Back to Journals » Clinical Optometry » Volume 13
Intraoperative Observation of a Macular Holes Using Optical Coherence Tomography
Authors Nishitsuka K , Nishi K , Namba H, Kaneko Y, Yamashita H
Received 9 February 2021
Accepted for publication 19 March 2021
Published 14 April 2021 Volume 2021:13 Pages 113—118
DOI https://doi.org/10.2147/OPTO.S305927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Mr Simon Berry
Koichi Nishitsuka, Katsuhiro Nishi, Hiroyuki Namba, Yutaka Kaneko, Hidetoshi Yamashita
Department of Ophthalmology and Visual Sciences, Yamagata University Faculty of Medicine, Yamagata, Japan
Correspondence: Koichi Nishitsuka
Department of Ophthalmology and Visual Sciences, Yamagata University Faculty of Medicine, Yamagata City, Yamagata, Japan
Tel +81 23-628-5374
Fax +81 23-528-5377
Email [email protected]
Purpose: This study aimed to observe intraoperative changes in macular hole (MH) form using intraoperative optical coherence tomography (iOCT).
Methods: A total of 10 eyes from 10 patients with MH who underwent vitrectomy using iOCT from May 2015 to October 2015 at the Yamagata University Hospital were retrospectively evaluated. Accordingly, 25-gauge pars plana vitrectomy using iOCT with internal limiting membrane (ILM) peeling and sulfur hexafluoride gas tamponade was performed on each patient. During surgery, MHs were observed using iOCT over four points, namely, before posterior vitreous detachment (PVD) formation, after PVD formation, after ILM peeling, and after fluid–gas exchange. Thereafter, basal MH diameter and minimum aperture MH diameter were postoperatively analyzed.
Results: Before PVD formation, after PVD formation, after ILM peeling, and after fluid–gas exchange, the mean basal MH diameters were 690.7 ± 268.4, 683.3 ± 274.2, 683.7 ± 269.5, and 668.3 ± 261.4 μm, while the mean minimum aperture MH diameters were 278.3 ± 165.2, 283.0 ± 170.2, 257.0 ± 127.8, and 188.0 ± 105.0 μm, respectively. The mean minimum aperture MH diameter decreased significantly after fluid–gas exchange (one-way repeated measures ANOVA, p < 0.05). None of the patients exhibited intraoperative closure of the MHs. However, MH closure was confirmed in all patients after the surgery.
Conclusion: None of the patients demonstrated intraoperative MHs closure. Accordingly, the minimum aperture MH diameter was the first change formation to close after fluid–gas exchange.
Keywords: macular hole, intraoperative optical coherence tomography, early change
Introduction
Idiopathic macular holes (MH) is a retinal defect located at the center of the fovea that has been associated with posterior vitreous detachment (PVD).1 Although some stage 1 MHs can resolve spontaneously, they still require close observation.2,3 Meanwhile, stage 2 and higher MHs are usually indications for surgical correction for better outcomes.2–4 Currently, primary MH repair has been performed using pars plana vitrectomy, which involves posterior cortical vitreous removal, epiretinal membrane stripping, and finally intraocular gas tamponade. Internal limiting membrane (ILM) peeling has also been proven to be beneficial for stage 2 to 4 MHs.2,5
One study showed that maintaining the face-down position during gas tamponade promoted MH closure.6 However, this position is not comfortable for patients and can be associated with complications, such as back pain or ulnar nerve palsies.6 To reduce pain during face-down positioning, confirmation of early MH closure is important. Accordingly, Yamashita et al7 published images of early MH closure in postoperative gas-filled eyes using spectral domain optical coherence tomography (SD-OCT), while Kikusima et al8 demonstrated very early dynamics of MH closure in gas-filled eyes within 24 h of surgery using swept source optical coherence tomography (SS-OCT).
Mohammad et al revealed that all MH dimensions remained stable during consecutive stages of surgery, except for the MH apex diameter, which showed a significant decrease after ILM peeling using intraoperative hand-held SD-OCT.9 Recently, the emergence of microscope-integrated intraoperative OCT (iOCT) has provided surgeons with valuable information needed during MH surgery.10 As such, the current study aimed to describe intraoperative changes in MH using iOCT. Moreover, we hypothesized that confirmation of MH closure during surgery could further reduce postoperative postural restrictions.
Methods
Medical records of 14 eyes from 14 consecutive patients (mean age, 65.6 ± 6.7 years) who underwent 25-gauge pars plana vitrectomy with ILM peeling and sulfur hexafluoride (SF6) tamponade for stage 3 MH from May 2015 to October 2015 were retrospectively analyzed. All work was approved by the institutional review board of Yamagata University Faculty of Medicine (approval number: H26-21). The procedure performed herein conformed with the tenets of the Declaration of Helsinki. All data were fully anonymized, with the institutional review board waiving the need for informed consent.
