Back to Journals » International Medical Case Reports Journal » Volume 11
Keratoconus treatment using femtosecond-assisted intrastromal corneal graft (FAISCG) surgery: a case series
Received 29 September 2017
Accepted for publication 2 November 2017
Published 23 January 2018 Volume 2018:11 Pages 9—15
DOI https://doi.org/10.2147/IMCRJ.S152884
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Dr Khosrow Jadidi.
Views: 253
Khosrow Jadidi,1 Seyed Aliasghar Mosavi2
1Department of Ophthalmology, Baqiyatallah University of Medical Sciences, Tehran, Iran; 2Vision Health Research Center, Tehran, Iran
Abstract: We describe an intrastromal corneal graft technique that uses femtosecond laser to create a desirable corneal lenticule with precise diameter, depth, and shape as well as an intrastromal pocket in keratoconus patients. The technique seems to be a feasible and safe treatment option to treat keratoconic eyes with reference to the irregularity and instability of cornea. The technique can be performed easily and appears safe and effective. At 7 days postoperatively, all eyes were white and quiet, and the grafts were clear. No graft folds or interface complications were observed at the 12-month follow-up using Visante optical coherence tomography.
Keywords: keratoconus, femtosecond assisted, intrastromal, corneal graft
Introduction
Keratoconus is a bilateral, progressive, noninflammatory disease of cornea which often leads to high myopia and astigmatism with an estimated prevalence of approximately 1 in 2000,1 although it may be much higher in other areas.2
Keratoconus seems to be a multifactorial disease with unknown exact etiology. However, biomechanical instability is thought to be one of the main causes.3
According to disease severity, many modalities exist for the treatment of keratoconus, including glasses, contact lenses, and intrastromal ring for mild-to-moderate disease.1,4,5 Several authors believe that adding volume to the peripheral cornea by implanting ring segments may reduce central corneal steepening in keratoconus.6,7 However, some complications were detected in previous studies.8 Additionally, a highly irregular shape of the cornea in advanced keratoconus still remains a main problem. Therefore, corneal graft as an option was preserved for more advanced disease.9
The presented technique was planned to fulfill the centralization easily, optimizing the optical properties, and to save the biomechanics of the cornea. So far as we know, this is the first report that demonstrates the effect of the positioning of a corneal intrastromal graft on the irregularity, instability, and refractive results of cornea using femtosecond laser.
Case reports
Surgical technique
The surgical procedures were performed at Basir Hospital, Tehran, Iran, and approved by the ethics committee. Four consecutive patients (men) were enrolled for the series. Written informed consent was obtained in all cases. The surgical femtosecond assisted intrastromal corneal graft (FAISCG) technique shown in the Video S1 is characterized by three-step procedure as follows:
Step 1: Creation of a corneal lenticule in donor eye
Preoperatively, epitheliums of donor eyes from the whole globe were removed with a blade number 15. Subsequently, a desirable corneal lenticule with precise diameter, depth, and shape was created using Ziemer LDV femtosecond laser (Ziemer AG, Port, Switzerland). To create the donor grafts, corneoscleral donor tissue was removed from storage solution (Optisol-GS; Bausch and Lomb Surgical, Irvine, CA, USA) and mounted on an artificial anterior chamber (LDV; Ziemer Ophthalmic Systems AG). The femtosecond laser keratoplasty software was programmed as follows: a donor lenticule thickness of 140–250 mm, applanation 7–9 mm, vacuum value 700 mbar, total cut in donor tissue 45 seconds, and an applanation time ranging from 10 to 20 seconds in the patients and donors. For donors and patients, we used 4.0 mm/s velocity of the stroma and 30 mm/s velocity of the border.
The proper depth of the cornea was defined by subtracting the normal corneal depth measure from the thinnest part of the recipient cornea. The shape of the lenticule was determined on the basis of keratoconus types; in central keratoconus, circular shape was chosen; in inferior keratoconus, crescent shape was chosen; and in asymmetric bow-tie keratoconus, mesopic pupil size and round shape were selected (Video S1) (Figure S1). In addition, the accurate diameter measurement was based on lenticule size.
