Back to Journals » Clinical Interventions in Aging » Volume 16
Intraoperative Oxygen Concentration and Postoperative Delirium After Laparoscopic Gastric and Colorectal Malignancies Surgery: A Randomized, Double-Blind, Controlled Trial
Authors Lin X, Wang P, Liu DW, Guo YW, Xie CH , Wang B, Dong R, Sun LX, Wang MS, Bi YL
Received 15 March 2021
Accepted for publication 3 June 2021
Published 15 June 2021 Volume 2021:16 Pages 1085—1093
DOI https://doi.org/10.2147/CIA.S311190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Xu Lin,1,* Pei Wang,1 Ding-Wei Liu,2 Yu-Wei Guo,1 Chun-Hui Xie,3 Bin Wang,1,* Rui Dong,4 Li-Xin Sun,1 Ming-Shan Wang,1 Yan-Lin Bi1
1Department of Anesthesiology, Qingdao Municipal Hospital Affiliated to Qingdao University, Qingdao, People’s Republic of China; 2Department of Laboratory, Qingdao Municipal Hospital Affiliated to Qingdao University, Qingdao, People’s Republic of China; 3Department of Anesthesiology, Weifang Medical University, Weifang, People’s Republic of China; 4Department of Anesthesiology, Drum Tower Hospital Affiliated to Nanjing University Medical School, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan-Lin Bi; Ming-Shan Wang
Department of Anesthesiology, Qingdao Municipal Hospital Affiliated to Qingdao University, Qingdao, People’s Republic of China
Tel +86 186 6167 5610
; +86 186 61675608
Email [email protected]; [email protected]
Purpose: Postoperative delirium (POD) is common in elderly patients undergoing laparoscopic surgery for gastric and colorectal malignancies. POD may be affected by different fraction of inspired oxygen (FiO2). The purpose of this study was to compare the effects of different FiO2 on POD.
Patients and Methods: A randomized, double-blind controlled trial was performed in Qingdao Municipal Hospital Affiliated to Qingdao University. A total of 662 patients aged 65 to 85 years old underwent isolated laparoscopic radical gastrectomy, radical resection of colon cancer, or radical resection of rectal cancer only. A random number table method was used to divide the patients into two groups: 40% FiO2 (group A) and 80% FiO2 (group B). The primary endpoint was the incidence of POD, which was assessed by the Confusion Assessment Method (CAM) twice daily during the first 7 postoperative days, and POD severity was measured by the Memorial Delirium Assessment Scale (MDAS). The secondary endpoints were the intraoperative regional cerebral oxygen saturation (rSO2), Bispectral (BIS) index, invasive arterial blood pressure (IABP), oxygen saturation (SpO2), end-tidal carbon dioxide partial pressure (PETCO2), the number of atelectasis cases and visual analogue scale (VAS) scores on days 1– 7 after surgery.
Results: The incidence of POD was 19.37% (122/630), including 20.38% (64/314) in group A and 18.35% (58/316) in group B. No statistical significance was found in the incidence of POD between the two groups (P > 0.05); compared with group B, SpO2, rSO2 and PaO2 decreased at T2 to T4 time point (P < 0.01), and the incidence of postoperative atelectasis decreased (P < 0.05) in group A.
Conclusion: The incidence of POD was not significantly affected by different FiO2 and the incidence of postoperative atelectasis was decreased at low FiO2.
