Back to Journals » Journal of Asthma and Allergy » Volume 16
Involvement of Transforming Growth Factor-β-Associated Kinase 1 in Fixed Airway Obstruction in Asthmatic Patients with Longer Disease Duration Independent on Airway Eosinophilia
Authors Maneechotesuwan K , Wongsurakiat P , Assawabhumi J, Kasetsinsombat K, Wongkajornsilp A
Received 4 January 2023
Accepted for publication 26 March 2023
Published 4 April 2023 Volume 2023:16 Pages 343—354
DOI https://doi.org/10.2147/JAA.S403645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Kittipong Maneechotesuwan,1 Phunsup Wongsurakiat,1 Jirawat Assawabhumi,1 Kanda Kasetsinsombat,2 Adisak Wongkajornsilp2
1Division of Respiratory Disease and Tuberculosis, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 2Department of Pharmacology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Kittipong Maneechotesuwan, Division of Respiratory Diseases and Tuberculosis, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Prannok Street, Bangkok, 10700, Thailand, Tel +662 419 7757, Fax +662 419 7760, Email [email protected]
Objective: Transforming growth factor-β-associated kinase 1 (TAK1) mediates non-canonical TGF-β signalling by promoting adhesive, migratory, proliferative and contractile responses of fibroblasts to TGF-β1. However, TAK1 expression status in asthmatic patients with or without fixed airway obstruction (FAO) is unknown.
Patients and Methods: A total of 60 adult asthmatics with FAO were recruited and compared to 43 those without FAO (nFAO). TGF-β1 concentrations, and total TAK1 and phosphorylated TAK1 (p-TAK1) levels were determined in sputum supernatants, cytospin, and whole cell lysate by ELISA, immunocytochemistry, and Western blot analysis, respectively, in asthmatics with and without FAO.
Results: Asthmatic patients with FAO had much greater sputum TGF-β1 concentrations than those without FAO. This was independent of airway eosinophilia as there was no significant difference in TGF-β1 levels between high and low eosinophil counts within FAO and nFAO groups. In contrast, patients with FAO in the presence of sputum eosinophilia had greater expression of TAK1 and p-TAK1 than those without sputum eosinophilia (P=0.0032 and P=0.0061, respectively). The Western Blot data of total TAK1 and p-TAK1 were consistent with the immunocytochemistry, showing upregulation in all sputum cell types (neutrophils, eosinophils, macrophages, lymphocytes and airway epithelial cells). In addition, total TAK1 expression negatively correlated with pre- and post-bronchodilator FEV1/FVC ratio.
Conclusion: TAK1 may play a key role in asthmatic patients with fixed airway obstruction, which was independent of eosinophilic airway inflammation. The interruption of TAK1 might have favourable clinical impact.
Keywords: transforming growth factor-β-associated kinase 1, fixed airway obstruction, asthma
Introduction
Asthma, a chronic respiratory disease characterized by typically reversible airway obstruction and airway inflammation, while causes variable and recurrent episodes of wheezing, breathlessness, and cough.1 Airway inflammation in asthma is usually effectively suppressed by inhaled corticosteroids (ICS) with low dose, which provides approximately 80–90% of the maximum achievable therapeutic benefit across the spectrum of severity.2 However, there is considerable individual variability in the response to ICS in adult asthma depending on clinical characteristics, such as type 2 inflammation.2 If this is the case, subsequent dose titration has been implicated in clinical practice.2 Asthma phenotypes and particularly inflammatory endotypes determine which patients will be the most likely to respond to ICS or who will benefit from the treatment with biologics.3,4 However, the underlying pathologies of asthma that are independent of the asthma phenotype and endotype are chronic airway inflammation and airway remodeling.3 In asthma, airway remodeling of the conducting airway walls leads to structural changes including loss of epithelial integrity, goblet cell and submucosal gland enlargement, basement membrane thickening, subepithelial fibrosis, increased smooth muscle mass, angiogenesis, and decreased cartilage integrity.5–7 Airway remodeling features have been documented for all stages of asthma severity, are associated with the greater use of asthma medications, and result in fixed airflow obstruction (FAO).3,8,9 Despite treatment with inhaled corticosteroids and long-acting bronchodilators, it has been shown longitudinally that they have no significant effects in improving fixed airflow obstruction over time if present in childhood.3 The hypothesis was previously mooted that Th2 inflammation-mediated chronic cycle of injury resulted in airway remodeling over the lifetime of an individual with asthma.3,10 However, recent studies have now demonstrated that pathological features of airway remodeling commence, often before allergic airway inflammation is observed or a clinical diagnosis of asthma is made.3,11 In addition, results from a systematic review of 39 studies analysing the relationship between inflammation and airway remodeling failed to prove the hypothesis that inflammation causes these changes, which could not be prevented by early ICS treatment but may be modestly improved by biologics.3,12,13 This is also supported by the evidence from a large cohort of adult asthmatics with active asthma or a history of asthma, that independent of asthma status and medication use, the extent of airway remodeling and fixed airflow obstruction did not alter over a 3-year time period.14 All above data, in addition to the fact that none of the drugs used for asthma therapy provides any suppressive effects on airway wall remodeling,5 suggest that there are additional mechanisms driving airway remodelling in chronic asthma. Intercepting these mechanisms may provide effective prevention and treatment of FAO and may enhance anti-remodeling effects of biologics in asthmatics.
