Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Inverse Psoriasis: From Diagnosis to Current Treatment Options
Authors Micali G , Verzì AE , Giuffrida G, Panebianco E, Musumeci ML, Lacarrubba F
Received 7 September 2019
Accepted for publication 4 December 2019
Published 3 January 2020 Volume 2019:12 Pages 953—959
DOI https://doi.org/10.2147/CCID.S189000
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Giuseppe Micali, Anna Elisa Verzì, Giorgia Giuffrida, Enrico Panebianco, Maria Letizia Musumeci, Francesco Lacarrubba
Dermatology Clinic, University of Catania, Catania, Italy
Correspondence: Giuseppe Micali
Dermatology Clinic, University of Catania, Via S. Sofia 78, Catania 95123, Italy
Tel + 39 095 321705
Fax + 39 095 3782425
Email [email protected]
Abstract: Inverse psoriasis represents a clinical variant of psoriasis that is sometimes difficult to diagnose due to its clinical similarity with other skin disorders involving the folds, mainly including mechanical intertrigo, fungal and bacterial infections, contact dermatitis, seborrheic dermatitis, and lichen planus. Dermoscopy represents a useful tool for an enhanced non-invasive diagnosis. The treatment of inverse psoriasis may be challenging and include topical corticosteroids, topical calcineurin inhibitors, vitamin D analogs, traditional oral systemic therapies such as cyclosporine and methotrexate, and biologic therapies.
Keywords: psoriasis, inverse, diagnosis, dermoscopy, confocal microscopy, treatment
Introduction
Inverse psoriasis (IP), also known as flexural or intertriginous psoriasis, is a variety of plaque psoriasis that involves the body folds, most often the axillary, anogenital, and inframammary ones.1
According to different studies and populations, the prevalence of IP is highly variable, ranging from 3 to 36%, because of the lack of precise diagnostic criteria and of the consensus whether genital localization is considered part of the disease.1–4 IP is typical in children, especially in young infants with involvement of the diaper area configuring the “napkin psoriasis”.5
The pathogenesis of IP does not differ from that of plaque psoriasis, and the possible role of fungal and/or bacterial colonization of the folds as possible trigger factor is still debated.1
Although IP may involve a limited percentage of skin body areas, it may have a significant impact on the quality of life, especially on sexual function, embarrassment, and shame,6 as demonstrated by a study using a specific tool to measure the burden of disease called Inverse Psoriasis Burden of Disease (IPBOD) questionnaire.7
It has also been suggested that the sudden onset of IP in adults might be an indicator of HIV infection.8,9
Clinical Presentation
Inverse psoriasis is clinically characterized by well demarcated, erythematous patches [Figures 1–5]. The most commonly affected areas are the inguinal folds, followed by axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Antecubital and popliteal fossae and interdigital spaces may also be involved. External genitalia involvement is considered part of IP presentation.1 IP may represent the exclusive localization of psoriasis or, more frequently, it may be accompanied by classical plaque psoriasis lesions localized in other body areas. Unlike plaque psoriasis, whitish scales are typically minimal or absent, and the surface of the lesions appear as moist, smooth and shiny.
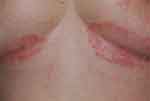 |
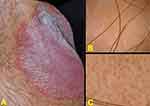
Figure 1 Inverse psoriasis of the inframammary folds in a 38-year-old woman: well demarcated, erythematous patches with fine scaling. |
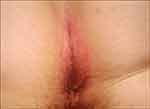 |
Figure 2 Inverse psoriasis of the perianal area in a 54-year-old woman: symmetrical, erythematous patch with minimal scaling. |
 |
Figure 3 Inverse psoriasis of the right axilla in an infant: minimal erythematosus patch. |
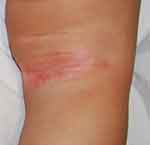 |
Figure 4 Inverse psoriasis of the right popliteal fossa in a 5-year-old child: moist, shiny, erythematous patches. |
In young infants, IP may often manifest as sharply demarcated, minimally elevated erythematous plaques in the diaper area, with a typical involvement of the inguinal folds (napkin psoriasis).5 Superficial erosions and maceration are frequently observed, resulting in intense itching, irritation from sweating, and soreness.10
Superinfection by bacteria and fungi (especially Candida spp) is frequent in IP as the moist skin provides an ideal environment for growth of microorganisms.11 On the other hand, colonization of flexural areas may also predispose to IP flares.1
Differential Diagnosis
Differential diagnosis of IP includes any erythematous rash involving the body folds, generically defined as “intertrigo”.12,13 This may be caused by simple mechanical friction, infectious disorders such as fungal and bacterial infections, and some inflammatory diseases.14 Diagnosis may be troublesome, especially in case of few/isolated lesions, and laboratory exams and/or histopathology are often required to reach a definitive diagnosis.
