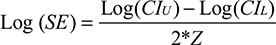Back to Journals » Clinical Epidemiology » Volume 10
Is dementia incidence declining in high-income countries? A systematic review and meta-analysis
Authors Roehr S , Pabst A, Luck T, Riedel-Heller SG
Received 27 January 2018
Accepted for publication 4 May 2018
Published 18 September 2018 Volume 2018:10 Pages 1233—1247
DOI https://doi.org/10.2147/CLEP.S163649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Susanne Roehr,1,2,* Alexander Pabst,1,* Tobias Luck,3 Steffi G Riedel-Heller1
1Institute of Social Medicine, Occupational Health and Public Health (ISAP), Medical Faculty, University of Leipzig, Leipzig, Germany; 2LIFE – Leipzig Research Center for Civilization Diseases, University of Leipzig, Leipzig, Germany; 3Department of Economic and Social Sciences, Institute of Social Medicine, Rehabilitation Sciences and Healthcare Research (ISRV), University of Applied Sciences Nordhausen, Nordhausen, Germany
*These authors contributed equally to this work
Purpose: To perform a systematic review and quantitative synthesis of studies on recent trends in dementia incidence in high-income countries (HIC), considering study quality.
Methods: PubMed and Web of Science were searched for eligible studies, that is, population-based/community-based prospective cohort studies investigating dementia incidence with similar methods over time, published after 1990. Study selection, data extraction, and quality assessment were performed independently by two investigators. Random-effect meta-analysis and meta-regression were used to estimate incidence change (IC) and to explore associations with study attributes. PRISMA standards were followed.
Results: The systematic review included seven studies (42,485 individuals), and the meta-analysis included five studies of sufficient quality. Relating dementia incidence of later cohorts to earlier cohorts (reference) yielded a nonsignificant decrease across HIC (IC =0.82; 95% CI 0.51–1.33), with high heterogeneity (I²=94.9%, P<0.001) and without publication bias (Egger’s t=–1.77; P=0.18). Excluding the Japanese Hisayama study, the only study suggesting an increase, indicated borderline evidence for a decrease across Western HIC (IC =0.69; 95% CI 0.47–1.00; I²=88.1%, P<0.001; Egger’s t=–0.34, P=0.77), again with high heterogeneity. Meta-regression did not reveal an association of incidence rate with calendar year or study attributes; however, analyses were low powered.
Conclusion: There is evidence of favorable trends in dementia incidence in Western HIC (stabilizing/decreasing). Reverse trends may occur in HIC of other regions, as exemplified by Japan. However, study number was small and heterogeneity was high. Further cohort studies using consistent methods are needed to draw definite conclusions. Our work may inform such future studies.
Keywords: dementia, Alzheimer’s disease, cohort study, incidence, trends, epidemiology
Plain language summary
Population aging around the world is driving an increase in numbers of people living with dementia. Already, dementia is among the leading causes of disability and dependency in old age, and effective interventions are currently not available. Therefore, it seems particularly encouraging that recent studies suggested a potential first-time decline in dementia incidence, that is, a smaller number of new cases of dementia in later periods of time, in high-income countries (HIC). The wealth advantage of HIC may have facilitated beneficial environments, for example, good access to education, nutrition, and health care, allowing for better population health, and hence, leading to fewer cases of dementia. Our aim was to systematically search for recent studies on trends in dementia incidence from HIC and to statistically synthesize data from identified studies of good methodological quality. We were able to identify seven studies, and five were included in the meta-analysis. Our results suggest favorable trends in dementia incidence in Western HIC (stabilizing/decreasing); however, the decline does not reach statistical significance. Moreover, there may be reverse trends in other HIC, as exemplified by an observed increase in dementia incidence in Japan. Overall, there may be differential trends in dementia incidence in HIC, reflecting varying life conditions and life experiences beyond beneficial environments in such wealthy societies. Ultimately, further well-conducted studies on trends in dementia incidence are needed to add to this growing research field in order to come to more robust conclusions. Our work may inform future studies.