Several studies of airway remodelling in asthma have focused on the role of different cytokines, chemokines, and growth factors. Several mediators of remodelling have been identified, including transforming growth factor-β (TGF-β), IL-4, IL-9, IL-13, IL-17, and vascular-endothelial growth factor (VEGF).15 TGF-β is of particular importance because it induces multiple effects in multiple cell types, depending on local microenvironmental and cellular conditions. Abundancy of TGF-β1 is associated with high eosinophilic infiltration and higher severity of asthma.16–18 Eosinophils are the major source of TGF-β in asthma that acts on eosinophils themselves in an autocrine fashion.19 Epithelial cells and macrophages also release TGF-β, which further triggers fibroblast proliferation and tissue remodelling.15,20 TGF-β signalling was conventionally emphasized on canonical Smad-dependent cascades to promote airway remodelling pathways in asthma.21–23 Much less attention has been paid to the non-canonical TGF-β-activated kinase 1 (TAK1), a serine/threonine kinase that becomes active after phosphorylation. This led us to hypothesize that the level of activated TAK1 in asthmatics with FAO was higher than those without FAO.
The present study investigated whether TGF-β1-induced non-canonical TAK1 activation played a possible role in asthmatics with FAO and there was a significant difference in TAK1 expression and cellular localization in airway inflammatory cells between asthmatic patients with FAO and those without FAO using induced sputum.
Materials and Methods
Study Design
This was cross-sectional, non-interventional, real-life study of adult non-smokers with asthma, with and without fixed airflow obstruction (FAO), which was conducted between 2015 and 2016. The conduct of this study complies with the Declaration of Helsinki. Written informed consent was obtained from each patient, and this study was approved by Siriraj IRB on the 23th of September 2015 (Si 527/2015). There is no conflict of interest of the authors related to the present study.
Inclusion and Exclusion Criteria
Patients had to meet the following inclusion criteria for the study: non-smoker participants (≤5 pack-years) aged over 40 years, have a current physician-diagnosed asthma and were prescribed at least 12 months of moderate-to high-dose inhaled corticosteroids (ICS) or fixed-dose combination ICS and long-acting β2 agonist therapy (ie, regular prescriptions of GINA Step 3 or 4) without adjustment of ICS dose in the year before study entry. Patients were excluded if they had a history of other lung diseases likely to influence lung function, or they developed an asthma exacerbation within 6 months or they had received oral corticosteroids and/or antibiotics for a lower respiratory condition or their asthma medications were adjusted within the 12 weeks before study entry, or they had received maintenance systemic therapy (ie, biologics and immunosuppressive agents) for asthma or other systemic diseases.
Asthma was defined according to the American Thoracic Society criteria.24 The subjects previously demonstrated the reversibility of FEV1 after therapy with nebulized albuterol (2.5 mg) of ≥12% and a provocative concentration of methacholine causing a 20% fall in FEV1 (PC20) of ≤4 mg/mL. However, some patients with asthma may not improve their FEV1, in response to bronchodilators during episodes of severe airways obstruction,24 and therefore, current bronchodilator reversibility was not included in inclusion criteria for studies of fixed airway obstruction in patients with asthma.25–27 In other words, those studies recruited asthmatic patients with FAO regardless of bronchodilator reversibility status.
Data Collection
Data, including patient demographics, medical history, and current asthma management, are collected from the asthma clinics of the Division of Respiratory Disease in Faculty of Medicine Siriraj Hospital at enrollment via an electronic case record form. At screening, previous pulmonary function data performed at least 3 months prior to study entry were used to review fixed airway obstruction (FAO) of individual participants and LLN values were calculated and utilized for classifying FAO and non-FAO (nFAO) groups using lung function data during enrolment period. Enrolled patients were requested to undergo asthma control assessment using asthma control test (ACT), pulmonary function testing for confirmation of FAO and used for the present study, fractional exhaled nitric oxide measurement, sputum induction, and complete blood count and total IgE.
Assessments of Fixed Airway Obstruction
The presence of fixed airway obstruction (FAO) was defined as postbronchodilator FEV1/FVC < LLN with the first lung function result at least 3 months before study entry and was confirmed the persistence with the second pulmonary function testing at study enrolment, and the absence of FAO (nFAO) was defined as FEV1/FVC ≥LLN using the Global Initiative Lung all-age reference equations for spirometry.28,29 FAO status was assigned based on whether FEV1/FVC was <LLN or ≥ LLN (FAO and nFAO, respectively) approximately 15–30 minutes after nebulized albuterol (2.5 mg).