Intertrigo by mechanical friction is more frequent in overweight and obese subjects and/or in people who practice sports (e.g. runners and bikers) being induced by friction from skin-to-skin rubbing. Other trigger factors include humidity, hyperhidrosis, poor hygiene and use of wool and synthetic fibers. It is clinically characterized by erythema, maceration, erosions and fissurations.
Fungal infections due to both dermatophytes (i.e. tinea cruris) and Candida spp. are very common on skin folds and are clinically characterized by erythematous patches. The presence of raised, scaly, annular borders in tinea cruris and of peripheral satellite papules and/or pustules in candidiasis can help differentiate these conditions from IP. Direct and cultural mycological exams may address to the correct diagnosis, although often IP may coexist with these infections.
Bacterial infections may also be the cause of intertrigo that may simulate IP. Erythrasma, which is caused by Corynebacterium minutissimum, appears as red-brownish patches with well-defined edges. Examination with Wood’s light can help identify this form by showing a coral red fluorescence,11 and a positive bacterial culture is diagnostic. Other bacteria may often complicate mechanical intertrigo including Staphylococcus aureus, Streptococcus, Pseudomonas aeruginosa, Proteus mirabilis and Proteus vulgaris.
Seborrheic dermatitis of the folds appears as an erythematous eruption, often with erosions and fissures. The presence of yellowish, greasy scales can help differentiate seborrheic dermatitis from IP, although they may be absent.11 The simultaneous involvement of typical areas (face, scalp) may address to the correct diagnosis.
Lichen planus may involve the folds simulating IP and the presence of concomitant typical cutaneous and/or mucosal lesions or the clinical recognition of the Wickham striae may address to the diagnosis that should be confirmed by histopathology.
Irritant and allergic contact dermatitis are frequently encountered on the skin folds. They are mainly due to the contact with deodorants, perfumes, and coloured synthetic underwear, and are generally characterized by the presence of poor-defined erythematous patches ad vesicles. Allergic tests (patch tests) may be useful for the diagnosis.
Other skin disorders that may present intertriginous patches/plaques include atopic dermatitis, Darier disease, Hailey-Hailey disease, extramammary Paget’s disease, glucagonoma syndrome, acrodermatitis enteropathica and Langerhans cell histiocytosis.
Finally, in case of genital involvement, Zoon’s plasma cell mucositis and erythroplasia of Queyrat should be taken in consideration as differential diagnoses.15,16
Diagnosis
The diagnosis of IP [Figure 6] is usually clinical, and physical examination should include beyond the skin folds, the entire body surface including mucosae in order to check for other areas affected by psoriasis. The nails and joints should be examined for any change indicative of psoriasis, and a family history should be taken in consideration to further enhance the diagnosis.
