Back to Journals » Drug Design, Development and Therapy » Volume 13
Jatrorrhizine inhibits colorectal carcinoma proliferation and metastasis through Wnt/β-catenin signaling pathway and epithelial–mesenchymal transition
Authors Wang P, Gao XY, Yang SQ, Sun ZX, Dian LL, Qasim M , Phyo AT, Liang ZS, Sun YF
Received 1 March 2019
Accepted for publication 21 May 2019
Published 8 July 2019 Volume 2019:13 Pages 2235—2247
DOI https://doi.org/10.2147/DDDT.S207315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Pan Wang,1 Xiao-Yan Gao,1 Si-Qian Yang,1 Zhi-Xin Sun,2,3 Lu-Lu Dian,1 Muhammad Qasim,1,4 Aung Thu Phyo,1,5 Zong-Suo Liang,1 Yan-Fang Sun1
1College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, People’s Republic of China; 2College of Life Sciences, Wenzhou Medical University, Wenzhou 325035, People’s Republic of China; 3Department of Life Sciences, Zaozhuang No.1 Middle School, Zaozhuang, 277100, People’s Republic of China; 4Institute of Sustainable Halophyte Utilization, University of Karachi, Karachi 75270, Pakistan; 5Department of Biotechnology, Mandalay Technological University, Mandalay 05072, Myanmar
Purpose: Jatrorrhizine (JAT) is a natural protoberberine alkaloid, possesses detoxification, bactericidal and hypoglycemic activities. However, its anti-cancer mechanism is not clear. This study aimed to investigate the mechanism of JAT through which inhibits colorectal cancer in HCT-116 and HT-29 cells.
Methods: MTT assay and colony formation assay were used to check the cell proliferation ability. Cell apoptosis and cell cycle were measured by Hoechst 33342 staining and flow cytometry, respectively. Cell migration and invasion were detected by scratch wound healing assay and trans-well assay, respectively. Further, expression of related proteins was examined via Western blotting and the in vivo anti-cancer effect of JAT was confirmed by nude mice xenograft model.
Results: The research showed that JAT inhibited the proliferation of HCT-116 and HT-29 cells with IC50 values of 6.75±0.29 μM and 5.29±0.13 μM, respectively, for 72 hrs. It has also showed a time dependently, cell cycle arrested in S phase, promoted cell apoptosis and suppressed cell migration and invasion. In addition, JAT inhibited Wnt signaling pathway by reducing β-catenin and increasing GSK-3β expressions. Increased expression of E-cadherin, while decreased N-cadherin, indicating that JAT treatment suppressed the process of cell epithelial–mesenchymal transition (EMT). In HCT-116 nude mice xenograft model, JAT inhibited tumor growth and metastasis, and induced apoptosis of tumor cells.
Conclusion: This study demonstrated that JAT efficiently inhibited colorectal cancer cells growth and metastasis, which provides a new point for clinical treatment of colorectal cancer.
Keywords: jatrorrhizine (JAT), colorectal cancer (CRC), Wnt/β-catenin signaling, epithelial-mesenchymal transition (EMT), xenograft model
Introduction
Colorectal cancer (CRC) belongs to malignant gastrointestinal tumor and is the third leading cause of death among all cancer.1 According to the National Cancer Research Center, 101,420 new cases of CRC are expected in the United States in 2019, while 51,020 cases of cancer deaths.2 CRC still remains a major challenge due to high mortality and metastasis, despite some progress in systemic therapy.3,4 Metastasis and invasiveness are the main causes of high recurrence and mortality in cancer patients.5 In addition, epithelial–mesenchymal transition (EMT) is a phenotypic change unique to tumor metastasis, accompanied by a loss of epithelial features (such as a decrease in E-cadherin) and production of mesenchymal cells (such as an increase in N-cadherin).6 Wnt signaling pathway can promote EMT, in which GSK-3β and β-catenin are two key players.7 Deregulation of Wnt signaling pathway is closely related to tumor initiation, growth, and metastasis.8
Rhizoma coptidis (RC) is one of the most important traditional Chinese herbs and has been used for more than two thousand years. The main component of RC is alkaloid, including jatrorrhizine (JAT), berberine, coptisine, palmatine, epiberberine, and columbamine (Figure 1).9 Jatrorrhizine, a natural protoberberine alkaloid has been demonstrated to possess detoxification, bactericidal, hypoglycemic, and hypolipidemic effects.10–12 JAT has a similar parent structure to berberine.13 The previous study has testified that berberine inhibited the growth and migration of colon cancer cells by JAK2/STAT3 signaling pathway.14 However, the underlying mechanisms of JAT-induced suppression colorectal cancer have not been fully elucidated. Therefore, in this experiment, we explored the anti-proliferation and anti-metastasis mechanism of JAT on colorectal cancer cells (HCT-116 and HT-29).