Introduction
Despite the global increase in numbers of people living with dementia due to population aging, a growing body of literature suggests a potential decline in dementia occurrence for the first time, specifically in dementia incidence in high-income countries (HIC).1–5
A potential decline in dementia incidence in HIC may likely be the result of the decades-long wealth advantage that may have facilitated beneficial environments allowing for better population health.6 However, it is unclear which particular factors could contribute to a potential decline, but rising levels of education and more successful management of cardiovascular diseases might likely be driving factors in such trends.7–9
We aimed to contribute to the growing knowledge on the topic by providing a quantitative synthesis of the literature on recent trends in the incidence of dementia in HIC, considering study quality, which, to our knowledge, has not been done previously. We chose to focus on trends in dementia incidence in HIC for two reasons. First, if previous studies or reviews reported on a decline in dementia occurrence, they seemed to primarily point to dementia incidence in HIC. Second, incidence may be the most sensitive indicator of change driven by, if in decline, primary prevention or compression of morbidity as a result of the improvement of modifiable risk factors for dementia.10
Materials and methods
Registration, protocol, and guidelines
This work was registered with PROSPERPO (registration number CRD42016043232).11 A study protocol is available.12 Our study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).13
Search strategy and study selection
Two reviewers (SR, clinical investigator; AP, clinical investigator/methodologist) independently searched the electronic databases MEDLINE and Web of Science on June 6, 2017. A prespecified search syntax (see Supplementary materials) comprised the terms dementia, Alzheimer’s disease, time, trend, secular, change, incidence, epidemiology, and cohort. We further performed a gray literature search in Google and Google Scholar, considering conference abstracts and unpublished studies. Additionally, a reference list check of initially identified full-texts was performed. The search was restricted to articles published in English and German. HIC categorization followed the World Bank classification.14 To report on recent trends, we considered articles published after 1990. Studies were included if they 1) were population-based or community-based prospective cohort studies collecting primary data on dementia incidence; 2) investigated change in dementia incidence over time, that is, comparing incidence rate (IR) estimates between cohorts from a minimum of two different time intervals, or using a longitudinal approach measuring incidence change (IC) over time within one cohort; 3) applied similar methods throughout the study; and 4) investigated individuals at least 60 years of age. We first screened titles and abstracts of all database returns. Then, we checked study eligibility against the preestablished criteria through full-text analysis. Discrepancies during study selection were resolved by discussion, which, if necessary, included a third researcher (SRH, TL).
Data extraction and data items
Data from each included study were extracted and collected independently by two investigators (SR and AP) based on a standardized data abstraction form. Whether data abstraction was reliable was tested on a random sample. Cases of disagreement were resolved by discussion, including the opinion of a third researcher (SRH and TL). Extracted data comprised information on study, participant, and outcome characteristics as well as methodology. Specifically, we have extracted the following variables: 1) study characteristics: study, publication, year of publication, country, study design, setting, sample size of cohorts, response rate(s), study begin, study end, person-years, follow-up intervals, time between cohorts; 2) participants’ characteristics: minimum age, mean age, sex (% female); 3) outcome characteristics: IR (including 95% CI), IR calculation, IC (including 95% CI), measure of incidence, adjustment; and 4) methodological aspects: diagnostic approach, diagnostic criteria, comparability of diagnostic approach. Additionally, we searched supplements and study protocols of the articles, or contacted study authors, if necessary.
Study quality
Methodological risk of bias of included studies was assessed independently by SR and AP using the framework proposed by Hoy et al.15 The tool consisted of ten items addressing external and internal validity. Each item was judged “high” or “low” risk of bias, in rare cases “unclear”. The summary item was based on the Grades of Recommendation, Assessment, Development and Evaluation and Cochrane approaches.16 Adaptations were made to the tool to apply to incidence studies (see Supplementary materials).
Statistical analysis
We applied two effect size measures. First, we used the estimates of ratios of IR reported in the studies, relating the IR of the follow-up cohort to the IR of the reference cohort (ie, IC). Hazard ratios and incidence rate ratios were used as equivalent risk measures.17 Second, to evaluate change in dementia incidence over calendar year and to account for the magnitude of dementia risk, we used the individual study estimates of IR, denoted as new cases of dementia per 1,000 person-years. Where available, we used age- and sex-adjusted (ie, standardized) IR estimates in order to adjust for the differences in the population structure of a given study. Where only crude IR was reported, information on age- and sex-adjusted IR was requested from study authors.
Differences in summary measures as well as in clinical assessments, study design, methodological and statistical approaches were inspected. If necessary, summary measures of IR were transformed to obtain comparable estimates.
For the pooled analysis, the natural logarithm of the effect size measures was calculated; the corresponding standard error was obtained using the formula
|
with CIU and CIL denoting the upper and lower bounds of the 95% CI around the effect size, and Z ≈ 1.960 assuming a two-sided significance level of P<0.05. To investigate consistency of effect across studies, pooled effect size measures with 95% CI were estimated using DerSimonian–Laird random-effect models to account for heterogeneity.18 The pooled measures were back-transformed and are presented on the ratio scale. Heterogeneity was inspected using forest plots and I2 statistics. The influence of individual studies on the overall meta-analysis summary estimates was evaluated by omitting each study in turn and reestimating the pooled effect size measure. Funnel plots and Egger’s regression-based test were used to explore publication bias.19 In case publication bias was detected, bias-corrected estimates of the pooled effect sizes were calculated using the nonparametric trim-and-fill method by Duval and Tweedie.20
Since measurable differences in the study characteristics are not sufficiently reflected in the ordinary weighted pooled effect, we additionally conducted random-effects meta-regression using aggregate-level data from the individual studies to examine whether statistical heterogeneity was associated with study characteristics. Different meta-regression models were tested. The first model tested design parameters, including response rates, diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders [DSM]-IV/DSM-5 vs DSM III/III-R), and an indicator of whether incidence measures were obtained from clinical or algorithmic diagnosis. The second model included the calendar year when the respective observation period started along with the length of the observation period. The third model added mean age and sex (percentage of females), assuming a uniform trend in dementia incidence across HIC. Last, we included an indicator of the country where the study was conducted to account for possible country variation. The continuous measures in the meta-regression models were centered to minimize multicollinearity. Where mean age was not reported, it was extrapolated from reports of the number of cases by age group. P-values and CI in meta-regression were computed using the Knapp–Hartung method.21 Estimates of the meta-regression models were exponentiated to indicate the percent change in the pooled estimate given a one-unit increase in the covariate.