Sputum Induction and Processing
Sputum induction was performed as previously described.30 The supernatants were kept frozen at –70°C until further analysis. For immunocytochemistry, cytospins were fixed with 4% paraformaldehyde (BDH Ltd., Poole, UK) and stored at –20°C. Total cell counts were recorded with a hemocytometer, using Kimura stain. Cell viability was determined by Trypan blue exclusion before cytospins were undertaken. The slides were stained with May-Grunwald-Giemsa stain, and differential cell counts were made by a blinded observer. Four hundred inflammatory cells were counted on two slides for each sample in a blinded manner. Differential cell counts are expressed as the percentages of total inflammatory cells. Samples with cell viability of greater than 70% and less than 30% squamous cell contamination were considered adequate for analysis.
Alkaline Phosphatase Immunostaining
Sputum cells were immunostained with the alkaline phosphatase-anti-alkaline phosphatase (APAAP) method using a commercial kit (Vectastain Laboratories, Burlingame, CA). Cells were permeabilized for 10 min with 0.5% Nonidet P-40 (NP-40, Sigma Chemicals, St Louis, MO) and blocked in 20% normal swine nonimmune serum (Vector Laboratories, Burlingame, CA) for 30 min at room temperature. Polyclonal anti-rabbit antibody to phosphorylated TAK1 (p-TAK1) (Cell Signaling, Beverly, MA) and TAK1 (Santa Cruz, CA) were used for immunocytochemistry staining. After incubating with the secondary biotinylated goat anti-rabbit or anti-mouse antibody, the immunoreaction was detected using the APAAP system to produce red positive staining. Slides were counterstained with hematoxylin for cellular identification and examined under light microscopy. All immunoreactive cells expressing targeted proteins, which were identified by red signals on sputum cytospins, were counted by an experienced observer blinded to the clinical characteristics of the subjects. The total immunoreactive cells were counted among 400 cells and expressed as a percentage of each cell type.
Enzyme-Linked Immunosorbent Assay (ELISA) for Transforming Growth Factor-b1
For detection of human TGF-β1 sandwich ELISA was performed according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN) with a sensitivity was 4.61 pg/mL. Cytokine output was normalized to the concentration of protein.
Western Blot Analysis
Whole cell lysates were prepared in NP-40 lysis buffer (0.5% Nonidet P-40, 20 mM Tris-HCl [pH 7.5], 150 mM NaCl) in the presence of complete protease cocktail inhibitor. Lysates were centrifuged at 4°C for 10 min at 12,000 rpm in an Eppendorf microcentrifuge to remove cellular debris. Western blot analysis was performed as previously described,31 using anti-p-TAK1 (Cell Signaling Technology) and anti-TAK1 (Santa Cruz). Immunoreactive proteins were detected using an enhanced chemiluminescence ECL kit (Amersham Biosciences).
Statistical Analysis
The data are expressed as the mean ± the SD. Demographic data were described by number and percentage of patients for categorical variables and median (IQR) for continuous variables. Comparisons of demographic data and the differences in the expression of signaling molecules between the groups were performed by the Mann–Whitney U-test and unpaired t-test as appropriate. Chi-square test for trend test was used to analyse categorical data for differences and Chi-square test for linear by linear association used for previous exacerbation numbers. The median was calculated if the distribution of the variables was not normal. The r value was determined for the correlation of p-TAK1 with other indicated parameters within the FAO groups using Spearman’s rank correlation test. All statistical tests were two-tailed and significance was accepted at the level of 95% and P<0.05 using PASW statistics 28 (SPSS, IBM, Chicago).
Results
Patient Characteristics
Table 1 summarizes the patients’ demographic data. Of 190 screened patients, 103 were included in this study. Patients excluded from the analysis were those without complete spirometry and demographic data, and those with inadequate induced sputum and incomplete clinical and laboratory data. Patients in fixed airway obstruction (FAO) group were significantly older (64.5±10.3 vs 54.6±10.9, P<0.001) with longer asthma duration (31.5±17.1 vs 24.6±13.9, P=0.039) and more comorbidities, particularly HT and dyslipidemia but not allergic rhinitis and CRSwNP and less GERD (P=0.048), and required higher intensity of treatment with ICS/LABA and LAMA than those without fixed airway obstruction (non-FAO) group (88.3% vs 74.4%, P=0.04; 21.7% vs 4.7%, P=0.01, respectively), whereas there was no significant differences in ACT score, atopy, total IgE, BMI, white blood cell and differential counts between groups, except the absolute monocyte number that was markedly higher in FAO group (median [IQR], 0.5 [0.4 to 0.6]×103/μL vs 0.44 [0.4 to 0.5] ×103/μL, P=0.009). There was much lower pre-and post-bronchodilator (BD) FEV1 values with markedly greater BD reversibility in FAO compared to non-FAO group (1.21±0.4L vs 1.75±0.5L and 1.41±0.42L vs 1.92±0.45L for absolute pre- and post-BD FEV1; 60.4%±19.1% vs 82.7±19.3% and 69.9%±17.7% vs 90.5% vs 16.7% for pre- and post-BD FEV1% of predicted, P<0.001 for all, respectively; 18.8%±16.4% vs 10.9%±9.7% for airway reversibility, P=0.0037). However, both groups showed no significant difference in the degree of airway inflammation in which no predominated inflammatory cell type was detected. In addition, despite lower FeNO levels in FAO group than non-FAO group, there was no statistically significant difference (31.5±17.1 ppb vs 40.5±35.6 ppb, P=0.23). Despite the presence of eosinophilic airway inflammation and elevated FENO levels, it suggested that persistent airflow obstruction was possibly unrelated to the severity and persistence of airway inflammation and might be driven by other underlying mechanisms.