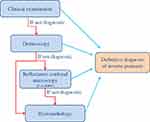 |
Figure 6 Diagnostic flow-chart of inverse psoriasis. |
In some cases, the diagnosis of IP may be difficult, especially when it appears as the only manifestation of the disease, thus requiring skin biopsy. At histopathology, IP presents the classical pattern of plaque psoriasis, consisting of epidermal hyperplasia and elongation of rete ridges, with acanthosis, parakeratosis, thinning of the suprapapillary plates, reduction of the granulosus layer and, in some cases, Munro microabcesses and Kogoj micropustules. Compared to classical plaque psoriasis, epidermal hyperplasia is less pronounced and spongiosis is more common.1
In the last years, dermoscopy has demonstrated to enhance the clinical diagnosis of psoriasis.17–19 In particular, low magnification (X10) dermoscopy shows the characteristic presence of whitish scales and “red dots” homogeneously distributed within the entire plaque on an erythematous background [Figure 5B]. At higher magnification (>X100), the red dots appear as typical “bushes” of convoluted capillaries [Figure 5C] that histopathologically correspond to the typical modification of microvascular architecture in psoriasis, i.e. dilated, elongated, and tortuous capillary loops in the papillary dermis.20–22 Dermoscopy may assist the diagnosis of psoriasis especially in unusual anatomic sites and/or presentation, such as palmo-plantar, genital, scalp and inverse psoriasis.16,23–27 In particular, a specific study demonstrated the usefulness of dermoscopy in the diagnosis in IP, in which the visualization of vascular structures is easy because of the absence of scales.26 Thirty patients with erythematous lesions localized to cutaneous body folds (axillary, inframammary, inguinal or intergluteal) were studied: 10 with concomitant typical plaque psoriasis and 20 with exclusive flexure involvement. High magnification dermoscopy showed the typical “bushy” capillaries in all cases of the first group, thus confirming the diagnosis of IP. In the second group of patients, the “bushy” capillary pattern was observed in 12 cases, suggesting the diagnosis of IP later confirmed by histological examination, while in the remaining 8 subjects, in which this pattern was not observed, other diagnoses (extramammary Paget’s disease, erythrasma, candidiasis and irritant dermatitis) were demonstrated by histological examination and/or other laboratory tests (mycological and bacteriological examinations, patch test, Wood lamp).
Dermoscopy is also useful for the diagnosis of genital involvement, both in males and females, by showing the same vascular pattern.28,29 In an open study on 12 subjects with different types of balanitis, only patients with biopsy-proven psoriatic balanitis showed the “bushy” capillaries, while in the remaining cases (2 lichen planus, 1 lichen sclerosus, 1 Zoon’s balanitis, and 2 candidiasis) a not specific capillary pattern was evident.24
Another non-invasive technique that has been reported to improve the diagnosis of genital psoriasis is in vivo reflectance confocal microscopy (RCM), which is currently used in the evaluation of melanocytic lesions, skin tumors, and some inflammatory and infectious diseases.30,31 In a study on 10 patients with biopsy-proven psoriatic balanitis, handheld RCM revealed a peculiar pattern, consisting of enlarged, irregular, blurred and dark (“non-rimmed”) dermal papillae filled with dilated blood vessels and separated by thin interpapillary rete ridges.29 This pattern correlates with the “red dots”/“bushy capillaries” observed at dermoscopy.22,29 RCM may be useful for the differential diagnosis with other inflammatory genital disorders, although it is currently available only in selected academic centers due to the high cost.29
Treatment
Treatment of IP [Table 1] significantly differs from that of plaque psoriasis due to the characteristics of the affected area, in which the skin is generally thin and more prone to local side effects. In addition, it is important to consider the skin to skin contact that may result in an occlusive effect.
 |
Table 1 Therapeutic Options for Inverse Psoriasis |
Topical corticosteroids and topical immunomodulators represent the most used treatments for IP. However, the evidence of the efficacy and safety of both topical and systemic treatments for IP is generally low, as shown by a recent systematic review.6
According to the guidelines of the Medical board of the National Psoriasis Foundation, the first-line therapy for IP should be represented by low- to mid-potency topical corticosteroids.32 This recommendation is supported by a randomized trial on 80 adult patients with IP in which betamethasone 0.1% applied for 4 weeks determined an 86% reduction of modified PASI score. Moreover, 78% of patients reported reduction in pruritus.33 In another open-label study on 20 adult patients, 0.005% fluticasone propionate ointment applied for two weeks resulted in an improvement of the physician’s global evaluation of disease status of target lesions greater than 75% in 85% of cases.34 However, corticosteroids are recommended only for a short-term therapy (<4 weeks) in order to avoid the well-known adverse effects, such as atrophy, telangiectasia, striae, absorption with systemic side effects and tachyphylaxis.32,33,35 As psoriasis starts improving, they may be gradually decreased.