As sensitivity analysis, we 1) repeated the meta-analyses using data on alternate dementia diagnoses (where available) to test the robustness of the primary analysis, and 2) performed the same analyses including data of all selected studies, regardless of the risk of bias assessment. All analyses were performed using RevMan 5.3 (Cochrane Collaboration) and Stata 13.1 SE (StataCorp LP, Collage Station, TX, USA).
Results
Study identification and study characteristics
The initial search returned 11,297 records. After screening titles and abstracts, 31 articles with the potential for inclusion were identified. Seven studies were found eligible after full-text analysis (Figure 1). References of excluded studies are given in the Supplementary materials. Table 1 gives an overview of study characteristics. Overall, the seven studies comprised a total of 42,485 individuals aged ≥60 years. Three studies were conducted in the USA (Chicago Health and Aging Project/CHAP;22 Framingham Heart Study/FHS;23 Indianapolis-Ibadan Dementia Project/IIDP24), three in Western Europe (France: PersonnesAgées Quid/PAQUID&Three-City/3C;25 Netherlands: Rotterdam Study;26 UK: Cognitive Function and Ageing Study/CFAS I&II9), and one in Japan (Hisayama Study27).
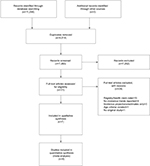  | Figure 1 Flowchart of study selection. |
Outcome characteristics
The main outcome considered was change in dementia incidence over time. Six out of seven studies reported incidence trends on all-cause dementia, other than CHAP,24 which investigated dementia due to Alzheimer’s disease (AD) only. All studies applied conventional criteria for dementia diagnosis across individual cohorts; primarily as of the DSM, though different versions were used (DSM-III-R,23,24,26,27 DSM-IV,9 and DSM-51,25). The IIDP study additionally applied the International Classification of Diseases, version 10 (ICD-10).24
Dementia subtypes, which were investigated exclusively in the CHAP study22 and in addition to all-cause dementia in two other studies,9,27 were classified according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for AD9,22,27,28 and the Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia (VaD).9,27,29
Approaches to establish dementia diagnosis varied between studies; however, the application of a multistage procedure was common. Instrument selection varied largely between studies with the Mini-Mental State Examination (MMSE) being the only tool that was administered in the majority of studies.9,22,25–27 Clinical evaluation and examination usually followed initial cognitive assessment, except in the CFAS I and II23 which relied on an algorithmic diagnosis using the Geriatric Mental State - Automated Geriatric Examination for Computer Assisted Taxonomy (GMS-AGECAT) and in the PAQUID&3C study25 which in addition to a clinical diagnosis used an algorithmic diagnosis based on MMSE scores and function in instrumental activities of daily living. Assessment and other relevant outcome characteristics are further detailed in Table 2.
Study quality
Item-based risk of bias assessment of domains of external and internal validity is depicted in Figure 2. Risk of bias assessment and justification for judgment are further detailed in the Supplementary materials. Overall, risk of bias was considered low in two studies,23,27 moderate in four,22,24–26 and high in one.9
Results of individual studies
Main outcomes
The majority of the studies, 5 out of 7, reported a decrease in dementia incidence over time. One study reported no change in dementia incidence, however, investigated AD only.22 A significant increase in overall dementia incidence was suggested by the Japanese Hisayama study.27 An overview of individual study results is given in Table 2.
