disease, and other studies are warranted to establish the role of OCT-A technology in this field.18
Natural history
The clinical course and spontaneous evolution of LMHs have been extensively assessed. Studies on the natural history of LMHs suggest that this condition tends to remain stable over time or eventually to progress very slowly.5,8–10 Patients with lamellar macular defects may experience decreased VA with or without metamorphopsia and develop anatomical signs of progression on OCT.4,19 Different groups have focused their attention on morphological and clinical changes of lamellar holes in the long term, also in order to assess the factors that are associated with visual loss (Table 1).
 |
Table 1 Studies on natural history of lamellar macular holes |
Several authors have pointed out that LMHs may be associated with different subtypes of ERM and argued that ERM may play a role in the pathogenesis and prognosis of LMH.7–9,27,28 Although they used different terminology and definitions, most agreed on the fact that lamellar holes are virtually always accompanied by an ERM and that two families of epiretinal tissue can be detected. A conventional type of ERM features as a hyperreflective band above the RNFL on OCT and usually shows tractional forces. Another form of ERM has been revealed by OCT as a thick homogeneous layer of material with medium reflectivity on the epiretinal surface without tractional features. This second type has been named “atypical“ ERM or LHEP. Müller cells seem to be major players in the pathophysiology of LHEP.27 It has been observed that the two types of ERM are often associated with specific features of LMHs that possibly involve Müller cells. Two clinical entities may be described by SD-OCT: “tractional” and “degenerative” lamellar holes. The tractional type is a conventional ERM that can exert tangential forces on the retinal surface, inducing a “schisis-like” appearance, due to stretching of the retinal layers, while the ellipsoid layer is usually intact. Degenerative LMHs are characterized by the presence of LHEP and round-edged intraretinal cavitation with ellipsoid-zone disruption and sometimes signs of atrophy of retinal pigment epithelium.8 Features of both conventional ERM and LHEP may coexist in a portion of lamellar holes — 10%–46%.7–9,29,30
Data on 35 idiopathic LMHs followed for 24 months showed no significant changes in BCVA in one study.9 However, residual FT significantly decreased and maximal diameter of intraretinal splitting significantly increased, demonstrating (according to the authors) that LMHs are a rather slowly progressing clinical entity, as opposed to a stable condition.9 The evolution of LMHs with and without LHEP has also been assessed for a mean period of 25 months. The number of eyes that showed a decline of 0.3 logMAR VA was three of 62 in the LHEP group (5%) and three of 83 in the group of eyes without LHEP (4%). SD-OCT revealed that 18% of eyes with LHEP showed morphological progression compared with 13% of eyes without LHEP (P=0.49).7 Other authors followed both degenerative and tractional LMH for a mean period of 34 months. They found no significant changes in terms of VA or macular thickness in either group at the end of follow-up.8 Therefore, even if in this large study LMH proved to be a stable condition with no significant clinical signs of progression, a slow tendency toward anatomical progression was observed in both subgroups, since mean inner and outer diameters of holes increased significantly from baseline to the end of follow-up in about 50% of eyes.8
Based on the different OCT features and clinical evolution of LMHs, authors have suggested that more studies are necessary to explain the specific differences among LMH subtypes. In particular, it is not clear if tractional and degenerative MHs are two completely different clinical entities or two sequelae of the same pathology. Some lamellar holes followed with OCT for many years show a conventional ERM exerting traction on the retina that over time transformed to a homogeneous material with medium reflectivity (LHEP), bridging from the bottom of the hole to the inner retinal surface.8 A sort of repair process may be hypothesized in these cases.27 A precise classification of LMHs based on their OCT characteristics and clinical evolution would be helpful to standardize indications for surgical treatment.