 |
Table 1 Patient Characteristics and Sputum Cytology |
TGF-β1 Production in Asthmatics with Fixed Airway Obstruction (FAO) Was Greater Than Those Without FAO
Table 2 demonstrates asthmatic patients with FAO had greater TGF-β1 concentrations than those without FAO (nFAO) (49.6 ± 6.7 pg/mL vs 7.7 ± 3.6 pg/mL, P<0.001) and Figure 1 shows that there was significantly higher level of TGF-β1 in FAO asthmatics with and without airway eosinophilia than nFAO counterpart (50.2±7.0 pg/mL vs 7.3±3.4 pg/mL; 49.1±6.6 pg/mL vs 7.8±3.7 pg/mL, P<0.001 for both). However, we could not detect any significant difference in TGF-β1 levels in FAO-inflicted asthmatics with or without airway eosinophilia (sputum eosinophils ≥3% and <3%) (mean concentrations 50.2±7.0 pg/mL vs 49.1±6.6 pg/mL, P=0.8) and this was also the case for nFAO groups (7.3±3.4 pg/mL vs 7.8±3.7 pg/mL, P=0.39).
 |
Table 2 Sputum Signaling Molecules |
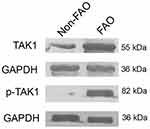
Markedly Higher Levels of Total TAK1 in Asthmatics with FAO Than Those Without FAO
Table 2 shows that the FAO group had markedly higher total TAK1 immunoreactive sputum cells than the non-FAO (nFAO) group (P< 0.0001), which was consistent with higher TAK1 protein level in Western blot analysis (Figure 2). Specifically, the high immunopositivity for TAK1 was detected in sputum neutrophils, eosinophils, macrophages, airway epithelial cells and lymphocytes from FAO group (P < 0.001 for the first four cell types, except lymphocytes which had P = 0.035).
Correlation Between Total TAK1 Level and the Degree of Airway Obstruction in Asthmatics with FAO
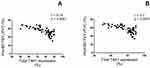
Figure 3 shows that the percentage of total TAK1 immunopositive sputum cells negatively correlated with the degree of airway obstruction as assessed by pre- and post-bronchodilator FEV1/FVC (r = −0.75, P < 0.0001 and r = −0.7, P < 0.0001, respectively) in asthmatics with FAO.
Comparison of Phosphorylated TAK1 Level in Asthmatics with and without FAO
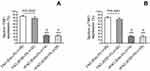
Table 2 shows significantly higher p-TAK1 level in total sputum cells from asthmatics with FAO than those with non-FAO (nFAO) (P<0.001) as demonstrated by Western blot analysis (Figure 2) and immunocytochemistry which demonstrated p-TAK1 localization in neutrophils, eosinophils, epithelial cells, macrophages, and lymphocytes (P<0.001 for the first three cell types, except macrophages and lymphocytes which had P=0.0037 and P=0.039, respectively). In addition, FAO asthmatics with airway eosinophilia had greater total TAK1 and p-TAK1 levels than those without airway eosinophilia (median [IQR], 84.8% [74.5% to 87.5%] and 81.1% [72.8% to 84.5%] vs 78.3% [65.3% to 83.7%] and 75.4% [61.3% to 80.9%], P=0.0032 and P=0.0061, respectively) (Figure 4). In addition, patients in FAO group, regardless of eosinophilia status exhibited higher expression of total TAK1 and p-TAK1 in sputum cells than nFAO counterpart (24.4% [18.1% to 27.4%] and 21.7 [15.5% to 24.1%] for nFAO with airway eosinophilia, 22.8% [19.8% to 26.9%] and 20.9% [17.9% to 24.7%] for nFAO without eosinophilia, respectively, P<0.001 for all) (Figure 4).
Discussion
Our results suggest that protein levels of total TAK1, phosphorylated TAK1 and its cellular localization in differential sputum cell types as well as TGF-β1 release were significantly increased in asthmatics with FAO over those without FAO. Moreover, the upregulation of TAK1 directly correlated with the degree of FAO. Despite no discernible differences in the cellular contribution to such up-regulation of these signaling molecules, the eosinophilic airway inflammation contrasted sharply with the levels of these proteins in patients with FAO over those with less sputum eosinophil counts. However, although, asthmatic patients with FAO and the absence of airway eosinophilia had total TAK1 and p-TAK1 expression to a lesser extent than those with sputum eosinophilia, they exhibited marked increase in these cellular signaling proteins compared with patients in the absence of FAO regardless of eosinophilic status.