Secondary results
Except for one study,22 various subgroup analyses were conducted. The Rotterdam study reported lower IR over time for all age strata (60–69, 70–79, 80–89) in both sexes, except for oldest men (80–89) for whom IR remained unchanged.26 In the IIDP, IR was also lower across all age groups (70–74, 75–79, 80–84, ≥85), but remained stable in the oldest age group for both sexes.24 Incidence dementia rate remained unchanged in the age strata 80–84 in women in the CFAS, but was lower across all other age groups (65–69, 70–74, 75–79, ≥85) for both sexes.9 The Hisayama study reported increasing IR for 65–84 year olds, but unchanged IR for those >85 years of age, applying to both sexes.27 Furthermore, the FHS suggested a 5-year delay in mean age of dementia onset from 80 years to 85 years over a time period of three decades.23
With regard to sex, overall trends in dementia incidence were predominantly seen in both sexes. However, the PAQUID&3C study reported a decreasing trend for women only.25 By contrast, the CFAS observed a decline in dementia incidence in men, particularly.23
In addition to all-cause dementia, three studies reported on trends in subtypes. The IIDP noted a decline not only in all-cause dementia, but also in AD dementia.24 A nonsignificant decrease was observed for AD dementia and a significant as well as more rapid decline for VaD in the FHS.23 The Hisayama study found that the overall increase was mainly attributable to AD cases, whereas risk for VaD did not change.27
Regarding education, the FHS reported dementia risk reduction particularly among individuals with at least a high school diploma.9 Likewise, the PAQUID&3C study found that higher educational levels in the later cohort accounted to some extent for dementia risk reduction.25 Moreover, the FHS observed a parallel improvement in cardiovascular health, specifically among individuals with higher education.9 Vascular factors, that is, more medication against cardiovascular diseases such as hypertension and hyperlipidemia as well as diabetes mellitus and less history of stroke in the later cohort, partly explained decreasing dementia incidence in the PAQUID&3C study.25
In the CHAP study, a biracial population of African-Americans and Caucasians, being African-American was associated with higher dementia incidence while there was no change in dementia incidence over time taking ethnicity into account. By contrast, within the African-American sample of the IIDP, a significant decline in dementia incidence was noted in the later cohort. The FHS, which reported a marked decline in dementia incidence, noted that their participants were overwhelmingly of European ancestry. Apart from the three US-based studies, ethnicity was not further considered in the studies.
Finally, some effect of deprivation, that is, higher IR in more deprived areas, was seen in CFAS II, but not in CFAS I. However, the effect did not remain significant after adjusting for age, sex, and area.23
Results across studies
Meta-analysis of IC across HIC
We included five studies in the quantitative synthesis. Two of the identified references were excluded – the CHAP22 cohort investigated incidence of AD dementia only, and the FHS9 was considered high risk of bias. The pooled overall estimate of IC was 0.82 (95% CI: 0.51–1.33) across HIC, indicating that dementia incidence was 18% lower in follow-up cohorts compared with the original cohorts across studies; however, the decrease was nonsignificant (Table S1). Heterogeneity was high (I2=94.9%, P<0.001). There was no evidence for publication bias (Egger’s t=–1.77; P=0.175). Results of the meta-regression revealed no evidence for differential IC associated with design and time parameters (Table S2).
Meta-analysis of IR across HIC
Pooled IR of dementia was 17.5 (95% CI: 11.8–25.8) per 1,000 person-years. Again, heterogeneity was high (I2=98.4% (P<0.001). Publication bias was present (Egger’s t=–3.08; P=0.015). Correction for publication bias using the trim-and-fill procedure suggested that two studies were missing at the left side of the mean effect. “Filling” these studies and reestimating the pooled effect revealed an imputed estimate of IR =14.4 (95% CI: 9.2–22.6).
Results of the meta-regression revealed no evidence for differential IR associated with study design parameters. Moreover, there was no effect of calendar year on the pooled IR estimate, both unadjusted and adjusted for sociodemographics. Assuming a uniform trend across HIC showed that higher incidence of dementia was associated with follow-up length, higher mean age, and a lower percentage of females in the study. Except for mean age, these effects disappeared when adjusting for country variance (Table S3).
Meta-analysis of IC and IR in western HIC
Omitting the Japanese Hisayama study (only study observing an increase in dementia incidence) revealed borderline evidence for a decline in dementia incidence across Western HIC (pooled IC =0.69; 95% CI: 0.47–1.00; I2=88.1%, P<0.001; Egger’s t=–0.34, P=0.767; Figure 3).
The pooled random-effect estimate for IR was 14.9 (95% CI: 9.8–22.7) when excluding the results of the Hisayama study, with high heterogeneity among estimates (I2=98.1%, P<0.001). There was no evidence for publication bias (Egger’s t=–2.16; P=0.074). Results of meta-regression models were similar to those without excluding the Hisayama study (Table S3, right panel). In particular, there was no evidence for differential IR by calendar year. Predictions of the mean IR over calendar year considering each study’s random-effect meta-analysis weight and adjusting for follow-up length, mean age, and female percentage are shown by country in Figure 4.
  | Figure 4 Results of meta-regression of log dementia incidence rate on calendar year of observation period in four Western high-income countries, adjusted for individual study characteristics reported for mean age, proportion of females and length of observation period. Notes: The gray shaded area marks lower and upper confidence intervals. The circles mark the incidence rate of each cohort, earlier vs later. The flags represent the included studies, namely (according to chronological appearance) representing the Personnes Agées Quid (PAQUID) & Three-City (3C) study (France)25, the Rotterdam study (The Netherlands)26, the Cognitive Function and Ageing Study (CFAS, UK)23, and the Indianapolis-Ibadan Dementia Project (IIDP, USA)24. |
Sensitivity analyses
Meta-analyses using data on alternate dementia diagnosis (applied to one study only: PAQUID&3C study) revealed comparable results with regard to IC and IR measures across HIC (results not shown). Only when excluding the Hisayama study, we observed a significant pooled measure for IC with follow-up study periods showing lower IR compared with the reference study periods (pooled IC =0.63; 95% CI: 0.46–0.85) across Western HIC.