All cases were treated using a 25-gauge PPV wide-angle noncontact viewing system (Resight®; Carl Zeiss Meditec AG, Jena, Germany) with the Constellation Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA). Posterior vitreous detachment was performed using the suction mode while disabling the cutter. The ILM was stained using indocyanine green (ICG 0.05%) to facilitate peeling. After successful ILM peeling, intraocular gas tamponade was performed via fluid–air exchange using a suction needle. During the surgery, MHs were detected using iOCT (RESCAN; Carl Zeiss Meditec AG, Jena, Germany). After the surgery, MH treatment was evaluated using OCT (Cirrus HD-OCT; Carl Zeiss Meditec AG, Jena, Germany).
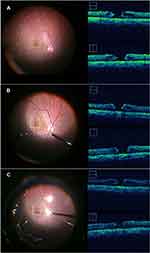
The main outcome measures were iOCT findings of MH. Accordingly, MHs were measured along horizontal and vertical lines using iOCT. To obtain high-quality images, the surgeon performed OCT adjustments during which they focused on the MH as much as possible. During surgeries, MHs were observed across four points, namely, before PVD formation, after PVD formation, after ILM peeling, and after fluid–gas exchange on vitrectomy (Figure 1). Recorded iOCT findings were analyzed by extracting still images from video recordings. Minimum aperture and basal MH diameters were measured in pixels using ImageJ software and calculated using OCT scan size (3 or 6 mm). The vertical and horizontal lines of the MH diameters then averaged, after which each MH size was compared using one-way repeated measures ANOVA. The Bonferroni correction was used for post-hoc analysis. A p value of <0.01 indicated statistical significance.
Results
iOCT produced good quality images across all phases in 10 of the 14 eyes, which had a mean age of 55.6 ± 13.7 years. Before PVD formation, after PVD formation, after ILM peeling, and after fluid–gas exchange, the mean minimum aperture MH diameters were 278.3 ± 165.2, 283.0 ± 170.2, 257.0 ± 127.8, and 188.0 ± 105.0 μm, while the mean basal MH diameters were 690.7 ± 268.4, 683.3 ± 274.2, 683.7 ± 269.5, and 668.3 ± 261.4 μm, respectively (Table 1). The mean minimum aperture MH diameter decreased significantly after fluid–gas exchange (one-way repeated measures ANOVA, p < 0.01) (Figure 2). None of the patients exhibited MH closure at the end of surgery. However, all patients showed MH closure on the first day after the surgery.
 |
Table 1 Macular Hole Size Measured Using Intraoperative Optical Coherence Tomography During Surgery |
Discussion
iOCT, which greatly enhances the precision of vitreoretinal surgical maneuvers and biometry, is an essential prerequisite for the implementation of real-time guided surgical techniques at the micrometer level.11–16 Recent advancements in the iOCT-guided inverted-ILM technique have improved its effectiveness in treating large MHs.17,18 Although the current study observed a decrease in the minimum aperture MH diameters during fluid–gas exchange, none of the patients demonstrated MH closure at the end of surgery. To the best of our knowledge, this has been the first report to evaluate MH closure immediately after surgery using iOCT.
One multicenter study in Japan showed that medium to large (400–550 µm) and extra-large (>550 µm) MHs had a MH closure rate of 95.2% and 88.4% following ILM peeling, respectively. Moreover, multiple logistic regression analysis in the study by Yamashita et al identified MH size as a factor independently associated with postoperative best corrected visual acuity at 6 months. MHs with diameters ≥400 µm have been found to have unsatisfactory surgical outcomes.19,20 In fact, one meta-analysis provided evidence that a face-down postoperative position seems to be unnecessary for MHs <400 μm in size.21 Meanwhile, Lally et al showed 85% of small MHs (<400 μm in size) had closed without the use of intraocular gas tamponade,22 with other cases of spontaneous closure of small MHs also have been reported.23 Based on such findings, small MHs have shown considerable success following surgery.