TGF-β1 is a potent fibrogenic growth factor. TGF-β1-mediated signaling involves both canonical (Smad-dependent) and non-canonical (Smad-independent) pathways.32 The canonical ALK5/Smad3 pathway mediates pro-fibrotic responses to TGF-β1 in a variety of fibroblasts.33,34 One of the non-canonical TGF-β pathways is mediated by TGF-β-associated kinase 1 (TAK1), a mitogen-activated kinase (MAP3K), that can activate downstream kinases including p38 and JNK MAPK.35 In human adult dermal fibroblasts, TAK1 pathway selectively mediates adhesive, migratory, proliferative and contractile responses to TGF-β1.36,37 However, the role of TAK1 in asthma with FAO is largely unknown. In the present study, we demonstrated that rising TGF-β1 was associated with TAK1 activation as reflected by significantly increased phosphorylation of TAK1 in sputum macrophages and eosinophils of asthmatics with FAO. This indicated that TAK1 responses in the context of its level and activation status may imply its role in asthmatics with FAO. Future investigation for the underlying mechanisms of TAK1 in regulating airway remodeling in asthma is warranted.
TAK1 is essential for the development of Th17 cells. It reprograms inducible regulatory T cells into IL-17+ effector T cells.38 Although Th17-polarizing effect of TAK1 could drive neutrophilic airway inflammation and possibly airway remodeling, we were unable to demonstrate any significant correlation between TAK1 activation with neutrophilic inflammation or fixed airway obstruction as there was no significant difference in neutrophil numbers between the FAO and nFAO groups (data not shown). Similarly, this was also the case with eosinophilic inflammation, making it unlikely that remodeling effects of TAK1 is directly mediated through inflammation. We found greater TGF-β1 level and increased TAK1 activation in asthmatics who had FAO with longer duration of asthma than those without this clinical feature. However, in agreement with other studies, we found no correlation between TGF-β1 and the duration of asthma.39–41
Circulating monocytes and airway macrophages play an important role in the development of airway fibrosis in asthma and pulmonary fibrosis.42,43 Circulating blood monocytes are rapidly recruited to the airway and differentiate into macrophages during asthmatic inflammation; these macrophages are defined as monocyte-derived macrophages.44 In certain polarizing milieu, they differentiate into M1 and M2 subsets with different phenotypic and functional characteristics.45 Along with Th2 cells, M2 macrophages are a major source of type 2 cytokines that potentiate asthmatic inflammation and airway remodeling that can be attenuated by inhibition of monocytes/macrophages axis.42 Therefore, higher circulating monocytes in FAO than nFAO group may suggest more recruitment into the airway and differentiation into monocyte-derived macrophages despite the fact that we failed to demonstrate increased sputum macrophage numbers in asthmatics with FAO to a greater extent than nFAO counterpart. However, FAO asthmatics had higher percentage and absolute numbers of airway macrophages with increased airway remodeling activity as demonstrated by TAK1 and p-TAK1 expression than those without FAO. In addition to monocytes/macrophages, there were significantly increased numbers of other inflammatory cell types with activated TAK1 expression (neutrophils, eosinophils, lymphocytes and airway epithelial cells), suggesting their roles in regulating airway remodeling through TAK1-associated mechanisms in FAO individuals. However, we could not exclude the possibility of airway basophils involved in eosinophilic asthma to mediate airway remodeling via TAK1 other than known IgE-related mechanisms, primarily involving mast cells, in asthma despite no report in TAK1 expression in this cell type.46–48
Although patients with FAO required more ICS and LABA for asthma control than the non-FAO group, there was no significant difference in previous asthma exacerbation numbers, sputum eosinophil percent and FeNO levels. In addition, there was significant upregulation of TAK1 and p-TAK1 expression and increase in TGF-β1 levels in patients with FAO in the presence of airway eosinophilia compared to non-FAO individuals with airway eosinophilia. This may suggest that the occurrence of FAO with underlying airway remodeling was independent on airway inflammation and frequent exacerbations, which was in contrast to previous studies showing the association between childhood and adolescent asthmatics with FAO and frequent exacerbations.49 This may result from differences in our study population comprising more advanced age, adult-onset asthma and longer disease duration being risk factors associated with FAO.49–51 Asthmatics treated with moderate ICS in FAO group had similar FeNO levels and sputum eosinophil percent to those of unselected asthmatics and those of well-controlled asthmatics,52–54 whereas both biomarkers were lower than those of uncontrolled asthmatics.55,56 We did not find the association between both biomarkers and FAO status and the relationship of eosinophil activation with FAO status as demonstrated by others.57 However, our results confirmed earlier studies that aging was associated with FAO and lower FeNO concentrations as demonstrated by the evidence that asthmatics with FAO were significantly older and tended to have lower FeNO levels, albeit statistically insignificant, than asthmatics with nFAO.58