Keywords: intraoperative oxygen concentration, postoperative delirium, malignancy
Introduction
Postoperative delirium (POD), as an acute central nervous system dysfunction, has a high incidence of up to 50% during laparoscopic abdominal surgery.1 The occurrence of POD is associated with worse outcomes, including prolonged length of hospital stay, increased morbidity and mortality, impaired long-term cognitive function and physical ability, and increased medical care costs.2–5
The causes of POD are multifactorial, including predisposing and precipitating factors.6 The study has shown that intraoperative hypoxia is an important factor for postoperative cognitive dysfunction,7 while the hyperoxia does not increase neurological complications.8 It is well known that perioperative hypoxia or hyperoxia can be regulated by the fraction of inspired oxygen (FiO2), which is the main factor affecting regional cerebral oxygen saturation (rSO2), and has a high correlation with rSO2.9 Moreover, the rSO2 is a non-invasive method for assessing the adequacy of the oxygen supply-demand balance in the fronto parietal brain areas at any position during surgery.10 The rSO2 value is a very sensitive indicator of cerebral hypoxia, which can cause cerebral hypoperfusion, resulting in POD.11,12 At present, there is no unified standards for the optimal FiO2 in operation, which is 30% ~ 100% in clinical operation. However, for elderly patients undergoing laparoscopic abdominal surgery, no unified conclusion has been reached on the effects of different FiO2 on POD.
In this study, FiO2 was controlled at 40% and 80%, to compare the incidence of POD after laparoscopic abdominal surgery in two groups of elderly patients. The primary outcome was the incidence of POD every 1–7 days postoperatively. We hypothesized that 80% FiO2 would reduce the incidence of POD, which is associated with increased rSO2.
Patients and Methods
Participants
Patients, in this study, who underwent laparoscopic surgery for gastric and colorectal malignancies had an American Society of Anesthesiologists (ASA) physical status I, II or III and a New York Heart Association Functional Classification (NYHA) class I or II. Their age was between 65 and 85 at the time of the study. Those who were excluded from this study met any of the following criteria: preoperative Mini Mental State Scale (MMSE) scores of 23 or less; history of severe mental or nervous system; history of drug or psychotropic substance abuse, long-term use of steroids and steroids; preoperative complicated with III or IV hepatic encephalopathy; severe visual and hearing impairment; preoperative asthma, chronic obstructive pulmonary disease, or pulmonary function test showing moderate or severe ventilation dysfunction; low hemoglobin (HB < 100g/L) and hypoproteinemia; operation time <2 h or >5 h; preoperative radiotherapy or chemotherapy; intraoperative blood loss >200 mL; patients were admitted to intensive care unit after operation with blood oxygen saturation (SpO2) <95% (Based on the results of our preliminary trial) or anesthesia complications occurred. Patients in this study were randomly divided into two groups according to the level of FiO2: Group A with 40% FiO2 and Group B with FiO2 80%.
Ethics Approval and Consent to Participate
This prospective, randomized, double-blind controlled trial was performed from February 2018 to February 2020 in accordance with the Declaration of Helsinki principles. This study was approved by the Ethics Committee for Clinical Trials of the Qingdao Municipal Hospital Affiliated to Qingdao University, China [approval no: 2018 PRO FORMA Y number 003]. The trial was registered in the Chinese Clinical Trial Registry prior to patient enrollment (ChiCTR1800014972). All patients submitted written informed consent.