The present study focused on relatively small, therapeutically sized MHs cases (average diameter, 278.3 μm) to investigate MH closure during surgery. The decrease in minimum aperture MH diameter after fluid–gas exchange observed herein may be an objective finding indicating that gas tamponade could have helped with MH closure by generating interfacial surface tension force between the retina and the gas bubble, thereby pulling the edges of the hole.2 Accordingly, Yamashita et al showed MH closure on day 1 after surgery using SD-OCT,7 while Kikusima et al also showed MH closure on day 0 after the surgery using SS-OCT.8 Similarly, the current study showed that while MHs did not close immediately after surgery, closure had been noted the day after surgery. These results indicate that MH closure does not occur during surgery but rather after surgery through postoperative Müller cell migration.24
Meanwhile, one case report showed that vitrectomy combined with ILM peeling without gas tamponade promoted closure of small MHs.22 ILM peeling has also been found to play a considerable role in MH closure. However, one meta-analysis25 comparing cases with and without ILM peeling showed that ILM peeling provided no benefit of in terms of the primary outcome (visual acuity at 6 months). Nonetheless, ILM peeling appears to be advantageous given that it offers favorable cost effectiveness by increasing the likelihood of primary anatomical closure and subsequently decreasing the likelihood of further surgery, with no differences in unwanted side-effects compared to no peeling.25 Considering that all patients included in the current study underwent ILM peeling, comparisons between the presence and absence of ILM peeling could not be conducted. More extensive future studies involving iOCT are therefore needed to elucidate MH repair.
This study has several limitations worth noting. First, measuring the exact same area using iOCT is difficult. Surgeons needed to perform an iOCT scan relatively quickly to limit the duration wherein patients are placed in an uncomfortable position. Therefore, video recordings were taken during iOCT, after which analysis was performed using still images obtained following after surgery while working on the measurements as much as possible. Second, limitations in the performance of the iOCT equipment had been noted. In particular, sufficient quality images could not be obtained for analysis from 4 of the 14 cases. Third, the current study included a small number of cases, all of whom had small MH sizes. Future studies comparing different MH sizes may provide further insights into effective treatment strategies for MH.
In summary, the current study evaluated MH formation during surgery using iOCT. Accordingly, while none of the patients exhibited intraoperatively MH closure, the minimum aperture MH diameter decreased after the fluid–gas exchange. As such, the minimum aperture MH diameter was the first change formation to close after fluid–gas exchange.
Acknowledgment
The 10th Congress of Asia Pacific Vitreo-retina society, Bangkok, 2016.
Funding
This work was supported by JSPS KAKENHI Grant Number JP25462704.
Disclosure
Mr Koichi Nishitsuka reports personal fees from Santen, personal fees from RE MEDICAL, INC, personal fees from Carl Zeiss, personal fees from Alcon, personal fees from Novartis, personal fees from Kowa Company.Ltd., personal fees from Johnson & Johnson, personal fees from Senju Pharmaceutical Co/,Ltd., personal fees from HOYA, and personal fees from Sanwa Kagaku Kenkyusyo CO.,LTD., outside the submitted work. Mr Hidetoshi Yamashita reports grants from Atsuzawa Prosthesis Co,Ltd., Alcon Japan Ltd., Santen Pharmaceutical Co.,Ltd., Eisai Co.,Ltd., Senju Pharmaceutical Co.,Ltd., B.LJ Company, Ltd., Bayer Yakuhin,Ltd., AMO Japan, Novartis Pharma Japan, Trust Medical Co.,Ltd., and Taisho Pharma Japan, outside the submitted work. The authors report no other conflicts of interest.
References
1. Gass JD. Idiopathic senile macular hole: its early stages and pathogenesis. 1988. Retina. 2003;23(6 Suppl):629–639.
2. Bikbova G, Oshitari T, Baba T, Yamamoto S, Mori K. Pathogenesis and management of macular hole: review of current advances. J Ophthalmol. 2019;2019:3467381. doi:10.1155/2019/3467381