Step 2: Creation of a corneal pocket in recipient eye
The intracorneal pocket entry in recipient eye was created on the steepest corneal topographic axis using femtosecond surgical laser. The pocket depth was set at 250 micron of the corneal thickness at the incision site. Then, the intracorneal pockets were gently separated from the underlying stroma using a Sinskey hook and a spatula.
Step 3: Graft implantation
After creation of the intrastromal pocket via the small incision while the pocket was easily opened using a dissecting spatula, an intrastromal corneal graft was implanted immediately into the corneal pocket. The graft implantation into the pocket is easily achievable. The implantation procedure and adjustment of the graft in the pocket can be performed by a Sinskey hook or forceps. The incision site is self-sealing and does not require suturing. The procedure takes approximately 30 minutes. Subsequently, a silicone-hydrogel bandage contact lens (Bausch and Lomb, Alcon Laboratories, Inc., Fort Worth, TX, USA) was placed on the cornea. Postoperatively, patients were given betamethasone drops (Sina Darou, Tehran, Iran) four times a day, chloramphenicol drops (Sina Darou) six times a day, and nonpreserved artificial tears (Artelac Advanced) (Baush and Lomb, Rochester, NY USA) six times daily. Chloramphenicol drops were discontinued 7 days postoperatively and betamethasone drops tapered off in 4 weeks. Bandage contact lenses were removed 1 day postoperation. Patients were scheduled for postoperative clinical examination on the 1st day, 1st week, and afterward in the 1st, 3rd, and 6th month, and yearly.
Clinical data
The characteristics of patients included in this case series are summarized in Table 1. No intraoperative and postoperative complications occurred. At 7 days postoperatively, all eyes were white and quiet, and the grafts were clear (Figures S2–S5).
  | Table 1 Patient characteristics and outcomes Abbreviations: Kmax, maximum K-value in diopters; Kmean, average K-value in diopters; Kmin, minimum K-value in diopters; UCVA, uncorrected visual acuity. |
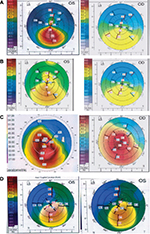
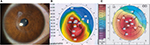
Figure S2A is a 7 days postoperative slit lamp photograph of the right eye of a 29-year-old man who was treated with a refraction of -4.00-3×20 (left image). The uncorrected visual acuity (UCVA) was 4/10. The preoperative topography is shown in Figure S2B (middle image). The maximum K-value was 42.1 diopters (D) and the central K-values were 41.5 D at the steepest and 40 D at the flattest meridian. Corneal thickness at the thinnest point was 377 µm.
The mesopic pupil size was 3.5 mm. Twelve months after implantation of the graft (7 mm diameter and 140 µm thickness) centered at the corneal reflex, UCVA was 7/10 with a refraction of -1.00 -2.00×90. The topography is shown in Figure S2C (right image).
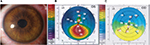
Figure S3A shows the left eye of a 28-year-old man 7 days after implantation of graft. While the preoperative refraction was -5×170, postoperatively it was -0.5-2×165. Twelve months postoperatively, the UCVA was 5 lines better than the UCVA before surgery. The changes in corneal topography (Figure S3B–C) demonstrate that cylinder of up to at least 3.0 D can be corrected. They also demonstrate a kind of ‘‘regularization’’ of the corneal surface.
Figure S4A shows a graft in situ in the right eye of a 23-year-old man 1 month after implantation. Cosmetically, the implant appears similar to a normal cornea. The preoperative refraction was -3-4×50. The uncorrected visual acuity was 5/100. One week postoperatively, the eye was emmetropic and the uncorrected visual acuity was 7 lines better than the uncorrected visual acuity before surgery. Figure S4(B–C) shows the corneal topography before surgery (middle), and 12 months after surgery (right). No graft folds or interface complications were observed in the postoperative course. The patient is very satisfied and has not reported daytime or night time problems.
Figure S5A shows the left eye of a 29-year-old man 7 days after implantation of graft. While the preoperative refraction was -4.5-2.5×117, postoperatively it was -3-2×119. Twelve months postoperatively, the uncorrected visual acuity was 2 lines better than the uncorrected visual acuity before surgery. Increased corneal thickness was observed with optical slit scanning topography in the area along with no graft folds or interface complications postoperatively (Figure S6). They also demonstrated a kind of ‘‘regularization’’ of the corneal surface postoperatively (Figure 1). Ocular Response Analyzer measurement was done for study cases (data not shown here) which showed that no significant changes were detected in corneal hysteresis and corneal resistance factor after graft implantation (Figure S7).