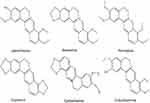 |
Figure 1 Chemical structure of jatrorrhizine, coptisine, berberine, palmatine, epiberberine, and columbamine. |
Materials and methods
Experimental materials
Jatrorrhizine (CAS: 3621-38-3) was purchased from National Institute for Food and Drug Control, China. For in vitro cell studies, JAT was dissolved in dimethyl sulfoxide (DMSO) to form 10 mM stock concentration and stored at 4°C in the dark. Then, it was further diluted in fresh medium for cell experiment. For in vivo assay, the stock was diluted in PBS.
Human colorectal carcinoma cell lines HCT-116 and HT-29 were obtained from Chinese Academy of Sciences, Shanghai Institutes for Cell Resource Center. The cell lines were cultured in RPMI 1,640 medium (Gibco, USA) containing 10% fetal bovine serum (Gibco, Qualified, Australia) and 1% penicillin-streptomycin (Gibco). The cells were cultured in an incubator with 5% CO2 and 95% humidity, the experiments were performed with cells in the logarithmic growth phase.
Cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.3 HCT-116 and HT-29 cells were collected and seeded at 1.0×104 cells/well in 96-well plates and treated with JAT for 24, 48, and 72 hrs. Then, 20 μL MTT (5.0 mg/mL) was added to each well and incubated for another 4 hrs at 37°C. The reaction was terminated by addition of 150 µL DMSO, and the absorbance at 570 nm was measured by a microplate reader (BioTek, USA) after shaking for about 10 mins. Fifty percent inhibition concentration (IC50) was calculated from growth-inhibitory curves of cells by SigmaPlot 12.5 software (Systat Software, Inc., Germany).
Cell proliferation was detected by clonal formation assay. Cells were collected and seeded in 6-well plates (3×105 cells/well) with JAT (0, 5, 10, 15 µM), and after 72 hrs, cells were collected and seeded in new 6-well plates (1,000 cells/well) and incubated for 2 weeks. Subsequently, cells were stained with 0.5% crystal violet for 15 mins, washed 3 times with PBS, and dried in a 37°C incubator. The clones of more than 50 cells were counted.
Detection of cell apoptosis
Hoechst 33342 fluorescence staining was used to analyze cell apoptosis by observing changes in nuclear morphology.15 Cells were seeded into 6-well plates and treated with selected concentrations of JAT for 72 hrs. Then, cells were cultured with Hoechst 33342 staining (20 μg/mL) for 30 mins at 37°C in the dark. The images of cell nuclei were visualized under fluorescence microscope (Olympus Co., Tokyo, Japan).
Mitochondrial membrane potential (△Ψm) was detected by flow cytometry. Metachromatic probe JC-1 was selectively used as the △Ψm sensitive dye, following a standard detection kit specification. Experiments were carried out in triplicate with at least 10,000 cells per test sample.
According to the manufacturer's instruction, cell apoptosis was monitored using Annexin V-FITC/PI detection kit (BD, Biosciences Pharmingen). HCT-116 and HT-29 cells were co-cultured with JAT for 72 hrs. Cells were collected and gently resuspended in AnnexinV-binding buffer. A total of 5 μL annexin V and PI were added to cells and incubated at room temperature for 15 mins in the dark. Finally, cell apoptosis was detected by flow cytometry (BD, FACSAria) and data were analyzed using BD AccuriTM C6 software (Accuri Cytometers, New Jersey, USA).