Options for a long-term topical therapy (>4 weeks) may include topical calcineurin inhibitors (TCI), such as tacrolimus or pimecrolimus, and vitamin D analogs, as supported by different studies.32,36–43
In particular, TCI are considered as first-line choices for IP due to the lack of significant side effects, although they may be less effective than topical corticosteroids. Their use in psoriasis, however, is off-label. A multi-center randomized controlled trial of tacrolimus versus placebo on 167 patients 16 years or older with facial or intertriginous psoriasis, resulted after 8 weeks in “excellent” clinical improvement (defined as >90% using the Physician’s Global Assessment) in 66.7% of subjects treated with tacrolimus 0.1% ointment compared to 36.8% of patients treated with vehicle.37 On the other hand, in a double-blind study on 57 adult patients with moderate to severe IP, pimecrolimus cream 1% applied twice daily was safe and effective in 71% of patients showing “clear” or “almost clear” status after 8 weeks of treatment compared to 21% in the vehicle cream group, with a very low incidence of adverse events reported and no atrophy.38
As regards vitamin D analogs, 0.005% calcipotriol ointment determined a 62% improvement in the modified PASI score in adult patients with IP.33 In another open-label study on 12 adult patients, 0.005% calcipotriol ointment applied twice daily for 6 weeks determined a significant improvement, with modified PASI score reduction from 31.7 to 6.2.44 However, 42% of patients presented lesional or perilesional skin irritation.44 In a randomized intra-individual comparison on 75 adult subjects between the twice-daily application of 3 μg/g calcitriol ointment versus 50 μg/g calcipotriol ointment on matched right-left lesions study, calcitriol was found to be better tolerated and more effective in the treatment of psoriasis in flexural areas: 20 of 30 (67%) subjects with calcitriol and only 10 (33%) subjects with calcipotriol were clear or almost clear.42
Second-line therapy for IP includes emollients and topical tar-based products.32 Emollients are well tolerated, and they may be used in association with topical corticosteroids. Tar-based products might be potentially irritant, so they are less frequently used. As bacterial and/or fungal superinfections are frequently encountered in IP, antimicrobial therapy may be useful for the potential role of these micro-organisms in producing inflammatory reactions and flares,45–47 although the evidence is low. The Medical board of the National Psoriasis Foundation suggests the use of topical antibacteric and/or antifungal agents in combination with a low- to mid-potency topical corticosteroid or calcipotriol. Few studies also suggest the use of antiseptics such as 0.5% chlorhexidine, 2% eosin and 0.3% chloroxylenol.48,49
The evidence regarding the efficacy of systemic treatments for IP (including traditional oral systemic therapies such as cyclosporine and methotrexate, biologics, PDE4 inhibitors and dimethyl fumarate) is very low, mainly because they are indicated for moderate-to-severe plaque psoriasis and have not specifically investigated in isolated IP.6 Some experience may arise from the treatment of psoriasis of the anogenital area with biologics. In particular, a patient with psoriatic arthritis and a significant involvement of genitalia and intergluteal folds experienced an almost complete regression of the genital lesions after 90 days of treatment with adalimumab.50 In another patient with psoriatic lesions involving groin, gluteal cleft, shaft and glans penis, as well as other body areas, a significant improvement was recorded after 4 injections of ustekinumab.51 Recently, a randomized, double-blinded, placebo-controlled phase IIIb study reported the efficacy of ixekizumab in the treatment of moderate-to-severe genital psoriasis, with 73% of patients achieving clear or almost clear genital skin at week 12.52 Although these few reports do not give sufficient data to support the efficacy of biological treatment in IP, their use could be recommended in recalcitrant, moderate-to-severe cases. Overall, traditional systemic treatments and biologics could be beneficial for severe IP resistant to topical treatment and/or widespread psoriasis with flexural/genital involvement.6