Results of the meta-analyses using data of all seven included studies, regardless of risk of bias assessment, were in agreement with the stricter analyses including only five studies of sufficient study quality. Meta-regression did not indicate a significant effect of calendar year on IC or IR across seven studies (Tables S4 and S5).
Discussion
The systematic review and meta-analysis comprised seven studies on recent trends in dementia incidence in HIC, considering study quality. Six out of seven studies, notably all from Western HIC (ie, one study from each of France, The Netherlands, UK, and three from the USA) reported either a decrease or stable dementia incidence between the late 1980s and early 2010s. By contrast, an increase in overall dementia incidence was observed in the Japanese Hisayama study. Quantitative synthesis, which included five studies of moderate-to-good methodology quality, suggested a nonsignificant decrease of dementia incidence (18%; IC =0.82; 95% CI: 0.51–1.33) across the HIC category, although heterogeneity between studies was high.
Synthesizing results of studies from Western HIC only revealed borderline significance for a decrease in dementia incidence (31%; IC =0.69; 95% CI: 0.47–1.00), again indicating a high amount of heterogeneity. However, pooled IR of individual cohorts was not associated with significant temporal variation (based on calendar year) in meta-regression. Adjusting for study and methodological characteristics (where feasible regarding number of observations) did not indicate much change in dementia incidence in HIC since the late 1980s. Overall, the results point to seemingly differential trends in dementia incidence regarding HIC region. While in Western HIC dementia incidence shows a rather stable, possibly decreasing trend, it may be increasing in East Asian HIC, exemplified by the Japanese Hisayama study. However, the limited number of studies at hand, particularly having identified only one study reporting on trends in dementia incidence representing the East Asian HIC, as well as the high heterogeneity among them, precludes a reliable conclusion. Nevertheless, it may suggest that trends in dementia incidence are less uniform across HIC. Ultimately, life conditions and life course experiences may vary a lot among HIC and may therefore differentially impact trends in dementia incidence in these societies beyond common favorable circumstances such as high life expectancy, stable environments after World War II, general wealth, good education, or advanced health care systems.30 Notably, primary data on trends in dementia incidence from HIC, particularly from countries other than Western Europe and the USA, are sparse.
Our meta-analytic findings are largely in line with previous works reviewing trends in dementia occurrence.1,3,5,6,30 A systematic review by Prince et al on global trends in prevalence, incidence, and mortality in dementia, found “moderately consistent evidence to suggest that the incidence of dementia may be declining in HIC”, while the age-specific prevalence seemed rather stable except for some evidence of an increase in East Asia, including Japan.1 A potential increase in dementia prevalence in Japan was also reported by two further reviews, while data from other countries pointed to rather stable prevalence and stable or declining incidence.3,30 Studies from two other East Asian HIC, that is, South Korea and Taiwan, suggested stable dementia prevalence; however, available data were insufficient to draw definite conclusions.30 Trend data regarding dementia incidence from East Asian HIC other than Japan are lacking. Results from the incidence trend study included in this work support the notion of an overall increase of dementia occurrence in Japan. Whether this is a phenomenon also occurring in other East Asian HIC needs further study. By contrast, evidence from Western HIC is a bit more convincing, consistently suggesting stable or declining dementia occurrence.3,5 Our analyses confirm an overall favorable trend in dementia incidence in Western HIC, though weighing in the results of our different analytical approaches would rather support stabilizing than declining dementia incidence. The divergent trend seen between Western HIC and Japan may also indicate variation due to ethnicity. Heterogeneity in dementia risk and dementia occurrence within different ethnic categories has been well described in research, specifically within the diverse US population.31 Thus, how this may affect the generalization of trends within countries and regions, however, is yet to be studied. Importantly, studies investigating dementia occurrence in Japanese living outside of Japan observed patterns of AD and VaD rather similar to Caucasian Americans than to Japanese in Japan.32,33 Then again, this may hint that environmental factors could be more important than ethnicity in the development of dementia.