Cell cycle distribution
Cell cycle was detected by flow cytometry using Cell Cycle and Apoptosis Analysis Kit (Beyotime Biotechnology, China). HCT-116 and HT-29 cells in log-phase growth were seeded into 6-well plates. After JAT treatment, cells were harvested, centrifuged, and fixed with ice-cold 70% ethanol overnight. Cells were further stained with RNase A (50X) and propidium iodide (PI, 20X) for 30 mins at 37°C in the dark. The cell cycle phase distribution and hypodiploid DNA were detected by flow cytometry at 488 nm and analyzed with FlowJo software (FlowJo, Oregon, USA). The histogram was obtained from GraphPad software (GraphPad Software Inc., San Diego, CA, USA).
Scratch wound healing assay
The migration ability of colon cancer cells was evaluated by scratch wound healing assay. HCT-116 and HT-29 cells were collected and seeded into 6-well plates at a density of 1×106 cells/well. When the cells attained more than 90% confluence, a linear scratch was made at the center of each well using a 200 μL pipette tip to create an artificial wound in the cell monolayer. Each well was gently washed with PBS three times to remove the cell debris created from the scratch. Then, the cells were cultured in serum-free medium and treated with selected concentrations of JAT for 48 hrs. The images were captured by a microscope (Olympus Co., Tokyo, Japan) at 0, 24, and 48 hrs, respectively. The scratch area was calculated by measuring the gap enclosed by the cells using Image J software (National Institutes of Health, Bethesda, MD). The wound healing percentage was calculated according to the following formula:
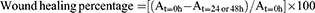
where, At=0 h, At=24 or 48 h represent the scratch area measured at 0, 24, and 48 hrs after scratching, respectively. The histogram was obtained from GraphPad software.
Trans-well assay
Trans-well assay was used to measure cell invasion. Trans-well chambers with 8 μM pore size (24-well inserts; Costar, Corning, NY, USA) were pre-coated with Matrigel (BD Biocoat, Corning, NY, USA), according to manufacturer’s instructions. HCT-116 and HT-29 cells were pre-treated with JAT (0, 5, 10 µM) for 24 hrs. Then, cells were collected and re-suspended with serum-free DMEM medium and seeded into the upper chambers at a density of 5×104 cells/well. A total of 500 μL DMEM medium containing 20% fetal bovine serum was added to the lower chamber. After incubating the cells for 24 hrs in a 37°C incubator, cells on the upper chamber surface were removed with a cotton swab and the invasive cells were fixed and stained with 0.5% crystal violet for 15 mins. Five fields on each chamber were randomly selected and the number of invaded cells was counted under a microscope (×100 magnification). The histogram was obtained from SigmaPlot 12.5 software.
Cell protein extraction and Western blotting
Total cellular protein was extracted by RIPA buffer containing 1 mM phenylmethanesulfonyl fluoride (Aidlab Biotechnologies Co., Ltd, Beijing, China). Protein quantification was performed according to the BCA Protein Quantitation Specification (Applygen Technologies Inc. Beijing, China), a standard curve was drawn, and protein loading volume was calculated. Equal amounts of protein (40 μg) were separated by 10–15% SDS-PAGE and transferred onto the PVDF membrane, where 5% skim milk was used to block the membrane. Membranes were then incubated for overnight with different antibodies including β-actin (catalog number: 3,700), β-catenin (catalog number: 8,480), GSK-3β (catalog number: 12,456), caspase-3 (catalog number: 9,665), caspase-8 (catalog number: 9,746), caspase-9 (catalog number: 9,508), PARP (catalog number: 9,532), E-cadherin (catalog number: 14,472), N-cadherin (catalog number: 13,116), and F-actin (catalog number: ab205) at 4°C. Subsequently, the membranes were washed with TBST and incubated with the corresponding HRP-linked secondary antibody at room temperature for 2 hrs, followed by visualization using the ECL system. Western blotting was quantified using Quantity One software (Bio-Rad). All the primary and secondary antibodies were purchased from Cell Signaling Technology (Boston, USA) and abcam (Cambridge, UK).