Other treatments anecdotally reported for IP resistant to traditional treatments include excimer laser, that has shown efficacy in some cases with minimal side effects,53 and botulin toxin injections that determined some improvement probably through preventing perspiration and the consequent skin maceration and secondary infections.54 Finally, topical dithranol, retinoids and salicylic acid might be used as third-line therapy, because they are generally irritant.32
Conclusions
Inverse psoriasis is considered a special site of involvement of psoriasis rather than a separate disease.1 It is sometimes difficult to diagnose due to its clinical similarity with other skin disorders involving the folds. Dermoscopy represents a useful tool for an enhanced non-invasive diagnosis. The treatment may be challenging as intertriginous areas are more prone to side effects induced by traditional topical therapies. According to a consensus and a recent systematic review, alternating TCI or a vitamin D analog with short-term topical corticosteroids may represent the best strategy.6
Disclosure
Maria Letizia Musumeci reports personal fees from Abbvie, personal fees from Biogen, personal fees from Janssen Cilag, personal fees from Eli Lilly, personal fees from Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Omland SH, Gniadecki R. Psoriasis inversa: a separate identity or a variant of psoriasis vulgaris? Clin Dermatol. 2015;33(4):456–461. doi:10.1016/j.clindermatol.2015.04.007
2. Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12(2):143–146. doi:10.2165/11532060-000000000-00000
3. Fan X, Yang S, Sun LD, et al. Comparison of clinical features of HLA-Cw*0602-positive and negative psoriasis patients in a Han Chinese population. Acta Derm Venereol. 2007;87:335–340. doi:10.2340/00015555-0253
4. Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;3(Suppl 19):2–6. doi:10.1111/j.1468-3083.2005.01329.x
5. Bronckers IM, Paller AS, van Geel MJ, van de Kerkhof PC, Seyger MM. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):373–384. doi:10.1007/s40272-015-0137-1
6. Reynolds KA, Pithadia DJ, Lee EB, Wu JJ. Treatments for inverse psoriasis: a systematic review. J Dermatolog Treat. 2019;1–23. doi:10.1080/09546634.2019.1620912
7. Cohen JM, Halim K, Joyce CJ, Patel M, Qureshi AA, Merola JF. Shedding light on the “hidden psoriasis”: a pilot study of the Inverse Psoriasis Burden of Disease (IPBOD) questionnaire. J Drugs Dermatol. 2016;15(8):1011–1016.
8. Zampetti A, Tiberi S. Inverse psoriasis. Clin Med (Lond). 2015;15(3):311. doi:10.7861/clinmedicine.15-3-311
9. Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet Infect Dis. 2010;10:470–478. doi:10.1016/S1473-3099(10)70101-8
10. Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589. doi:10.1111/dth.2018.31.issue-3
11. Wilmer EN, Hatch RL. Resistant “candidal intertrigo”: could inverse psoriasis be the true culprit? J Am Board Fam Med. 2013;26(2):211–214. doi:10.3122/jabfm.2013.02.120210
12. Weisenseel P, Reich K. Psoriasis inversa. Hautarzt. 2015;6:408–412. doi:10.1007/s00105-015-3628-7
13. Janniger CK, Schwartz RA, Szepietowski JC, Reich A. Intertrigo and common secondary skin infections. Am Fam Physician. 2005;72(5):833–838.
14. Brandon A, Mufti A, Gary Sibbald R. Diagnosis and management of cutaneous psoriasis: a review. Adv Skin Wound Care. 2019;32(2):58–69. doi:10.1097/01.ASW.0000550592.08674.43