Previous studies reported that non atopy, asthma duration, and BMI before antiasthma treatment are important factors related to airway remodeling in patients with asthma,59 which was consistent with the present data showing that there was no significant difference in atopic status and serum total IgE levels between groups and FAO individuals had significantly longer disease duration than nFAO counterpart despite no BMI association. Our study also confirmed prior studies that there was no association between asthmatics with concurrent allergic rhinitis and FAO.60 In addition, significantly greater numbers of FAO asthmatics with comorbid dyslipidemia than nFAO group may suggest the clinical relevance of dyslipidemia in specific asthma phenotypes, particularly those with asthma with fixed airflow limitation.61
The present study has demonstrated the involvement of TGF-β1 and TAK1 in airway remodeling, both of which were upregulated in the airways with extensive expression among sputum inflammatory cell types including airway epithelial cells, in asthmatic patients with FAO being on controller therapy. This with disease duration-dependent manner indicated the importance of early diagnosis and early treatment that is necessary for preventing airway remodeling and TAK1-mediated fixed airway obstruction still occurred in asthmatics even currently receiving proper treatment with ICS and ICS/LABA. However, there was some limitations of this study to be addressed. This study was cross-sectional and therefore spatial relationship between the commencement of TGF-β1 /TAK1 activation and the occurrence of fixed airway obstruction. The degree of airway inflammation that is directly or indirectly involved in TAK1-mediated airway remodeling and the appropriate timing of therapeutic intervention remain unknown. Finally, due to technical limitation in detection, we did not find the potential role of airway basophils, particularly eosinophilic phenotype, in the development of fixed airway obstruction in asthma.
Abbreviations
FEV1, forced expiratory volume in one second; FAO, fixed airway obstruction; FVC, forced vital capacity; ICS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting agonist; LLN, lower limit of normal; TAK1, transforming growth factor-β-associated kinase 1; p-TAK1, phosphorylated TAK1; TGF-β1, transforming growth factor 1.
Acknowledgments
We would like to thank Faculty of Medicine Siriraj Hospital for providing research funding. We express our gratitude to Suthipol Udompunturak in the Department of Clinical Epidemiology and Biostatistics in Faculty of Medicine Siriraj Hospital, for valuable advice regarding the statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bush A. Pathophysiological Mechanisms of Asthma. Front Pediatr. 2019;7:68. doi:10.3389/fped.2019.00068
2. Beasley R, Harper J, Bird G, Maijers I, Weatherall M, Pavord ID. Inhaled corticosteroid therapy in adult asthma. time for a new therapeutic dose terminology. Am J Respir Crit Care Med. 2019;199(12):1471–1477. doi:10.1164/rccm.201810-1868CI
3. Hsieh A, Assadinia N, Hackett TL. Airway remodeling heterogeneity in asthma and its relationship to disease outcomes. Front Physiol. 2023;14:1113100. doi:10.3389/fphys.2023.1113100
4. Poddighe D, Brambilla I, Licari A, Marseglia GL. Omalizumab in the therapy of pediatric asthma. Recent Pat Inflamm Allergy Drug Discov. 2018;12(2):103–109. doi:10.2174/1872213X12666180430161351
5. Fang L, Roth M. Airway wall remodeling in childhood asthma-a personalized perspective from cell type-specific biology. J Pers Med. 2021;11(11). doi:10.3390/jpm11111229
6. Hough KP, Curtiss ML, Blain TJ, et al. Airway remodeling in asthma. Front Med. 2020;7:191. doi:10.3389/fmed.2020.00191
7. James AL, Donovan GM, Green FHY, et al. Heterogeneity of airway smooth muscle remodeling in asthma. Am J Respir Crit Care Med. 2023;207(4):452–460. doi:10.1164/rccm.202111-2634OC
8. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38(6):872–897. doi:10.1111/j.1365-2222.2008.02971.x
9. Osei ET, Mostaço-Guidolin L, Hsieh A, et al. Epithelial-interleukin-1 inhibits collagen formation by airway fibroblasts: implications for asthma. Sci Rep. 2020;10(1):8721. doi:10.1038/s41598-020-65567-z
10. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi:10.1152/physrev.1997.77.4.1033
11. Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1(8637):520–524. doi:10.1016/s0140-6736(89)90067-6
12. Chen YZ, Busse WW, Pedersen S, Tan W, Lamm CJ, O’Byrne PM. Early intervention of recent onset mild persistent asthma in children aged under 11 yrs: the steroid treatment as regular therapy in early asthma (START) trial. Pediatr Allergy Immunol. 2006;17(Suppl 17):7–13. doi:10.1111/j.1600-5562.2006.00379.x
13. Castro-Rodriguez JA, Rodriguez-Martinez CE, Ducharme FM. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: a systematic review. Pediatr Pulmonol. 2018;53(12):1670–1677. doi:10.1002/ppul.24176
14. Broekema M, Timens W, Vonk JM, et al. Persisting remodeling and less airway wall eosinophil activation in complete remission of asthma. Am J Respir Crit Care Med. 2011;183(3):310–316. doi:10.1164/rccm.201003-0494OC
15. Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44(2):127–133. doi:10.1165/rcmb.2010-0027TR
16. Redington AE, Madden J, Frew AJ, et al. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997;156(2 Pt 1):642–647. doi:10.1164/ajrccm.156.2.9605065
17. Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029–1036. doi:10.1172/JCI17974
18. Minshall EM, Leung DY, Martin RJ, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17(3):326–333. doi:10.1165/ajrcmb.17.3.2733
19. Moore B, Murphy RF, Agrawal DK. Interaction of tgf-beta with immune cells in airway disease. Curr Mol Med. 2008;8(5):427–436. doi:10.2174/156652408785160943
20. Aghasafari P, George U, Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm Res. 2019;68(1):59–74. doi:10.1007/s00011-018-1191-2
21. Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62(4):307–313. doi:10.1136/thx.2006.063487
22. McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol. 2005;174(9):5774–5780. doi:10.4049/jimmunol.174.9.5774
23. Karagiannidis C, Hense G, Martin C, et al. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117(1):111–118. doi:10.1016/j.jaci.2005.09.017
24. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American thoracic society was adopted by the ATS board of directors, November 1986. Am Rev Respir Dis. 1987;136(1):225–244. doi:10.1164/ajrccm/136.1.225
25. Sekiya K, Taniguchi M, Fukutomi Y, et al. Persistent airflow obstruction in young adult asthma patients. Allergol Int. 2012;61(1):143–148. doi:10.2332/allergolint.11-OA-0331
26. Sexton P, Black P, Wu L, et al. Fixed airflow obstruction among nonsmokers with asthma: a case-comparison study. J Asthma. 2013;50(6):606–612. doi:10.3109/02770903.2013.793706
27. Kaminska M, Foley S, Maghni K, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol. 2009;124(1):45–51e1–4. doi:10.1016/j.jaci.2009.03.049
28. Quanjer PH, Brazzale DJ, Boros PW, Pretto JJ. Implications of adopting the global lungs initiative 2012 all-age reference equations for spirometry. Eur Respir J. 2013;42(4):1046–1054. doi:10.1183/09031936.00195512
29. Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–1051. doi:10.1136/thx.2008.098483
30. Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2,3-dioxygenase. J Allergy Clin Immunol. 2010;126(4):754–762e1. doi:10.1016/j.jaci.2010.08.005
31. Ito K, Jazrawi E, Cosio B, Barnes PJ, Adcock IM. p65-activated histone acetyltransferase activity is repressed by glucocorticoids: mifepristone fails to recruit HDAC2 to the p65-HAT complex. J Biol Chem. 2001;276(32):30208–30215. doi:10.1074/jbc.M103604200
32. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi:10.1096/fj.03-1273rev
33. Piek E, Ju WJ, Heyer J, et al. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276(23):19945–19953. doi:10.1074/jbc.M102382200
34. Thompson K, Murphy-Marshman H, Leask A. ALK5 inhibition blocks TGFbeta-induced CCN1 expression in human foreskin fibroblasts. J Cell Commun Signal. 2014;8(1):59–63. doi:10.1007/s12079-014-0229-7
35. Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42(5):585–589. doi:10.1016/j.biocel.2009.12.023
36. Guo F, Hutchenreuther J, Carter DE, Leask A. TAK1 is required for dermal wound healing and homeostasis. J Invest Dermatol. 2013;133(6):1646–1654. doi:10.1038/jid.2013.28
37. Shi-wen X, Parapuram SK, Pala D, et al. Requirement of transforming growth factor beta-activated kinase 1 for transforming growth factor beta-induced alpha-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009;60(1):234–241. doi:10.1002/art.24223
38. Joetham A, Schedel M, Ning F, Wang M, Takeda K, Gelfand EW. Dichotomous role of TGF-beta controls inducible regulatory T-cell fate in allergic airway disease through Smad3 and TGF-beta-activated kinase 1. J Allergy Clin Immunol. 2020;145(3):933–946e4. doi:10.1016/j.jaci.2019.09.032
39. Manuyakorn W, Kamchaisatian W, Atamasirikul K, Sasisakulporn C, Direkwattanachai C, Benjaponpitak S. Serum TGF-beta1 in atopic asthma. Asian Pac J Allergy Immunol. 2008;26(4):185–189.