Others articles have focused on CFT with OCT during follow-up of patients with LMHs. This parameter allows broad quantification of retinal defects in lamellar holes. Recently, it has been showed that CFT appears to be lower in LMHs with LHEP than in eyes with LMHs without LHEP.30 This seems to be related to greater involvement of the outer retinal layers by LHEP and may correlate with worse BCVA in patients with LHEP.7–10,29 Another study found no difference between eyes with and without LHEP for any given parameter, except for the presence of disruption in the ellipsoid zone.23
Damage to the ellipsoid zone has been reported as a common finding in patients affected by the degenerative form of LMHs, rather than eyes without LHEP.7,30 Oster et al showed a significant correlation between disruption of the ellipsoid zone and worse VA in patients affected by LMHs with LHEP.6 They proved that the combination of macular thickness and presence or absence of photoreceptors on the ellipsoid layer was the best predictor of poor VA in follow-up of 54 eyes with LMHs and LHEP. It should be mentioned that although it is a rare occurrence, some LMHs naturally evolve into FTMHs — 1%–4%.7,8,11,30
Surgical strategies
Among retina surgeons, no agreement has yet been reached on the management of patients with LMHs. It is debated whether these entities should be treated or just observed. Currently, the procedure proposed by most vitreoretinal surgeons is pars plana vitrectomy with ERM and internal limiting membrane (ILM) peeling. The goal is to reestablish the typical foveal profile, remove tractional forces, and allow resolution of the intraretinal edema.31 The utility of vitreous tamponade (air or gases) at the end of surgery is debated. Vitrectomy without tamponade seems to be as effective as using air or gas.32,33 Sato et al recently investigated the surgical outcomes of vitrectomy for LMHs with or without air tamponade (23 vs 18 eyes). BCVA improved in both groups after surgery, with no significant differences between the two groups, suggesting that air–fluid exchange is not required for LMH surgery.34
However, there is no full consensus on surgery. In particular, surgical recommendations seem to be more frequent in some regions (ie, Europe) than in others (ie, the US). Vitrectomy and ERM peeling is usually proposed when a patient complains of worsening of VA, OCT shows signs of anatomical progression, such as reduction in central macular thickness and/or enlargement of the LMH, and above all the presence of metamorphopsia.27 Promising results have been shown on postoperative metamorphopsia after ERM peeling, possibly because surgery removed the tractional forces distorting the photoreceptor layer.21 The value of retina surgery with ERM/ILM peeling seems to be especially relevant in tractional LMHs, where conventional ERM plays a principal role in LMH pathogenesis. Surgical treatment of degenerative lamellar holes remains controversial.28,35,36
Discordant results have been reported in the literature regarding visual gain after vitrectomy in patients with LMHs (Table 2). Numerous studies have shown improvements in mean VA in patients affected by LMHs.28,33,37–42 In 2008, Garretson et al operated on 27 eyes with significant visual loss from LMHs. Mean follow-up was 9 months. Vitrectomy with indocyanine green staining and ILM peeling was performed in all patients, and the majority also underwent air–fluid exchange with SF6 tamponade. VA improved in 25 eyes (93%), with a mean gain of three Snellen lines. One patient developed an FTMH and had VA of 20/400 after repair.39 Androudi et al operated on 20 LMHs associated with ERM performing a 25 G three-port vitrectomy with ILM peeling preceded by staining with trypan blue and followed by C3F8 tamponade. After 12 months, BCVA had improved in 17 (85%) eyes by a mean of 2.6 Snellen lines (P=0.002) and remained stable in three eyes.38 Casparis et al followed for a median 8 months 45 operated eyes with LMHs and ERM. Mean BCVA improved from 0.4 to 0.13 logMAR (P<0.0001), with 26 (58%) eyes gaining at least two ETDRS lines after surgery.37 Another retrospective study compared 19 eyes with LMHs that had undergone pars plana vitrectomy with ILM peeling and 21 eyes with LMHs that were just observed. Mean BCVA improved from 0.54 to 0.33 logMAR in the operated group and decreased from 0.51 to 0.55 logMAR in the control group. Two eyes developed FTMHs after surgery.40 Parolini et al performed vitrectomy with ERM/ILM peeling in 19 eyes. Mean BCVA improved from 0.4 to 0.2 logMAR (P=0.01). The improvement in VA was significant in patients with both LHEP and tractional ERM. Three LHEP patients developed FTMHs.27
 |
Table 2 Studies describing surgical outcomes of vitrectomy for lamellar macular holes |