Nude mice xenograft modeling
BALB/c nude mice were used in the experiment, which was authorized by the Ethics Committee for the Shanghai Lab. Animal Research Center, China. HCT-116 at 2×106 cells/mouse subcutaneously inoculated into the right flanks of the athymic nude mice to build a human colorectal carcinoma xenograft model. On the seventh day after tumor cells implantation, the mice were randomly divided into 4 groups (8 mice/group) treated by daily intraperitoneal injection: negative control group (normal saline); positive control group (cis-Diammineplatinum (II) dichloride, PDD, 2.0 mg/kg); low dose group (JAT 2.5 mg/kg); and high dose group (JAT 5 mg/kg). Tumor growth and survival of each animal were recorded meanwhile. The body weight of mice was weighed every 5 days after tumor cells implantation. And the mice were sacrificed after 4 weeks of treatment, the tumors and lungs were removed, fixed, sectioned, and stained with hematoxylin and eosin (H&E). Apoptotic cells in tumor tissue were measured by In Situ Cell Death Detection Kit, Fluorescein (RocheMolecular Systems, Inc., US). Signally, the tumors were harvested for further immunohistochemical (IHC) analyses according to our lab protocol.
Statistical analysis
All values were presented as the mean±standard deviation (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA) using SigmaPlot 12.5 software. *P<0.05 was considered significant, **P<0.01 was considered very significant, and ***P<0.001 was considered much significant.
Results
Effect of JAT on the growth and proliferation of colon cancer cells
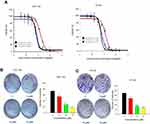
In order to explore the effect of JAT on the growth of CRC, we measured cell viability of HCT-116 and HT-29 by MTT assay. Cells were exposed to JAT concentrations range from 0 to 200 μM and monitored for 24, 48, and 72 hrs. As shown in Figure 2A, JAT efficiently inhibited the growth of HCT-116 and HT-29 cells in both time-and dose-dependent manner. IC50 of JAT on HCT-116 and HT-29 cells were 16.21±0.83 μM and 13.64±1.61 μM after 24 hrs, 8.01±0.64 μM and 6.27±0.17 μM after 48 hrs, 6.99±0.29 μM and 5.46±0.13 μM after 72 hrs, respectively. In comparison, IC50 values of JAT on HT-29 were lower than HCT-116 cells, which demonstrated a better inhibition of proliferation. MTT results also showed that JAT had the best inhibitory effect at 72 hrs.
The effects of JAT on the proliferation of colon cancer cells were examined by colony formation assay. With the increasing concentrations of JAT, the number of clones in the cell community and the colony formation rate were gradually decreased. This indicated that JAT can inhibit the proliferation of HCT-116 and HT-29 cells in a concentration-dependent manner (Figure 2B and C).
JAT induced apoptosis of colon cancer cells
The nuclear morphological changes of HCT-116 and HT-29 cells were examined through Hoechst 33342 staining. HCT-116 and HT-29 cells increased brightness, chromatin condensation and marginalization, and nuclear fragmentation, after treatment with different concentrations of JAT (0 μM, 5 μM, 10 μM, and 15 μM). These results stated a characteristic feature of apoptotic cells, suggesting JAT induced cell apoptosis in a concentration-dependent manner (Figure 3A).
In this study, JC-1 cationic dye was used to detect the effect of JAT on mitochondrial transmembrane potential (△Ψm). After JAT treatment for 72 hrs, flow cytometric analysis showed a marked shift from upper right quadrant to lower right quadrant (Figure 3B), indicating dissociation of JC-1 from dimer to monomer form. A total of 5 μM JAT exposures significantly increased the monomer/dimer ratio and up to 3-fold of 15 μM JAT treatment. These findings confirmed the disruptive effect of JAT on mitochondrial △Ψm in HCT-116 and HT-29 cells, suggesting mitochondrial dysfunction and early apoptosis.
Cell apoptosis was detected by flow cytometry with Annexin V-FITC/PI double staining. The results showed that the percentages of apoptotic HCT-116 cells treated with different concentrations of JAT (0 μM, 5 μM, 10 μM, and 15 μM) for 72 hrs were 4.96±0.22%, 26.64±3.20%, 29.30±5.00%, and 50.47±5.66%, respectively; while apoptosis of HT-29 cells was 4.51±0.71%, 70.18±24.78%, 84.48±13.91%, and 94.47±7.82%, respectively (Figure 3C). The results indicated that JAT promoted the apoptosis of HCT-116 and HT-29 cells in a dose-dependent manner.