Largely, changes in dementia occurrence are thought to be driven by multifaceted factors effective over the full life span, and many of the factors remain unexplained to date.34 However, a particular set of determinants is repeatedly discussed. First, there is a well-established link between vascular risk factors, that is, stroke, hypertension, hypercholesterolemia, smoking, diabetes, obesity in midlife, and the development of dementia.35–37 Therefore, favorable trends in vascular risk factors, for example, better management and treatment of cardiovascular disease, could contribute to less dementia incidence.38 Indeed, differential trends between VaD and AD dementia were indicated in some of the studies in this work. For example, the FHS reported a less marked, even nonsignificant decrease in AD dementia, but a more rapid and steep decrease in VaD.9 The IIDP noted a significant decline in both all-cause dementia and AD; however, there seemed to have been a much more marked decline in non-AD cases, of which VaD is the largest group.24 Furthermore, the PADQUID&3C study found that vascular factors would explain part of the decrease in their study.25 Interestingly, the increase in dementia incidence in the Hisayama study was predominantly seen in AD dementia while the incidence of VaD was stable,27 despite worsening cardiovascular risk profiles observed in the region.1 Notably, diagnosing subtypes of dementia based on research criteria is associated with some inaccuracy. For example, the NINDS-ADRDA criteria have limited specificity to differentiate AD against other types of dementia.39 In the absence of large-scale histopathological assessment in many cohort studies, dementia subtype classification remains probabilistic and likely ignores common mixed pathologies. Thus, it may be even more difficult assessing dementia subtypes stable across cohorts over time.3 On the other hand, the study results suggesting a trend toward less of a burden through VaD may support the hypothesis that a reduction of the vascular component of dementia may drive change in dementia incidence. Improvement in blood pressure and other stroke risk factors has been observed over the same period as a decline in dementia incidence in the FHS.38 Rocca suggested that trends could possibly take opposite directions regarding the two major subtypes of dementia, that is, the neurovascular type and the neurodegenerative type, which may be supported, for example, by declining stroke incidence, but parallel increasing Parkinson disease incidence.34 Moreover, trends in dementia occurrence should be viewed in a broader context of trends in related major disorders and diseases. In conjunction with trends in dementia, Jones and Greene recall the rise and fall and again rise of coronary artery disease (CAD) that demonstrated how fragile success may be.8 Particularly, the increase in obesity may have accompanied the reversal in CAD occurrence.40 Likewise, the upward trend in midlife obesity, but also hypertension and diabetes, may ultimately counterbalance favorable trends in dementia as well. At large, adverse trends in some risk factors for dementia can offset favorable trends in others.40 Latest results suggest that 35% of dementia is attributable to a set of nine modifiable risk factors, which include education in early life (8%), hearing loss (9%), hypertension (2%) and obesity in midlife (1%), and smoking (5%), depression (4%), physical inactivity (3%), social isolation (2%), and diabetes (1%) in late life. Furthermore, another 7% are attributed to the apolioprotein E ε4 allele, the major genetic risk factor for dementia.10 Changes in dementia incidence may be the effect of a complex interplay of life style changes with regard to the above named factors. Decreasing dementia incidence, however, would not only indicate fewer new cases due to other competing risks of death. Also, it likely indicates a delay of dementia onset. For example, higher education or physical activity could build cognitive reserve against cognitive and functional decline in old age,41 ultimately leading to a compression of morbidity from dementia. Particularly, the results of the FHS support this hypothesis as a 5-year delay in the mean age of dementia onset rising to 85 years from 80 years was reported during the last three decades.23 Similarly, the Rotterdam study as well as the IIDP reported lower dementia incidence over time for all age groups but the oldest for which incidence remained stable.24,26 In the CFAS I and II, incidence of dementia risk remained unchanged in the age strata 80–84 in women, but was lower across all other age groups (65–69, 70–74, 75–79, >85) for both sexes.9 Conversely, the Hisayama study reported increasing dementia incidence risk for younger age groups, while it remained unchanged for those >85 years of age.27 Future studies investigating trends in dementia onset and competing risk of death could give more insight into the patterns of changing dementia incidence.
However, the latest estimates on population attributable fraction by Livingston et al also suggest that 65% of dementia is attributable to potentially nonmodifiable or yet not identified factors.10 This points to the need to take an even broader perspective, for example, on the historical context in which life style factors and life conditions are effective.2
Conducting studies on secular trends introduces methodological challenges. To compare dementia IR, a minimum requirement is applying similar methods throughout the study. This was basically fulfilled by the included studies; however, six out of seven studies relied on a clinical diagnostic approach primarily. Though based on objective data, final dementia diagnosis involved clinical judgment. However, clinical and sociocultural awareness of dementia have changed immensely over time.42 Increased recognition of dementia may more likely lead to a diagnosis more recently. The results from Grasset et al support this assumption.25 When they reanalyzed trends in dementia incidence based on an algorithmic diagnosis, the decrease was more pronounced compared with the clinical diagnostic approach.25 Thus, dementia diagnosis involving clinical judgment may underestimate decrease.