Michalewska et al reported on 26 operated LMHs followed for 12 months.32 Mean VA went from 20/100 presurgery to 20/40 postsurgery, with 24 eyes improving by at least two Snellen lines. The size of the lamellar defect had no influence on final VA. Sun et al examined the surgical results of 30 patients with LMHs secondary to ERM that underwent ERM and ILM peeling.33 Mean BCVA improvement was 3.4 Snellen lines after a mean follow-up of 17 months. Lee et al presented data on 30 patients that underwent pars plana vitrectomy with ERM/ILM peeling for LMH.41 BCVA improved in 19 (63%), stayed the same in six (20%), and decreased in five (17%) eyes. Mean BCVA went from 20/63 to 20/50 after a mean period from surgery of 18 months (p=0.002). Visual benefit from surgery was only observed in patients with an intact ellipsoid layer, FT >100 µm, and initial BCVA better than 20/100. Lee et al reported on 31 eyes with LMH, showing BCVA improved by two Snellen lines in 18 eyes (58%) and decreased in two eyes (6.5%), leading to a mean gain of 0.18 logMAR after surgery (mean follow-up 39 months).42
Notably, less favorable results were disclosed in a series of 16 eyes with LMHs followed for a mean 32 months after surgery.13 Postoperative mean VA did not significantly increase after pars plana vitrectomy (20/158 to 20/118). Only 31% of patients had any improvement in VA, and 44% regained relatively normal retinal contour on OCT postoperatively. Two patients with foveoschisis on preoperative OCT developed a FTMHs postoperatively.13
Authors have recently focused on different visual outcomes in patients with and without LHEP. It has been reported that vitreous surgery cannot produce a significant improvement in VA in patients with degenerative LMH and LHEP.43 Ko et al compared surgical outcomes of LMHs with and without LHEP. BCVA significantly improved after surgery in patients without LHEP (58 eyes, P<0.001), but showed no change when LHEP was present (15 eyes, P=0.185).43 dell’Omo et al compared 84 LMHs with or without LHEP. A total of 26 patients underwent vitrectomy with ERM/ILM peeling. LHEP did not seem to influence the natural course of the disease or response to surgery. FTMHs developed in three eyes after surgery.30
Surgical treatment in LMH patients carries not-inconsiderable risks. Some vitreoretinal surgeons suggest caution before recommending vitrectomy. due to the not-uncommon risk of inducing an iatrogenic FTMH or disruption of the photoreceptor layer.35These complications of vitrectomy appear to be more frequent in patients with degenerative lamellar holes and LMHs with LHEP rather than without LHEP, possibly because of the connection of LHEP with the inner retina.44 Cataract is a quite common consequence of vitrectomy. Several surgeons have suggested performing cataract surgery at the same time as vitrectomy.36,43 Although clinical reports on outcomes of vitrectomy for LMHs often lack details on lens status, a large study on LMHs focused on the effects of different timing for cataract extraction on VA (phacoemulsification was performed before, concomitantly, or after retina surgery). This study reported on 106 patients affected by symptomatic lamellar holes of tractional, degenerative, or mixed nature. After vitreous surgery, patients were followed for a mean 36 months. Mean BCVA improved from 20/50 to 20/43 at 6 months and was 20/33 at the last follow-up (P<0.001). Subgroup analysis showed that VA improved in tractional-form (P<0.00001) and mixed-form groups (P=0.021), but not in the degenerative group (P=0.27).28
Conclusion
We have discussed whether LMHs are a stable clinical entity or if they should be resolved by surgery. Most studies on LMH natural history have demonstrated that LMHs are a slowly progressing disease with usually preserved photoreceptor layer and good VA. Therefore, most mild forms of intraretinal splitting may be monitored by clinical evaluation and OCT. The relative stability of LMHs should always be explained to patients seeking surgical advice by a retinal specialist. In fact, the fear of disease progression and further visual loss is often one of the main reasons that drive patients to consent to surgery. If the patient complains of moderate–severe visual impairment and/or metamorphopsia, crystalline lens status and outer retinal layers should be assessed before proposing vitreous surgery for LMHs. The role of cataract is often underestimated in these patients. Indeed, in some patients with LMH, vision after cataract surgery may improve to a point that they may not desire more surgery. Similarly, outcomes of vitreous surgery may be overestimated when cataract surgery is performed at the time of vitrectomy: vision may improve mainly because of cataract removal (and not ERM/ILM peeling). OCT is also key in evaluating outer retinal layers: if damage to the ellipsoid layer can be demonstrated before surgery, then then the patient should be made aware of potentially poor visual outcomes after vitrectomy.