To further explore the mechanism of JAT-induced apoptosis, we examined the expressions of apoptosis-related proteins by Western blotting. After treatment of JAT, the procaspase-9 was up-regulated in HCT-116 cells, but its expression was down-regulated in HT-29 cells. Procaspase-8 expression was slightly down-regulated in HT-29 cells, but there was no significant change in HCT-116 cells. Procaspase-3 is the opposite of procaspase-8. PARP was significantly decreased in both cells (Figure 3D). The above data suggested that JAT induced apoptosis through caspases family in HCT-116 and HT-29 cells.
JAT affected cell cycle of HCT-116 and HT-29 cells
To further investigate the cytotoxic mechanism of JAT on CRC, the cell cycle distribution of HCT-116 and HT-29 cells was monitored by flow cytometry after PI DNA staining. According to the experimental results, the proportions of HCT-116 cells in G0/G1 phase and G2/M phase decreased from 52.11±1.26% to 42.21±1.26% and 27.79±1.77% to 23.78±1.54%, respectively, when treated with JAT, while the number of cells in S phase increased from 20.10±0.52% to 34.01±0.67% (Figure 4A). For HT-29 cells, the proportion of cells in G0/G1 phase decreased from 57.33±1.02% to 48.24±0.83%, while the number of cells in S phase increased from 31.14±1.66% to 39.34±1.38% (Figure 4B). To sum up, JAT affected HCT-116 and HT-29 cells proliferation by blocking cell cycle in S phase.
JAT suppressed the migration and invasion of HCT-116 and HT-29 cells
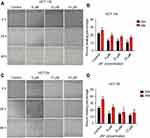
Cancer cell metastasis includes cell migration and invasion. Scratch wound healing assay was used to explore the effects of JAT on the migration of colon cancer cells. As shown in Figure 5A and B, the wound healing percentage of HCT-116 cells treated with different concentrations of JAT (0 μM, 5 μM, 10 μM, and 15 μM) were 22.16±1.39%, 12.81±0.94%, 12.44±0.93%, and 10.51±1.22% for 24 hrs, respectively; 26.20±3.26%, 19.21±2.11%, 17.73±1.57%, and 16.95±10.62% for 48 hrs, respectively. For HT-29 cells, when treated with JAT for 24 and 48 hrs, the wound healing percentage decreased from 22.52±2.54% to 11.72±0.93% and 35.41±3.86% to 15.84±3.31%, respectively (Figure 5C and D). The above experimental data indicated that JAT inhibited HCT-116 and HT-29 cells migration, and the inhibitory ability was related to time and drug concentration.
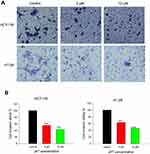
The effects of JAT on colon cancer cells invasion were assessed by trans-well assay. Results showed that the number of invasive cells in HCT-116 and HT-29 cells was reduced when treated with JAT. And the invasive potential of HCT-116 cells was inhibited by 44.36% and 57.16% when treated with JAT (5 and 10 μM) for 24 hrs, respectively; while it was inhibited by 36.58% and 53.96% in HT-29 cells, respectively (Figure 6A and B). The data implied that JAT could suppress the invasion of HCT-116 and HT-29 cells in a concentration-dependent manner.
JAT inhibited canonical Wnt/β-catenin signaling and EMT
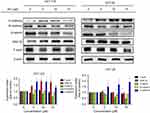
To further investigate the metastasis mechanism of JAT on CRC, Western blotting was used to detect the expression of key proteins in Wnt signaling pathway. Analysis of experimental results showed that the expression of β-catenin was down-regulated in HCT-116 and HT-29 cells in a concentration-dependent manner, after treatment with JAT for 72 hrs. The expression of GSK-3β was up-regulated in HCT-116 cells, while HT-29 cells were unchanged. Expression of epithelioid marker protein E-cadherin was increased significantly in HCT-116 and HT-29 cells, which is closely related to cell adhesion, while the expression of interstitial marker protein N-cadherin was distinctly decreased in HT-29 cells, and it was not altered greatly in HCT-116 cells. In addition, we also examined the expression of the cytoskeletal protein F-actin. After treatment with JAT, the expression of F-actin was decreased (Figure 7). This data suggested that JAT effectively inhibited the Wnt/β-Catenin signaling pathway and regulated the expressions of key proteins in EMT.