Management of General Anesthesia and Analgesia
None of the enrolled patients received any sedative or analgesic treatment prior to induction of anesthesia. Routine venous access was established after patients entered the operating room. Vital parameters, including oxygen saturation (SpO2), invasive radial arterial pressure, non-invasive arterial blood pressure (NABP), heart rate (HR), electrocardiogram (ECG), and end-tidal carbon dioxide partial pressure (PETCO2), were monitored using a Drager monitor (model: Primus, Qingdao unity medical co.). Two near-infrared spectral sensors spectroscopy (model: EGOS-600B, Jiangsu Aiqin Bio-Medical Electronics Co.) were pasted on the left and right sides of each patient’s forehead for measuring the rSO2 value. The rSO2 baseline data were collected before induction of anesthetic while patients breathing room air. For the BIS monitor (the Germany, Philips, M1034A Co.), a disposable BIS sensor was applied to the patient’s forehead after the skin was wiped with an alcohol swab. Provide 100% oxygen to the mask before intubation. Sufentanil 0.5 μg·kg−1 and etomidate 0.2 mg·kg−1 were used for induction of general anesthesia, and cisatracurium 0.2 mg·kg−1 was used for muscle relaxation. For tracheal intubation, the mechanical ventilation was set to the target of PETCO2 in the 35 to 45 mmHg range and SpO2 ≥ 95%. Radial artery and central venous catheterization were performed to monitor invasive arterial pressure and central venous pressure (CVP). Maintaining perioperative blood pressure and HR at ± 20% of baseline value is often the ideal control goal. The two groups of FiO2 were set to 40% and 80%, respectively. Anesthesia was kept with propofol (6–8 mg·kg−1·h−1), remifentanil (0.1–0.3 μg·kg−1·min−1), sevoflurane (1–2%), and cisatracurium (0.1–0.2 mg·kg−1·h−1). The (Bispectral) BIS index was held in the 40 to 60 range. The patient’s body temperature, monitored by an ear thermometer (Jiangsu, Jiruida, Xingsheng co.), was maintained at 36.0 °C and 37.0 °C. Cisatracurium besylate infusion and sevoflurane inhalation were terminated about 40 minutes and 20 minutes before the end of surgery, respectively. Oxycodone was administered with 10 mg to provide the analgesia postoperatively, meanwhile propofol and remifentanil were discontinued. However, glucocorticoid drugs, dexmedetomidine nonsteroidal analgesics, and midazolam were avoided during surgery. Early recovery was managed in a post-anesthesia care unit (PACU) after surgery, while patients were returned to the ward and headed by an anesthesiologist, with a Steward resuscitation score of above 6.13 All patients were treated with patient-controlled intravenous analgesia (PCIA) for 48 hours after surgery. The PCIA opioid composed of 2.5 μg·kg−1 sufentanil and 5 mg tropisetron (total volume of 100 mL, including 0.9% normal saline, bolus 2 mL, basal rate 2 mL/h, and lockout time 15 mins). Pulmonary ultrasound (the Germany, SIEMENS, ACUSON SC2000) was performed 24 hours after surgery to assess and record the incidence of atelectasis.
Outcome Measures
The primary endpoint was the incidence of POD on every 7 days postoperatively, which was evaluated twice daily at 8 a.m. and 2 p.m. by an anesthesiologist who was blinded to group assignment. POD was defined by the Confusion Assessment Method (CAM), and the severity of POD was measured by the Memorial Delirium Assessment Scale (MDAS).14,15 The CAM and MDAS in the Chinese research have been proven to have high reliability and validity in the Chinese elderly population.16,17 In addition to the baseline, mean arterial pressure (MAP), rSO2, BIS, HR, SpO2 and arterial blood gas (PH value, PaO2, PaCO2) were also recorded at the following endpoints, which were continuously measured and recorded before anesthesia induction (T1); (T2) after 45 minutes; (T3) after 90 minutes; 10 min before the end of the operation (T4). Additionally, preoperative hemoglobin, operation time, anesthetic time, type of surgery, estimated blood loss, postoperative the highest MDAS and visual analogue scale (VAS) score, length of PACU stay,18 length of hospital stays, and 6 months postoperative mortality were recorded.
Randomization and Blinding
A study statistician at the leading center generated random numbers without restriction (simple randomization) by a computerized system. The random numbers were sealed in sequentially numbered envelopes and sent to a research coordinator of our research team the day before surgery by a research nurse. The coordinator communicated the group assignment to an anesthesiologist, and upon consent, assigned participants to study groups according to the random numbers. An allocated random number was used to perform block randomization in a 301:301 ratio. The anesthesiologist was not blind to trials, who should know the FiO2 target for each assigned participant and make proper adjustment to achieve the target FiO2 level. The outcome assessment and statistical analyses were conducted by researchers independently. Outcome assessors and the surgical team were blind to the distribution of participants in the study.