On the other hand, it could be conservatively concluded that a select group of patients with symptomatic LMHs and significant epiretinal traction may benefit from surgery. Nevertheless, it should be mentioned to the patient that up to 20% of patients undergoing vitrectomy for LMHs experience significant visual loss,28 and after surgery, the occurrence of a full-thickness macular lesion is not uncommon in these patients.27,28,35,36,39,40 LMHs are an extremely interesting condition where intraretinal and epiretinal forces play a unique role. Clinical monitoring of lamellar holes by OCT over time may be laborious, but may save some patients from useless surgery or complications of vitrectomy. Surgery for LMHs may be challenging, since special care should be used to reduce the occurrence of postoperative FTMHs (ie, minimizing centripetal tractions to the fovea during ERM/ILM peeling). Prospective controlled studies are warranted to establish the correct management of these patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gass JD. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction: a clinicopathologic case report. Trans Am Ophthalmol Soc. 1976;73:231–250.
2. Haouchine B, Massin P, Tadayoni R, Erginay A, Gaudric A. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004;138(5):732–739. doi:10.1016/j.ajo.2004.06.088
3. Duker JS, Kaiser PK, Binder S, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi:10.1016/j.ophtha.2013.07.042
4. Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with Lamellar Macular holes. Retina. 2014;34(8):1513–1523. doi:10.1097/iae.0000000000000163
5. Michalewska Z, Michalewski J, Odrobina D, Nawrocki J. Non-full-thickness macular holes reassessed with spectral domain optical coherence tomography. Retina. 2012;32(5):922–929. doi:10.1097/IAE.0b013e318227a9ef
6. Oster SF, Freeman WR, Cheng L, Mojana F, Yuson RM, Brar M. Disruption of the photoreceptor inner segment/outer segment layer on spectral domain-optical coherence tomography is a predictor of poor visual acuity in patients with epiretinal membranes. Retina. 2010;30(5):713–718. doi:10.1097/iae.0b013e3181c596e3
7. Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015;35(4):720–726. doi:10.1097/IAE.0000000000000390
8. Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016;164:99–109. doi:10.1016/j.ajo.2016.02.008
9. Zampedri E, Romanelli F, Semeraro F, Parolini B, Frisina R. Spectral-domain optical coherence tomography findings in idiopathic lamellar macular hole. Graefe’s Arch Clin Exp Ophthalmol. 2017;255(4):699–707. doi:10.1007/s00417-016-3545-1
10. Bottoni F, Deiro AP, Giani A, Orini C, Cigada M, Staurenghi G. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(2):467–475. doi:10.1007/s00417-012-2044-2
11. Bottoni F, Carmassi L, Cigada M, Moschini S, Bergamini F. Diagnosis of macular pseudoholes and lamellar macular holes: is optical coherence tomography the “gold standard”? Br J Ophthalmol. 2008;92(5):635–639. doi:10.1136/bjo.2007.127597
12. Fujimoto J, Swanson E. The development, commercialization, and impact of optical coherence tomography. Investig Ophthalmol Vis Sci. 2016;57(9):OCT1–OCT13. doi:10.1167/iovs.16-19963
13. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113(3):388–397. doi:10.1016/j.ophtha.2005.10.047
14. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi:10.1002/jcp.24872.The
15. Adhi M, Duker JS. Optical coherence tomography – current and future applications. Curr Opin Ophthalmol. 2013;24(3):213–221. doi:10.1097/ICU.0b013e32835f8bf8.Optical
16. Keane PA, Bhatti RA, Brubaker JW, Liakopoulos S, Sadda S, Walsh AC. Comparison of clinically relevant findings from high-speed fourier-domain and conventional time-domain optical coherence tomography. Am J Ophthalmol. 2009;148:242–248. doi:10.1097/IEB.0b013e3181d5dbd0
17. Rao P, Yonekawa Y, Thomas BJ, Drenser KA. Spectral versus time-domain OCT in detecting preoperative epiretinal membranes that accompany macular holes. Eur J Ophthalmol. 2017;27(2):185–189. doi:10.5301/ejo.5000862
18. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi:10.1016/j.preteyeres.2017.11.003
19. Theodossiadis PG, Grigoropoulos VG, Emfietzoglou I, et al. Evolution of lamellar macular hole studied by optical coherence tomography. Graefe’s Arch Clin Exp Ophthalmol. 2008;247(1):13–20. doi:10.1007/s00417-008-0927-z
20. Acquistapace A, Cereda MG, Cigada M, Staurenghi G, Bottoni F. Imaging of tangential traction types in lamellar macular holes. Graefe’s Arch Clin Exp Ophthalmol. 2017;255(12):2331–2336. doi:10.1007/s00417-017-3806-7
21. Hirano M, Morizane Y, Kimura S, et al. Assessment of lamellar macular hole and macular pseudohole with a combination of en face and radial B-scan optical coherence tomography imaging. Am J Ophthalmol. 2018;188:29–40. doi:10.1016/j.ajo.2018.01.016
22. Spaide R. Autofluorescence from the outer retina and subretinal space: hypothesis and review. Retina. 2008;28:5–35. doi:10.1016/s0084-392x(09)79084-7
23. dell’Omo R, Virgili G, Bottoni F, et al. Lamellar macular holes in the eyes with pathological myopia. Graefe’s Arch Clin Exp Ophthalmol. 2018;256(7):1281–1290. doi:10.1007/s00417-018-3995-8
24. Teke MY, Cakar-Ozdal P, Sen E, Elgin U, Nalcacioglu-Yuksekkaya P, Ozturk F. Fundus autofluorescence imaging of patients with idiopathic macular hole. Int J Ophthalmol. 2013;6(5):685–689. doi:10.3980/j.issn.2222-3959.2013.05.26
25. Pierro L, Rabiolo A, Iuliano L, Gagliardi M, Panico D, Bandello F. Vascular density of retinal capillary plexuses in different subtypes of macular hole. Ophthalmic Surg Lasers Imaging Retina. 2017;48(8):648–654. doi:10.3928/23258160-20170802-07
26. Ahn J, Yoo G, Kim JT, Kim SW, Oh J. Choriocapillaris layer imaging with swept-source optical coherence tomography angiography in lamellar and full-thickness macular hole. Graefe’s Arch Clin Exp Ophthalmol. 2017;256(1):11–21. doi:10.1007/s00417-017-3814-7
27. Parolini B, Schumann RG, Cereda MG, Haritoglou C, Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Investig Ophthalmol Vis Sci. 2011;52(12):9074–9083. doi:10.1167/iovs.11-8227
28. Coassin M, Mastrofilippo V, Stewart JM, et al. Lamellar macular holes: surgical outcome of 106 patients with long-term follow-up. Graefe’s Arch Clin Exp Ophthalmol. 2018;256(7):1265–1273. doi:10.1007/s00417-018-3989-6
29. Compera D, Schumann RG, Cereda MG, et al. Progression of lamellar hole-associated epiretinal proliferation and retinal changes during longterm follow-up. Br J Ophthalmol. 2017:1–7. doi:10.1136/bjophthalmol-2016-310128.