JAT suppressed growth and metastasis of tumor in nude mice xenograft model
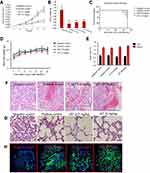
In order to study the in vivo anti-tumor efficacy of JAT, HCT-116 cells were injected into nude mice. After tumor cells implantation, tumor volume was measured every 5 days for 30 days and calculated as follows: tumor volume=(length × width2)/2. As shown in Figure 8A and B, JAT reduced tumor volume and weight compared with negative control group, and its depressor effect was similar to PDD. One mouse of negative control died at 29 d, and the survival rate of negative control group was 62.5% at 35 d. What’s more, the survival rates of positive control group, low dose group, and high dose group were 75%, 87.5%, and 100% (Figure 8C). This indicated that JAT (5 mg/kg) could prolong the survival of HCT-116 mice compared with the negative control group. There was no significant difference in body weight and organ index of liver and kidney between each group of mice (Figure 8D and E), indicating that JAT showed no toxicity to mice and no effect on liver and kidney functions of mice.
 |
|
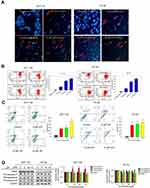
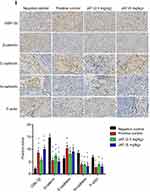
HE staining manifested that cells necrosis and nuclear pyknosis occurred in positive control and JAT groups, while the nuclei were intensely stained in negative control group (Figure 8F). Significant lung metastasis was observed in negative control group, while not in positive control group and JAT-treated groups (Figure 8G). Subsequently, we monitored the apoptosis of different tumor tissues by TUNEL method. The results showed that both PDD and JAT could induce distinct apoptosis in tumor tissues compared with negative control group (Figure 8H). Immunohistochemical analysis showed that the positive expression of GSK-3β and E-cadherin was increased successively in negative control, positive control, low dose, and high dose groups, while β-catenin, N-cadherin, and F-actin were decreased in tumor tissue (Figure 8I). The in vivo results indicated that JAT suppressed tumor growth and lung metastasis, and promoted tumor cell apoptosis.
Discussion
Jatrorrhizine has been reported to counteract the death of PC12 cell lines caused by H2O2 and has an antioxidant effect.16 Studies have found that JAT had a lower cytotoxicity than berberine and exerted anti-hypercholesterolemia effect by up-regulating the mRNA and protein of LDLR and CYP7A1.13 Now the research on JAT is mostly focused on its pharmacological activities; however, its anti-tumor activity is rarely reported. In this study, we explored the effects of JAT on the growth, apoptosis, and metastasis of colorectal carcinoma cells. Our results indicated that JAT could effectively inhibit the proliferation of HCT-116 and HT-29 cells in both time- and concentration-dependent manner. Furthermore, JAT induced cell apoptosis, arrested cell cycle in S phase of HCT-116 and HT-29 cells, and inhibited cell migration and invasion. These results were subsequently confirmed by in vivo experiment in which JAT suppressed tumor growth and metastasis, and promoted tumor cell apoptosis in nude mice xenograft model.
Wnt signaling pathway plays an important role in regulating cell cycle and controlling the proliferation of normal and malignant epithelial cells, and has become an important new target for cancers drug development.17,18 Wnt signaling pathway is divided into canonical and non-canonical pathways. Anxin-GSK-3β-β-catenin protein complex is degraded after activation of Wnt signaling pathway, released β-catenin, which is a key cytoplasmic and nuclear mediator of the canonical pathway and can cause cell proliferation and differentiation.17–19 In this study, the expression of β-catenin in HCT-116 and HT-29 cells was significantly decreased with dosage dependence, while GSK-3β was up-regulated in HCT-116 cells and unchanged in HT-29 cells, when treated with JAT for 72 hrs. Wnt signaling could regulate the expression level of intermediate mesenchymal-specific marker N-cadherin during the EMT process, which plays an important role in cancer metastasis.20 Western blotting results indicated that JAT could inhibit colon cancer cells metastasis by up-regulating the expression of E-cadherin. Furthermore, N-cadherin was decreased in HT-29 cells after treated with JAT, suggesting that JAT can reverse EMT in HT-29 cells.
Conclusion
In a word, the results revealed that JAT suppressed the proliferation and metastasis of colorectal carcinoma cells via blocking Wnt signaling pathway and EMT. This offers a new possible therapeutic operation in the future of JAT which is able to combat colorectal carcinoma.