3. Osawa S, Oshima Y. 27-Gauge vitrectomy. Dev Ophthalmol. 2014;54:54–62.
4. Yu Y, Liang X, Wang Z, Wang J, Liu W. Clinical and morphological comparisons of idiopathic macular holes between stage 3 and stage 4. Graefes Arch Clin Exp Ophthalmol. 2018;256(12):2327–2333. doi:10.1007/s00417-018-4158-7
5. Ittarat M, Somkijrungroj T, Chansangpetch S, Pongsachareonnont P. Literature review of surgical treatment in idiopathic full-thickness macular hole. Clin Ophthalmol. 2020;14:2171–2183. doi:10.2147/OPTH.S262877
6. Yamashita T, Sakamoto T, Yamashita T, et al. Individualized, spectral domain-optical coherence tomography-guided facedown posturing after macular hole surgery: minimizing treatment burden and maximizing outcome. Retina. 2014;34(7):1367–1375. doi:10.1097/IAE.0000000000000087
7. Yamashita T, Yamashita T, Kawano H, Sonoda Y, Yamakiri K, Sakamoto T. Early imaging of macular hole closure: a diagnostic technique and its quality for gas-filled eyes with spectral domain optical coherence tomography. Ophthalmologica. 2013;229(1):43–49. doi:10.1159/000343061
8. Kikushima W, Imai A, Toriyama Y, Hirano T, Murata T, Ishibashi T. Dynamics of macular hole closure in gas-filled eyes within 24 h of surgery observed with swept source optical coherence tomography. Ophthalmic Res. 2015;53(1):48–54. doi:10.1159/000368437
9. Mohammad RE, Khademi MR, Mazloumi M, Khodabandeh A, Riazi-Esfahani H. Macular surgery using intraoperative spectral domain optical coherence tomography. J Ophthalmic Vis Res. 2015;10(3):309–315. doi:10.4103/2008-322X.170355
10. Tew TB, Chen TC, Yang CH, Yang CM. Vitreomacular changes after intravitreal gas injection for idiopathic impending or early macular hole: an Optical Coherence Tomography Study. Ophthalmologica. 2018;239(1):1–10. doi:10.1159/000478666
11. Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol. 2014;158(5):999–1007. doi:10.1016/j.ajo.2014.07.034
12. Ehlers JP. Intraoperative optical coherence tomography: past, present, and future. Eye (Lond). 2016;30(2):193–201. doi:10.1038/eye.2015.255
13. Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER study 3-year results: feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery. Ophthalmology. 2018;125(7):1014–1027. doi:10.1016/j.ophtha.2017.12.037
14. Maier M, Bohnacker S, Klein J, et al. [Vitrectomy and iOCT-assisted inverted ILM flap technique in patients with full thickness macular holes]. Ophthalmologe. 2019;116(7):617–624. German. doi:10.1007/s00347-018-0769-y
15. Nishitsuka K, Nishi K, Namba H, Kaneko Y, Yamashita H. Intraoperative optical coherence tomography imaging of the peripheral vitreous and retina. Retina. 2018;38(3):e20–e2. doi:10.1097/IAE.0000000000001979
16. Nishitsuka K, Nishi K, Namba H, Kaneko Y, Yamashita H. Quantification of the peripheral vitreous after vitreous shaving using intraoperative optical coherence tomography. BMJ Open Ophthalmol. 2021;6(1):e000605. doi:10.1136/bmjophth-2020-000605
17. Maier M, Hattenbach LO, Klein J, et al. [Real-time optical coherence tomography-assisted high-precision vitreoretinal surgery in the clinical routine]. Ophthalmologe. 2020;117(2):158–165. German. doi:10.1007/s00347-019-01007-2
18. Bleidissel N, Friedrich J, Klaas J, Feucht N, Lohmann CP, Maier M. Inverted internal limiting membrane flap technique in eyes with large idiopathic full-thickness macular hole: long-term functional and morphological outcomes. Graefes Arch Clin Exp Ophthalmol. 2021. doi:10.1007/s00417-021-05082-7
19. Freeman WR, Azen SP, Kim JW, El-Haig W, Mishell DR
20. Gupta B, Laidlaw DA, Williamson TH, Shah SP, Wong R, Wren S. Predicting visual success in macular hole surgery. Br J Ophthalmol. 2009;93(11):1488–1491. doi:10.1136/bjo.2008.153189
21. Ye T, Yu JG, Liao L, Liu L, Xia T, Yang LL. Macular hole surgery recovery with and without face-down posturing: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2019;19(1):265. doi:10.1186/s12886-019-1272-1
22. Lally DR, Kasetty MA. Closure of small macular holes using vitrectomy surgery with internal limiting membrane peeling without the use of intraocular gas tamponade: broadening the understanding of the macular hole pathophysiology. Retin Cases Brief Rep. 2020;14(2):104–109. doi:10.1097/ICB.0000000000000919
23. Zhang W, Grewal DS, Jaffe GJ, Mahmoud TH, Fekrat S. Spontaneous closure of full-thickness macular hole with epiretinal membrane in vitrectomized eyes: case series and review of literature. Ophthalmic Surg Lasers Imaging Retina. 2017;48(2):183–190. doi:10.3928/23258160-20170130-15
24. Wu AL, Liu YT, Chou HD, et al. Role of growth factors and internal limiting membrane constituents in muller cell migration. Exp Eye Res. 2021;202:108352. doi:10.1016/j.exer.2020.108352
25. Spiteri Cornish K, Lois N, Scott N, et al. Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full-thickness macular hole (FTMH). Cochrane Database Syst Rev. 2013;(6):CD009306. doi:10.1002/14651858.CD009306.pub2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.