30. dell’Omo R, Virgili G, Rizzo S, et al. Role of lamellar hole–associated epiretinal proliferation in lamellar macular holes. Am J Ophthalmol. 2017;175:16–29. doi:10.1016/j.ajo.2016.11.007
31. Hirakawa M, Uemura A, Nakano T, Sakamoto T. Pars plana vitrectomy with gas tamponade for lamellar macular holes. Am J Ophthalmol. 2005;140(6):1154–1156. doi:10.1016/j.ajo.2005.07.022
32. Michalewska Z, Michalewski J, Odrobina D, et al. Surgical treatment of lamellar macular holes. Graefe’s Arch Clin Exp Ophthalmol. 2010;248(10):1395–1400. doi:10.1007/s00417-010-1400-3
33. Sun JP, Chen SN, Chuang CC, et al. Surgical treatment of lamellar macular hole secondary to epiretinal membrane. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(12):2681–2688. doi:10.1007/s00417-013-2364-x
34. Sato T, Emi K, Bando H, Ikeda T. Retrospective comparisons of vitrectomy with and without air tamponade to repair lamellar macular hole. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):38–43. doi:10.3928/23258160-20150101-06
35. Figueroa MS, Noval S, Contreras I. Macular structure on optical coherence tomography after lamellar macular hole surgery and its correlation with visual outcome. Can J Ophthalmol. 2011;46(6):491–497. doi:10.1016/j.jcjo.2011.09.011
36. Choi WS, Merlau DJ, Chang S. Vitrectomy for macular disorders associated with lamellar macular hole epiretinal proliferation. Retina. 2017;8:1–6. doi:10.1097/IAE.0000000000001591
37. Casparis H, Bovey EH. Surgical treatment of lamellar macular hole associated with epimacular membrane. Retina. 2011;31(9):1783–1790. doi:10.1097/IAE.0b013e31820a6818
38. Androudi S, Stangos A, Brazitikos PD. Lamellar macular holes: tomographic features and surgical outcome. Am J Ophthalmol. 2009;148(3):420–426.e1. doi:10.1016/j.ajo.2009.04.009
39. Garretson BR, Pollack JS, Ruby AJ, Drenser KA, Williams GA, Sarrafizadeh R. Vitrectomy for a symptomatic lamellar macular hole. Ophthalmology. 2008;115(5):884–886.e1. doi:10.1016/j.ophtha.2007.06.029
40. Sanisoglu H, Elbay A, Sevim MS, Celik U, Aktaş BF, Durmus E. Surgical therapy versus observation for lamellar macular hole: a retrospective comparison study. Clin Ophthalmol. 2013;7:1843–1848. doi:10.2147/opth.s46283
41. Lee CS, Koh HJ, Lim HT, Lee KS, Lee SC. Prognostic factors in vitrectomy for lamellar macular hole assessed by spectral-domain optical coherence tomography. Acta Ophthalmol. 2012;90(8):1–6. doi:10.1111/j.1755-3768.2012.02456.x
42. Lee SJ, Jang SY, Moon D, Choi KS, Jung GY. Long-term surgical outcomes after vitrectomy for symptomatic lamellar macular holes. Retina. 2012;32(9):1743–1748. doi:10.1097/IAE.0b013e3182551c3c
43. Ko J, Kim GA, Lee SC, et al. Surgical outcomes of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Acta Ophthalmol. 2016;95(3):e221–e226. doi:10.1111/aos.13245
44. Lai TT, Chen SN, Yang CM. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: clinical and surgical findings. Graefe’s Arch Clin Exp Ophthalmol. 2015;254(4):629–638. doi:10.1007/s00417-015-3133-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