Sample Size Estimation
In this study, PASS 11.0 (NCSS, LLC. Kaysville, Utah, USA) software was used to estimate the required sample size, with a sensitivity of 0.9, a sensitivity tolerance of 0.05, a specificity tolerance of 0.05, α = 0.05, 1–β = 0.8, a bilateral, and a 10% dropout rate. The sample size was 662.
Statistical Analyses
SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Shapiro–Wilk test was used to assess the normality of continuous data. Normally distributed continuous data were presented as mean ± standard deviation, and abnormal distributions were expressed as median (25–75% percentile). Categorical variables were expressed by numbers (%), and tested by χ2 test. Differences in continuous variables were measured by independent sample t-test or Kruskal–Wallis rank-sum test as appropriate, and the intra-group comparisons were performed by repeated measurement design. P < 0.05 was statistically significant.
Results
Participants’ Demographic Characteristics
The present study enrolled 662 participants, including 10 people refused to participate. Six hundred and fifty-two participants were randomly divided into two groups: 40% FiO2 maintenance (group A), 80% FiO2 maintenance (group B). Twenty-two participants were excluded. The criteria are shown in Figure 1. Finally, 314 participants in group A and 316 participants in group B were included in the analysis. All patients underwent isolated laparoscopic radical gastrectomy, radical resection of colon cancer or radical resection of rectal cancer only.
 |
Figure 1 Flow chart of the trial. Abbreviations: FiO2, fraction of inspired oxygen; SpO2, oxygen saturation; h, hour. |
Participants’ Characteristics and Operative Data
No significant differences in gender, age, years of education, BMI, dependence on smoking, alcohol abuse, types of surgery, comorbidity, ASA class, preoperative Hb, time of anesthesia, time of surgery, BIS value, estimated blood loss, postoperative the highest VAS score, length of PACU stay, length of stay in hospital and mortality in 6 months were observed among two groups in Table 1.
 |
Table 1 Characteristics of Participants |
Primary Outcomes
The incidence of POD in the Group B was 18.35% (58/316) compared to 20.38% (64/314) in Group A (P = 0.402) in Table 2.
 |
Table 2 Postoperative Characteristics of Participants |
Secondary Outcomes
The incidence of postoperative atelectasis in Group A (19.11%) was lower than that in Group B (41.77%) (P = 0.026). However, the incidence of adverse events including wound infection, cerebral hemorrhage and cerebral infarction, myocardial infarction, pulmonary embolism, pneumonia and reoperation was not statistically significantly different between two groups in Table 2.
Oxygen Administration and Protocol Adherence
The included participants were subjected to the following protocol immediately after intubation. FiO2 was maintained 40% (group A) and 80% (group B) by a Drager monitor (model: Primus, Qingdao unity medical co.). There was no statistical significance of PaO2, SpO2 and rSO2 at T1 time point in the two groups included, so we primarily analyzed at T2-4 time points. Thus, compared with Group B, PaO2, SpO2 and rSO2 of Group A at T2-4 time points decreased significantly. The difference was statistically significant (P < 0.01) in Figure 2A–C.
Preoperative MMSE Score and Postoperative MDAS Score
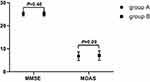
The preoperative MMSE score (25.16±1.02) in the Group B compared to (25.10±0.93) in Group A (P = 0.40); postoperative MDAS score (7.03±2.08) in the Group B compared to (6.75±1.86) in Group A (P = 0.09) in Figure 3.