Acknowledgments
This work was supported by grants as follows: National Natural Science Foundation of China (No.81602648), Natural Science Foundation of Zhejiang Province, China (No. LQ16H280004) and the Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences (201718).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Piyanuch R, Sukhthankar M, Wandee G, Baek SJ. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007;258(2):230–240. doi:10.1016/j.canlet.2007.09.007
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
3. Han B, Jiang P, Li Z, et al. Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Akt and mitochondrial-associated apoptotic pathway. Phytomedicine. 2018;48:152–160. doi:10.1016/j.phymed.2017.12.027
4. Ambrož M, Šmatová M, Šadibolov M, et al. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019;69:121–128. doi:10.2478/acph-2019-0003
5. Peng L, Yuan XQ, Zhang CY, et al. The emergence of long non-coding RNAs in hepatocellular carcinoma: an update. J Cancer. 2018;9(14):2549–2558. doi:10.7150/jca.24560
6. Lee JH, Cho HS, Lee JJ, et al. Plasma glutamate carboxypeptidase is a negative regulator in liver cancer metastasis. Oncotarget. 2016;7(48):79774–79786. doi:10.18632/oncotarget.12967
7. Zhang J, Tian XJ, Xing J. Signal transduction pathways of EMT induced by TGF-beta, SHH, and WNT and their crosstalks. J Clin Med. 2016;5(4). doi:10.3390/jcm5040041
8. Guo YH, Wang LQ, Li B, et al. Wnt/β-catenin pathway transactivates microRNA-150 that promotes EMT of colorectal cancer cells by suppressing CREB signaling. Oncotarget. 2016;7(27):42513–42526. doi:10.18632/oncotarget.9893
9. Chai FN, Ma WY, Zhang J, et al. Coptisine from Rhizoma coptidis exerts an anti-cancer effect on hepatocellular carcinoma by up-regulating miR-122. Biomed Pharmacother. 2018;103:1002–1011. doi:10.1016/j.biopha.2018.04.052
10. Choi SU, Baek NI, Kim SH, et al. Cytotoxic isoquinoline alkaloids from the aerial parts of corydalis incisa. Arch Pharm Res. 2007;30(2):151–154.
11. Ito C, Itoigawa M, Itoigawa M, Kuchide M, Nishino H, Furukawa H. Chemopreventive activity of isoquinoline alkaloids from corydalis plants. Planta Med. 2001;67(5):473–475. doi:10.1055/s-2001-15815
12. Zhu SL, Lei T, Gao X, Tu J. Jatrorrhizine regulates GLUTs with multiple manners for hypoglycemic effect in insulin-resistance 3T3-L1 adipocytes. Zhongguo Zhong Yao Za Zhi. 2018;43(6):1215–1220. doi:10.19540/j.cnki.cjcmm.20180104.005
13. Wu H, He K, Wang Y, et al. The antihypercholesterolemic effect of jatrorrhizine isolated from Rhizoma coptidis. Phytomedicine. 2014;21(11):1373–1381. doi:10.1016/j.phymed.2014.05.002
14. Liu X, Ji Q, Ye N, et al. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One. 2015;10(5):e0123478. doi:10.1371/journal.pone.0123478
15. Sun Y, Wu P, Sun Y, et al. Lycorine possesses notable anticancer potentials in on-small cell lung carcinoma cells via blocking Wnt/beta-catenin signaling and epithelial-mesenchymal transition (EMT). Biochem Biophys Res Commun. 2018;495(1):911–921. doi:10.1016/j.bbrc.2017.11.032
16. Luo T, Zhang H, Zhang WW, et al. Neuroprotective effect of Jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neurosci Lett. 2011;498(3):227–231. doi:10.1016/j.neulet.2011.05.017
17. Tai D, Wells K, Arcaroli J, et al. Targeting the WNT signaling pathway in cancer therapeutics. Oncologist. 2015;20(10):1189–1198. doi:10.1634/theoncologist.2015-0057
18. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
19. Tian D, Shi Y, Chen D, Liu Q, Fan F. The Wnt inhibitor LGK-974 enhances radiosensitivity of HepG2 cells by modulating Nrf2 signaling. Int J Oncol. 2017;51(2):545–554. doi:10.3892/ijo.2017.4042
20. Yang S, Liu Y, Li MY, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/beta-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124–136. doi:10.1186/s12943-017-0700-1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.