Another source of inaccuracy may be low and differing response rates between cohorts. It is a well-described phenomenon that participation in epidemiologic studies has been continuously declining over the past decades.43 This was also seen throughout the studies included in this work. Reasons for declining participation include increased requests for study participation, complex and demanding research protocols, declining volunteerism, lower trust in science, and less time capacities.44 The extent to which nonresponse creates bias in estimates on health outcomes sparks debate. It is known that nonresponse is associated with lower socioeconomic status, less education, poorer health, and lower level of functioning44 – all factors that are adversely associated with brain health in old age. Indeed, in a community survey of older individuals, nonresponders appeared to be disproportionately cognitively impaired.45 By contrast, another study reported that the impact of nonresponse on rates of dementia incidence was small and nonsignificant.46 However, if nonresponse continues to increase, it could lead to biased conclusions on secular trends. In such a scenario, the observed decrease in dementia incidence would be overestimated.
Generalizability of findings constitutes another issue. Five of the included studies acknowledged that it was unclear whether results were representative of the older population,22,24,26 or that it was clear that the sample was not.9,25 In consequence, generalization remains a matter of judgment and interpretation of the results, however, if determined to be internally valid.47 Our risk of bias assessment suggested predominantly low risk of bias for internal validity. Limitations were rather associated with items concerning external validity. It has been argued that internal validity is the priority for research, which might be particularly true for incidence studies.48 However, it is important that the external validity is strengthened in future studies.
Finally, it is noteworthy that there are other approaches to study secular trends, for example age-period-cohort (APC) analysis or analyses based on secondary data (medical records, health claims, etc). APC analysis may be specifically useful to understand risk differences in dementia based on year of birth. Likewise, it could give insight into variation due to larger external factors from the historical perspective (eg, war, economic crisis, and famine), that is, period effects. Secondary data may reveal different, but also important aspects of time trends than primary data, for example, information on changes in health care service use, number of dementia diagnoses in a specific period, or changing awareness of dementia in clinicians.
Limitations
The main limitations arose from the small number of studies included in this work based on our eligibility criteria and the high heterogeneity among them. High heterogeneity was not surprising as 1) one study would show opposite results, 2) the magnitude of IC and IR varied among studies, and 3) study attributes differed. Omitting studies one by one would not reveal significantly lower heterogeneity. To account for heterogeneity, we used DerSimonian–Laird random-effect models and conducted weighted random-effects meta-regression to examine associations of heterogeneity with study characteristics. However, exploring heterogeneity did not suggest significant variation due to design effects. Moreover, only a small effect of follow-up length was present once country variation was not taken into account. Importantly, the exploration of sources of heterogeneity was limited by low power, again owing to the small number of studies at hand. Likewise, there may be other factors causing heterogeneity that could not have been considered due to data availability. Overall, the limited number of studies and the high heterogeneity preclude definite conclusions until more studies become available. Furthermore, except for country variation, subgroup analysis was dropped out because of insufficient data (eg, comparable age groups or sex).
Pooling IR of dementia incidence across HIC revealed the presence of publication bias. Correction using the trim-and-fill procedure suggested that two studies were missing at the left side of the mean effect which would have lowered IR by three cases per 1,000 person-years. Possibly, we did not identify all studies, even though we have tried to ensure literature saturation by backing the database searches with gray literature and hand searches of reference lists of eligible studies.
In general, meta-analysis of aggregate data may carry risk of ecological bias, particularly when average participant’s characteristics do not adequately reflect individual-level associations.49 Individual participant data analysis could avoid such bias.
Conclusions
We found evidence to suggest favorable trends in dementia incidence in Western HIC (stabilizing/decreasing). However, there may be reverse trends in other HIC regions, as exemplified by an observed increase in dementia incidence in Japan. Overall, there may be differential trends in dementia incidence in HIC, reflecting varying life conditions and life experiences beyond beneficial environments in such wealthy societies. Adding to previous reviews, we may imply that the reason for not finding consistent and substantial trends over time in dementia incidence in earlier works is unlikely a result of inadequate methodological rigor. In other words, we can be slightly more confident than before that, although there are a number of individual studies that suggest a decline over time in dementia incidence, the data do not yet support a robust conclusion to that effect. Notably, primary data on incidence trends are sparse, the heterogeneity between studies was high, and power to isolate sources of variation was low which preclude drawing definite conclusions. Ultimately, further well-conducted studies on trends in dementia incidence, but also mortality and prevalence, from a broad range of countries, regions, and sociocultural backgrounds are needed to get a better picture of current developments in dementia occurrence, let alone to gain understanding of factors that drive change.