Discussion
This prospective, randomized, double-blind controlled trial assessed the effects of different FiO2 on POD, and measured CAM and MDAS scores in elderly patients undergoing laparoscopic surgery for isolated gastric and colorectal malignancies. In this study, the incidence of POD in Group B was 18.35% compared to 20.38% in Group A (P = 0.402). At the same time, the POD and its severity mainly diagnosed on the first and second days postoperatively by CAM and MDAS scores, which was the same as the previous study.19 Different FiO2 had no significant effect on the incidence of POD in 1 to 7 days postoperative between two groups. Although low FiO2 did not impact the length of PACU and hospital stay, nor patient mortality, it indeed reduced the incidence of postoperative atelectasis. All the above results benefited from the strict exclusion criteria in this study, such as anemia, hypoalbuminemia, massive intraoperative bleeding and prolonged surgery, which could have influence on the development of POD.2
Currently, the optimal interoperative FiO2 is still controversial. “Miller Anesthesiology” points out that whether spontaneous inhalation or mechanical ventilation following muscle relaxation, anesthesia would lead to lung function damage, because most of the blood oxygenation function of the subjects after anesthesia has been damaged. Therefore, it is recommended to add air to the inhaled gas to maintain the FiO2 at 30% to 40%. The oxygen-departure curve shows that when SpO2 is 99% to 100%, PaO2 is about 160 mmHg by. It is well known that the oxygenation index of normal lung is 400 to 500, while the oxygenation index is equal to PaO2/FiO2, so the FiO2 is 32% to 40%. Although the evidence regarding the exact association between lower FiO2 and clinically outcomes intraoperatively is weak, a FiO2 of ≤0.4 was recommended in the latest consensus.20 However, the included patients were treated with FiO2 32% to 40% and SpO2 nearly less than 95%, while the use of FiO2 40% improved SpO2 in the preliminary trial. In the meantime, World Health Organization (Geneva, Switzerland) recommended that FiO2 should be less than or equal to 80% in patients with tracheal intubation under general anesthesia to reduce complications such as postoperative atelectasis.21 Therefore, 40% and 80% of FiO2 were selected for this study.
Nevertheless, the results of this study did not clarify the optimal FiO2 for POD patients undergoing isolated laparoscopic radical gastrectomy, radical resection of colon cancer or radical resection of rectal cancer only. Other randomized controlled trials also demonstrated that the titration of intraoperative oxygenation resulted in no significant differences in postoperative cognition after cardiac surgery,22 potentially because both high and low levels of FiO2 can be monitored with rSO2 to ensure changes in cerebral oxygen supply and consumption. As we known, rSO2 is the weighted average of the regional cerebral oxygenation, and near-infrared spectroscopy is the only way to continuously and noninvasively monitor rSO2.23 Additionally, FiO2 is the main factor affecting rSO2, which has a high correlation with FiO2.9 In this study, compared with high FiO2, rSO2 and PaO2 decreased at low FiO2; however, SpO2 remained at 95% to 100% intraoperatively, so low FiO2 could meet the balance of oxygen supply and consumption in the brain during operation. And yet, atelectasis occurred more frequently, which might be related to the damage of lung function caused by high FiO2.24
The results of this study show that different levels of FiO2 lead to different rSO2, but there is no significant difference in the incidence of POD. Although previous studies have shown that decreased intraoperative rSO2 is associated with an increased incidence of POD,25 the latest research results do not support the correlation between the intraoperative reduction of rSO2 and the incidence of POD.26
At present, the relationship between the optimal perioperative FiO2 and POD is still under discussion. The lack of a consensus definition of hypoxia and hyperoxia makes clinical work and studies difficult to carry out effectively. Nowadays, both of the World Health Organization (Geneva, Switzerland) and the Centers for Disease Control and Prevention (Atlanta, Georgia) recommend high FiO2 to reduce surgical site infection.20,27 These recommendations are controversial because of the potential damage of hyperoxia, such as postoperative atelectasis.28–30 Our results confirm that low levels of FiO2 can reduce the incidence of postoperative atelectasis. In our study, low FiO2 may still provide adequate tissue oxygenation during the perioperative surgery without affecting the incidence of POD and survival after six months, even in patients with malignant tumors included. In fact, apart from postoperative atelectasis, our study did not find any significant differences in postoperative complications with low levels of FiO2, although these results must be interpreted carefully to support the safety of low FiO2. Therefore, it should be continuously concerned about the relationship between intraoperative low FiO2 and POD, and their severity scores and postoperative survival rate in patients with malignant tumors, so as to improve the quality of life of these patients.