15. Errichetti E, Lacarrubba F, Micali G, Stinco G. Dermoscopy of zoon’s plasma cell balanitis. J Eur Acad Dermatol Venereol. 2016;30(12):e209–e210. doi:10.1111/jdv.13538
16. Errichetti E, Lallas A, Di Stefani A, et al. Accuracy of dermoscopy in distinguishing erythroplasia of queyrat from common forms of chronic balanitis: results from a multicentric observational study. J Eur Acad Dermatol Venereol. 2019;33(5):966–972. doi:10.1111/jdv.2019.33.issue-5
17. Micali G, Lacarrubba F, Massimino D, Schwartz RA. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64(6):1135–1146. doi:10.1016/j.jaad.2010.03.010
18. Lacarrubba F, Ardigò M, Di Stefani A, Verzì AE, Micali G. Dermatoscopy and reflectance confocal microscopy correlations in nonmelanocytic disorders. Dermatol Clin. 2018;36(4):487–501. doi:10.1016/j.det.2018.05.015
19. Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79(6):1117–1132.e1. doi:10.1016/j.jaad.2018.06.021
20. Micali G, Lacarrubba F, Musumeci ML, Massimino D, Nasca MR. Cutaneous vascular patterns in psoriasis. Int J Dermatol. 2010;49(3):249–256. doi:10.1111/ijd.2010.49.issue-3
21. Lacarrubba F, Musumeci ML, Ferraro S, Stinco G, Verzì AE, Micali G. A three-cohort comparison with videodermatoscopic evidence of the distinct homogeneous bushy capillary microvascular pattern in psoriasis vs atopic dermatitis and contact dermatitis. J Eur Acad Dermatol Venereol. 2016;30(4):701–703. doi:10.1111/jdv.12998
22. Nasca MR, Lacarrubba F, Caltabiano R, Micali G. Image gallery: reproduction of the auspitz sign by videodermatoscopy, confocal microscopy and horizontal histopathology. Br J Dermatol. 2019;180(6):e178. doi:10.1111/bjd.2019.180.issue-6
23. Micali G, Nardone B, Scuderi A, Lacarrubba F. Videodermatoscopy enhances the diagnostic capability of palmar and/or plantar psoriasis. Am J Clin Dermatol. 2008;9(2):119–122. doi:10.2165/00128071-200809020-00005
24. Lacarrubba F, Nasca MR, Micali G. Videodermatoscopy enhances diagnostic capability in psoriatic balanitis. J Am Acad Dermatol. 2009;61(6):1084–1086. doi:10.1016/j.jaad.2009.04.012
25. Musumeci ML, Lacarrubba F, Verzì AE, Micali G. Evaluation of the vascular pattern in psoriatic plaques in children using videodermatoscopy: an open comparative study. Pediatr Dermatol. 2014;31(5):570–574. doi:10.1111/pde.2014.31.issue-5
26. Musumeci ML, Lacarrubba F, Catalfo P, Scilletta B, Micali G. Videodermatoscopy evaluation of the distinct vascular pattern of psoriasis improves diagnostic capability for inverse psoriasis. G Ital Dermatol Venereol. 2017;152(1):88–90. doi:10.23736/S0392-0488.16.05212-3
27. Rosina P, Zamperetti MR, Giovannini A, Girolomoni G. Videocapillaroscopy in the differential diagnosis between psoriasis and seborrheic dermatitis of the scalp. Dermatology. 2007;214(1):21–24. doi:10.1159/000096908
28. Borghi A, Virgili A, Corazza M. Dermoscopy of inflammatory genital diseases: practical insights. Dermatol Clin. 2018;36(4):451–461. doi:10.1016/j.det.2018.05.013
29. Lacarrubba F, Verzì AE, Ardigò M, Micali G. Handheld reflectance confocal microscopy, dermatoscopy and histopathological correlation of common inflammatory balanitis. Skin Res Technol. 2018;24(3):499–503. doi:10.1111/srt.2018.24.issue-3
30. Lacarrubba F, Pellacani G, Gurgone S, Verzì AE, Micali G. Advances in non-invasive techniques as aids to the diagnosis and monitoring of therapeutic response in plaque psoriasis: a review. Int J Dermatol. 2015;54(6):626–634. doi:10.1111/ijd.2015.54.issue-6
31. Verzì AE, Lacarrubba F, Caltabiano R, Broggi G, Musumeci ML, Micali G. Reflectance confocal microscopy features of plaque psoriasis overlap with horizontal histopathological sections: a case series. Am J Dermatopathol. 2019;41(5):355–357. doi:10.1097/DAD.0000000000001297
32. Khosravi H, Siegel MP, Van Voorhees AS, Merola JF. Treatment of inverse/intertriginous psoriasis: updated guidelines from the medical board of the national psoriasis foundation. J Drugs Dermatol. 2017;16(8):760–766.