Discussion
A high grade of safety and efficacy was demonstrated by adding volume to the corneal periphery for myopia correction.6,7. However, a highly irregular shape of the cornea in advanced keratoconus remains a major problem.
The femtosecond laser technology that was commercially introduced in the year 2002 for creation of a corneal flap in laser in-situ keratomileusis allows a new type of intrastromal lamellar keratoplasty with removal of a mid-stromal segment for both donor and recipient corneas in animal studies.10 However, to the best of our knowledge, no report has been published regarding femtosecond-assisted intrastromal cornea graft in humans so far.
Our clinical data show that this technique enables correcting irregularity as well as instability and refractive errors. In this study, we made a corneal punch into the required shape and reimplanted the reshaped layer into the eye, similar to the Jose Barraquer11 technique. However, a femtosecond laser was used in our technique, which made it different from Barraquer’s procedure.
Moreover, the geometry of the corneal surface improved into a generally regular shape. Consequently, sources of higher-order aberrations were somehow eliminated, triggering correction of refractive errors. We believe that using normal corneal tissue may possibly terminate the side effects of corneal ring implantation, holding back the need for corneal grafting and improving the quality of life.
In summary, this technique is easy to perform and appears to be safe and effective, particularly for the treatment of irregularly shaped corneas such as in keratoconus. However, because of the progressive characteristics of keratoconus disease, it remains unclear whether this technique is able to control the progression of the disease. Therefore, we suggest cornea cross-linking as an approach to halt the progression of corneal ectatic disorders along with intrastromal corneal graft. Moreover, further investigations and case series analysis and long-term follow-up are required to confirm our finding.
Disclosure
The authors report no conflicts of interest in this work.
References
Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. | ||
Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;4:359–369. | ||
Daxer A, Fratzl P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci. 1997;38:121–129. | ||
Weed KH, MacEwen CJ, Giles T, Low J, Mc Ghee CN. The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye. 2008;22:534–541. | ||
Bilgin LK, Yilmaz S, Araz B, Yüksel SB, Sezen T. 30 years of contact lens prescribing for keratoconic patients in Turkey. Cont Lens Anterior Eye. 2006;32:16–21. | ||
Ertan A, Colin J. Intracorneal rings for keratoconus and keratectasia. J Cataract Refract Surg. 2007;33:1303–1314. | ||
Miranda D, Sartori M, Francesconi C, Allemann N, Ferrara P, Campos M. Ferrara intrastromal corneal ring segments for severe keratoconus. J Refract Surg. 2003;19:645–653. | ||
Jadidi K, Mosavi SA, Nejat F, Alishiri A. Complications of intrastromal corneal ring implantation (Keraring 355) using a femtosecond laser for channel creation. Int J Kerat Ectatic Corneal Dis. 2014;3(2):53. | ||
Funnell CL, Ball J, Noble BA. Comparative cohort study of the outcomes of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Eye (Lond). 2006;20:527–532. | ||
Jonas JB. Intrastromal lamellar femtosecond laser keratoplasty with superficial flap. Br J Ophthalmol. 2003;87(9):1195. | ||
Barraquer JI. The history and evolution of keratomileusis. Int Ophthalmol Clin. 1996;36(4):1–7. |
Supplementary material
  | Figure S2 (A) Slit-lamp photograph 7 days after graft implantation, (B) Corneal topography before graft implantation (middle), (C) 12 months postoperatively (right). |
  | Figure S3 (A) Slit-lamp photograph 7 days after graft implantation, (B) Corneal topography before graft implantation (middle), (C) 12 months postoperatively (right). |
  | Figure S5 (A) Slit-lamp photograph 7 days after graft implantation, (B) Corneal topography before graft implantation (middle), (C) 12 months postoperatively (right). |
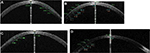  | Figure S6 Visante optical coherence tomography cut of study cases (A–D) through the cornea 12 months after graft implantation. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.