This study has several limitations. First, since it is a single-center study, more multi-center studies are needed to confirm the results of this study. Second, only 40% and 80% of FiO2 were studied, and our team will continue to study other levels of FiO2 to further explore the optimal interoperative FiO2 associated with perioperative neurocognitive disorder. Third, we did not assess neurocognitive function for the follow up six months of survival time. By including patients with malignant tumors, we sought to reduce the rate of loss, lessen possible missed data, and improve the assessment of 6-month survival after surgery. Unfortunately, only about 68% of patients could be contacted, which may affect the evaluation of the 6-month survival rates of intraoperative FiO2 in elderly patients with malignant tumors.
Conclusion
In conclusion, although there was no significant effect of FiO2 on postoperative delirium in elderly patients after laparoscopic gastric and colorectal malignancies surgery, low FiO2 was beneficial to decrease postoperative atelectasis, suggesting that low FiO2 may be safely employed without postoperative delirium.
Abbreviations
POD, postoperative delirium; FiO2, fraction of inspired oxygen; rSO2, regional cerebral oxygen saturation; BIS, Bispectral; IABP, invasive arterial blood pressure; SpO2, oxygen saturation; PETCO2, end-tidal carbon dioxide partial pressure; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association Functional Classification; MMSE, Mini-mental State Examination; PACU, post-anesthesia care unit; NABP, non-invasive arterial blood pressure; MAP, mean arterial pressure; HR, heart rate; ECG, electrocardiogram; CVP, central venous pressure; PCIA, Patient-controlled intravenous analgesia; VAS, visual analogue scale; CAM, Confusion Assessment Method; MDAS, Memorial Delirium Assessment Scale; SD, standard deviation; BMI, body mass index.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Informed Consent
The study was approved by the Research Ethics Committee of Qingdao Municipal Hospital Affiliated to Qingdao University and registered at ClinicalTrials.gov (ChiCTR1800014972). Written informed consent was obtained from each participant.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Xu Lin and Bin Wang are co-first authors for this study. The authors have no conflicts of interest to report.
References
1. Olin K, Eriksdotter-Jönhagen M, Jansson A, Herrington MK, Kristiansson M, Permert J. Postoperative delirium in elderly patients after major abdominal surgery. Br J Surg. 2005;92:1559–1564. doi:10.1002/bjs.5053
2. Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010;97:273–280. doi:10.1002/bjs.6843
3. Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42:68–73. doi:10.1176/appi.psy.42.1.68
4. Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi:10.1097/01.CCM.0000119429.16055.92
5. Bickel H, Gradinger R, Kochs E, Förstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26:26–31. doi:10.1159/000140804
6. Steiner LA. Postoperative delirium. In: part 1. pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28:628–636. doi:10.1097/EJA.0b013e328349b7f5
7. Tang L, Kazan R, Taddei R, Zaouter C, Cyr S, Hemmerling TM. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth. 2012;108(4):623–629. doi:10.1093/bja/aer501
8. Abou-Arab O, Huette P, Guilbart M, Dupont H, Guinot P-G. Hyperoxia during cardiopulmonary bypass does not increase respiratory or neurological complications: a post hoc analysis of the CARDIOX study. Br J Anaesth. 2020;125(5):e400–1. doi:10.1016/j.bja.2020.06.031
9. Hou XL, Teng YC, Ding HS, Ding HY, Zhou CL. Near infrared spatial resolution spectroscopy and its application to the detection of hypoxic-ischemic brain damage in newborn pigs. Spectrosc Spectral Anal. 2008;28(10):2263–2267.