33. Kreuter A, Sommer A, Hyun J, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: a double-blind, randomized controlled study. Arch Dermatol. 2006;142(9):1138–1143. doi:10.1001/archderm.142.9.1138
34. Lebwohl MG, Tan MH, Meador SL, Singer G. Limited application of fluticasone propionate ointment, 0.005% in patients with psoriasis of the face and intertriginous areas. J Am Acad Dermatol. 2001;44(1):77–82. doi:10.1067/mjd.2001.110046
35. Kalb RE, Bagel J, Korman NJ, et al., National Psoriasis Foundation. Treatment of intertriginous psoriasis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2009;60(1):120–124. doi:10.1016/j.jaad.2008.06.041
36. Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153–163. doi:10.2147/PTT.S101233
37. Lebwohl M, Freeman AK, Chapman MS, Feldman SR, Hartle JE, Henning A. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51(5):723–730. doi:10.1016/j.jaad.2004.07.011
38. Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51(5):731–738. doi:10.1016/j.jaad.2004.06.010
39. Freeman AK, Linowski GJ, Brady C, et al. Tacrolimus ointment for the treatment of psoriasis on the face and intertriginous areas. J Am Acad Dermatol. 2003;48:564–568. doi:10.1067/mjd.2003.169
40. Ezquerra GM, Regana MS, Acosta EH, Millet PU. Topical tacrolimus for the treatment of psoriasis on the face, genitalia, intertriginous areas and corporal plaques. J Drugs Dermatol. 2006;5:334–343.
41. Brune A, Miller DW, Lin P, Cotrim-Russi D, Paller AS. Tacrolimus ointment is effective for psoriasis on the face and intertriginous areas in pediatric patients. Pediatr Dermatol. 2007;24:76–80. doi:10.1111/j.1525-1470.2007.00341.x
42. Ortonne JP, Humbert P, Nicolas JF, et al. Intra-individual comparison of the cutaneous safety and efficacy of calcitriol 3 ug g-1 ointment and calcipotriol 50 ug g-1 ointment on chronic plaque psoriasis localized in facial, hairline, retroauricular or flexural areas. Br J Dermatol. 2003;148(2):326–333. doi:10.1046/j.1365-2133.2003.05228.x
43. Dattola A, Silvestri M, Bennardo L, et al. Update of calcineurin inhibitors to treat inverse psoriasis: a systematic review. Dermatol Ther. 2018;31(6):e12728. doi:10.1111/dth.v31.6
44. Kienbaum S, Lehmann P, Ruzicka T. Topical calcipotriol in the treatment of intertriginous psoriasis. Br J Dermatol. 1996;135(4):647–650. doi:10.1111/j.1365-2133.1996.tb03851.x
45. Noah PW. The role of microorganisms in psoriasis. Semin Dermatol. 1990;9(4):269–276.
46. Rosenberg EW, Noah PW, Skinner RB
47. Flytström I, Bergbrant IM, Bråred J, Brandberg LL. Microorganisms in intertriginous psoriasis: no evidence of candida. Acta Derm Venereol. 2003;83(2):121–123. doi:10.1080/00015550310007463
48. Arnold WP, Van Der Meer YG. Does adding of chlorhexidine 0.5% to topical corticosteroids enhance efficacy in the treatment of atopic dermatitis and inverse psoriasis? Results of a double-blind study. Ned Tijdschr Dermatol Venereol. 2005;15(8):340–342.
49. Leigheb G, Gallus D, Spano G. Treatment of psoriasis with an antiseptic combination, in a double-blind comparison versus eosin. G Ital Dermatol Venereol. 2000;135(1):
50. Ješe R, Perdan-Pirkmajer K, Dolenc-Voljč M, Tomšič M. A case of inverse psoriasis successfully treated with adalimumab. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23(1):21–23.
51. Campos MA, Varela P, Baptista A, Moreira AI. Inverse psoriasis treated with ustekinumab. BMJ Case Rep. 2016;2016. doi:10.1136/bcr-2015-212769
52. Ryan C, Menter A, Guenther L, et al.; IXORA-Q Study Group. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179(4):844–852. doi:10.1111/bjd.2018.179.issue-4
53. Mafong EA, Friedman PM, Kauvar AN, Bernstein LJ, Alexiades-Armenakas M, Geronemus RG. Treatment of inverse psoriasis with the 308 nm excimer laser. Dermatol Surg. 2002;28(6):530–532. doi:10.1046/j.1524-4725.2002.12005.x
54. Zanchi M, Favot F, Bizzarini M, Piai M, Donini M, Sedona P. Botulinum toxin type-A for the treatment of inverse psoriasis. J Eur Acad Dermatol Venereol. 2008;22(4):431–436. doi:10.1111/j.1468-3083.2007.02457.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.