40. Chu HW, Halliday JL, Martin RJ, Leung DY, Szefler SJ, Wenzel SE. Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. Am J Respir Crit Care Med. 1998;158(6):1936–1944. doi:10.1164/ajrccm.158.6.9712073
41. Joseph J, Benedict S, Badrinath P, et al. Elevation of plasma transforming growth factor beta1 levels in stable nonatopic asthma. Ann Allergy Asthma Immunol. 2003;91(5):472–476. doi:10.1016/S1081-1206(10)61516-5
42. Mo Y, Kim Y, Bang JY, et al. Mesenchymal stem cells attenuate asthmatic inflammation and airway remodeling by modulating macrophages/monocytes in the IL-13-overexpressing mouse model. Immune Netw. 2022;22(5):e40. doi:10.4110/in.2022.22.e40
43. Ogger PP, Albers GJ, Hewitt RJ, et al. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. 2020;5(52). doi:10.1126/sciimmunol.abc1884
44. Tomita K, Tanigawa T, Yajima H, et al. Identification and characterization of monocyte subpopulations from patients with bronchial asthma. J Allergy Clin Immunol. 1995;96(2):230–238. doi:10.1016/s0091-6749(95)70012-9
45. Saradna A, Do DC, Kumar S, Fu QL, Gao P. Macrophage polarization and allergic asthma. Transl Res. 2018;191:1–14. doi:10.1016/j.trsl.2017.09.002
46. Suzuki Y, Wakahara K, Nishio T, Ito S, Hasegawa Y. Airway basophils are increased and activated in eosinophilic asthma. Allergy. 2017;72(10):1532–1539. doi:10.1111/all.13197
47. Brooks CR, van Dalen CJ, Hermans IF, Gibson PG, Simpson JL, Douwes J. Sputum basophils are increased in eosinophilic asthma compared with non-eosinophilic asthma phenotypes. Allergy. 2017;72(10):1583–1586. doi:10.1111/all.13185
48. Boulet L-P. Airway remodeling in asthma. Curr Opin Pulm Med. 2018;24(1):56–62. doi:10.1097/mcp.0000000000000441
49. Sousa AW, Barros Cabral AL, Arruda Martins M, Carvalho CRF. Risk factors for fixed airflow obstruction in children and adolescents with asthma: 4-year follow-up. Pediatr Pulmonol. 2020;55(3):591–598. doi:10.1002/ppul.24625
50. Bennett GH, Carpenter L, Hao W, Song P, Steinberg J, Baptist AP. Risk factors and clinical outcomes associated with fixed airflow obstruction in older adults with asthma. Ann Allergy Asthma Immunol. 2018;120(2):164–168 e1. doi:10.1016/j.anai.2017.10.004
51. Lee JH, Haselkorn T, Borish L, Rasouliyan L, Chipps BE, Wenzel SE. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR study. Chest. 2007;132(6):1882–1889. doi:10.1378/chest.07-0713
52. Schleich FN, Seidel L, Sele J, et al. Exhaled nitric oxide thresholds associated with a sputum eosinophil count >/=3% in a cohort of unselected patients with asthma. Thorax. 2010;65(12):1039–1044. doi:10.1136/thx.2009.124925
53. Volbeda F, Broekema M, Lodewijk ME, et al. Clinical control of asthma associates with measures of airway inflammation. Thorax. 2013;68(1):19–24. doi:10.1136/thoraxjnl-2012-201861
54. Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005;35(9):1175–1179. doi:10.1111/j.1365-2222.2005.02314.x
55. Gao J, Chen Z, Jie X, Ye R, Wu F. Both fractional exhaled nitric oxide and sputum eosinophil were associated with uncontrolled asthma. J Asthma Allergy. 2018;11:73–79. doi:10.2147/JAA.S155379
56. Crespo-Lessmann A, Curto E, Mateus Medina EF, et al. Characteristics of induced-sputum inflammatory phenotypes in adults with asthma: predictors of bronchial eosinophilia. J Asthma Allergy. 2023;16:95–103. doi:10.2147/JAA.S389402
57. Mogensen I, Alving K, Dahlen SE, et al. Fixed airflow obstruction relates to eosinophil activation in asthmatics. Clin Exp Allergy. 2019;49(2):155–162. doi:10.1111/cea.13302
58. Wang J, Zhang X, Zhang L, et al. Age-related clinical characteristics, inflammatory features, phenotypes, and treatment response in asthma. J Allergy Clin Immunol Pract. 2023;11(1):210–219 e3. doi:10.1016/j.jaip.2022.09.029
59. Jang AS, Lee JH, Park SW, Park JS, Kim DJ, Park CS. Risk factors related to fixed airway obstruction in patients with asthma after antiasthma treatment. Ann Allergy Asthma Immunol. 2007;99(5):408–412. doi:10.1016/S1081-1206(10)60564-9
60. Jang AS, Park JS, Lee JH, et al. Asthmatics without rhinitis have more fixed airway obstruction than those with concurrent rhinitis. Allergy Asthma Immunol Res. 2010;2(2):108–113. doi:10.4168/aair.2010.2.2.108
61. Liu L, Liu Y, Zhang X, et al. Dyslipidemia is associated with worse asthma clinical outcomes: a prospective cohort study. J Allergy Clin Immunol Pract. 2022;2022:1. doi:10.1016/j.jaip.2022.11.037
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.