10. Edmonds HL
11. Green DW, Kunst G. Cerebral oximetry and its role in adult cardiac, non-cardiac surgery and resuscitation from cardiac arrest. Anaesthesia. 2017;72(Suppl 1):48–57. doi:10.1111/anae.13740
12. Wang X, Feng K, Liu H, et al. Regional cerebral oxygen saturation and postoperative delirium in endovascular surgery: a prospective cohort study. Trials. 2019;20(1):504. doi:10.1186/s13063-019-3586-y
13. Navarese EP, Austin D, Gurbel PA, et al. Drug-coated balloons in treatment of instent restenosis: a meta-analysis of randomised controlled trials. Clin Res Cardiol. 2013;102(4):279–287. doi:10.1007/s00392-012-0532-3
14. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi:10.7326/0003-4819-113-12-941
15. Schuurmans MJ, Deschamps PI, Markham SW, Shortridge-Baggett LM, Duursma SA. The measurement of delirium: review of scales. Res Theory Nurs Pract. 2003;17:207–224. doi:10.1891/rtnp.17.3.207.53186
16. Leung J, Leung V, Leung CM, Pan PC. Clinical utility and validation of two instruments (the confusion assessment method algorithm and the Chinese version of nursing delirium screening scale) to detect delirium in geriatric inpatients. Gen Hosp Psychiatry. 2008;30:171–176. doi:10.1016/j.genhosppsych.2007.12.007
17. Shi Z, Wu Y, Li C, et al. Using the Chinese version of memorial delirium assessment scale to describe postoperative delirium after hip surgery. Front Aging Neurosci. 2014;6:297. doi:10.3389/fnagi.2014.00297
18. Hesse S, Kreuzer M, Hight D, et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. Br J Anaesth. 2019;122(5):622–634. doi:10.1016/j.bja.2018.09.016
19. Lin X, Tang J, Liu C, et al. Cerebrospinal fluid cholinergic biomarkers are associated with postoperative delirium in elderly patients undergoing Total hip/knee replacement: a prospective cohort Study. BMC Anesthesiol. 2020;20:246–257. doi:10.1186/s12871-020-01166-9
20. Young C, Harris E, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth. 2019;123:898–913. doi:10.1016/j.bja.2019.08.017
21. Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative measures for surgical site infection prevention: an evidence based global perspective. Lancet Infect Dis. 2016;16:e288–303.
22. Shaefi S, Shankar P, Mueller AL, et al. Intraoperative oxygen concentration and neurocognition after cardiac surgery. Anesthesiology. 2020.
23. Choi SH, Kim SH, Lee SJ, Soh SR, Oh YJ. Cerebral oxygenation during laparoscopic surgery jugualar bulb versus regional cerebral oxygen saturation. Yonsei Med J. 2013;54(1):225–230. doi:10.3349/ymj.2013.54.1.225
24. Staehr-Rye AK, Meyhoff CS, Scheffenbichler FT, et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth. 2017;119(1):140–149. doi:10.1093/bja/aex128
25. Kim J, Shim J-K, Song JW, Kim E-K, Kwak YL. Postoperative cognitive dysfunction and the change of regional cerebral oxygen saturation in elderly patients undergoing spinal surgery. Anesth Analg. 2016;123(2):436–444. doi:10.1213/ANE.0000000000001352
26. Eertmans W, De Deyne C, Genbrugge C, et al. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br J Anaesth. 2020;124(2):146–153. doi:10.1016/j.bja.2019.09.042
27. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al.; Healthcare Infection Control Practices Advisory Committee. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017;152:784–791. doi:10.1001/jamasurg.2017.0904
28. Meyhoff CS. Perioperative hyperoxia: why guidelines, research and clinical practice collide. Br J Anaesth. 2019;122:289–291. doi:10.1016/j.bja.2018.12.016
29. Mattishent K, Thavarajah M, Sinha A, et al. Safety of 80% vs. 30–35% fraction of inspired oxygen in patients undergoing surgery: a systematic review and meta-analysis. Br J Anaesth. 2019;122:311–324. doi:10.1016/j.bja.2018.11.026
30. De Jonge S, Egger M, Latif A, et al. Effectiveness of 80% vs. 30–35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta-analysis. Br J Anaesth. 2019;122:325–334. doi:10.1016/j.bja.2018.11.024
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.