Acknowledgments
This work is published in affiliation with the German Study on Aging, Cognition and Dementia in Primary Care Patients (AgeCoDe; funded by the German Federal Ministry of Education and Research grants: 01GI431 and 01GI0714) and the Study on Needs, Health Service Use, Costs and Health-related Quality of Life in a Large Sample of Oldest-old Primary Care Patients (85+) (AgeQualiDe; funded by the German Federal Ministry of Education and Research grant: 01GY1322A). Susanne Roehr has also been supported by LIFE – Leipzig Research Center for Civilization Diseases, University of Leipzig. Her collaboration within LIFE was funded by means of the European Social Fund and the Free State of Saxony. We further acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Author contributions
Susanne Roehr conceived and designed the study. All authors contributed to the acquisition, analysis and interpretation of data, and drafting and critically revising for important intellectual content. All authors approved the final version of the manuscript, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. | ||
Skoog I. Dementia: dementia incidence - the times, they are a-changing. Nat Rev Neurol. 2016;12(6):316–318. | ||
Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13(6):327–339. | ||
Ganguli M. The times they are a-changin’: cohort effects in aging, cognition, and dementia. Int Psychogeriatr. 2017;29(3):353–355. | ||
Wu YT, Fratiglioni L, Matthews FE, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15(1):116–124. | ||
Wu YT, Lee HY, Norton S, et al. Period, birth cohort and prevalence of dementia in mainland China, Hong Kong and Taiwan: a meta-analysis. Int J Geriatr Psychiatry. 2014;29(12):1212–1220. | ||
Langa KM. Is the risk of Alzheimer’s disease and dementia declining? Alzheimers Res Ther. 2015;7(1):34. | ||
Jones DS, Greene JA. Is dementia in decline? Historical trends and future trajectories. N Engl J Med. 2016;374(6):507–509. | ||
Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. | ||
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–2734. | ||
Roehr S, Pabst A, Luck T, Riedel-Heller SG. Secular trends in the incidence of dementia in high income countries: a systematic review and meta-analysis. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016043232. Accessed June 12, 2017. | ||
Roehr S, Pabst A, Luck T, Riedel-Heller SG. Secular trends in the incidence of dementia in high-income countries: a protocol of a systematic review and a planned meta-analysis. BMJ Open. 2017;7(4):e013630. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. | ||
World Bank Country and Lending Groups. World Bank Data Help Desk. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed October 4, 2017. | ||
Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. | ||
Terracciano L, Brozek J, Compalati E, Schünemann H. GRADE system: new paradigm. Curr Opin Allergy Clin Immunol. 2010;10(4):377–383. | ||
Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893–899. | ||
Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3–28. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. | ||
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. | ||
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. | ||
Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93. | ||
Matthews FE, Stephan BC, Robinson L, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7:11398. | ||
Gao S, Ogunniyi A, Hall KS, et al. Dementia incidence declined in African-Americans but not in Yoruba. Alzheimers Dement. 2016;12(3):244–251. | ||
Grasset L, Brayne C, Joly P, et al. Trends in dementia incidence: evolution over a 10-year period in France. Alzheimers Dement. 2016;12(3):272–280. | ||
Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining?: trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456–1463. | ||
Ohara T, Hata J, Yoshida D, et al. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology. 2017;88(20):1925–1932. | ||
Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. | ||
Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250. | ||
Wu YT, Brayne C, Matthews FE. Prevalence of dementia in East Asia: a synthetic review of time trends. Int J Geriatr Psychiatry. 2015;30(8):793–801. | ||
Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72–83. | ||
White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276(12):955–960. | ||
Graves AB, Larson EB, Edland SD, et al. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. The Kame Project. Am J Epidemiol. 1996;144(8):760–771. | ||
Rocca WA. Time, sex, gender, history, and dementia. Alzheimer Dis Assoc Disord. 2017;31(1):76–79. | ||
Skoog I. Status of risk factors for vascular dementia. Neuroepidemiology. 1998;17(1):2–9. | ||
Knopman DS, Wang W, Whitmer RA, et al. Epidemiology of vascular related risk factors for dementia. Alzheimers Dement. 2016;12(7):P276. | ||
Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. Br Med J. 2001;322(7300):1447–1451. | ||
Pase MP, Satizabal CL, Seshadri S. Role of improved vascular health in the declining incidence of dementia. Stroke. 2017;48(7):2013–2020. | ||
Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry.1999;66(2):184–188. | ||
Jones DS, Greene JA. The decline and rise of coronary heart disease: understanding public health catastrophism. Am J Public Health. 2013;103(7):1207–1218. | ||
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. | ||
Grimmer T, Beringer S, Kehl V, et al. Trends of patient referral to a memory clinic and towards earlier diagnosis from 1985-2009. Int Psychogeriatr. 2015;27(12):1939–1944. | ||
Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol. 2006;163(3):197–203. | ||
Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–653. | ||
Norton MC, Breitner JC, Welsh KA, Wyse BW. Characteristics of nonresponders in a community survey of the elderly. J Am Geriatr Soc. 1994;42(12):1252–1256. | ||
Knopman DS, Roberts RO, Pankratz VS, et al. Incidence of dementia among participants and nonparticipants in a longitudinal study of cognitive aging. Am J Epidemiol. 2014;180(4):414–423. | ||
Kukull WA, Ganguli M. Generalizability: the trees, the forest, and the low-hanging fruit. Neurology. 2012;78(23):1886–1891. | ||
Steckler A, Mcleroy KR. The importance of external validity. Am J Public Health. 2008;98(1):9–10. | ||
Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G. Meta-analysis: pitfalls and hints. Heart Lung Vessel. 2013;5(4):219–225. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.