Back to Journals » Clinical Ophthalmology » Volume 15
Intra-Arterial Tissue Plasminogen Activator for Central Retinal Artery Occlusion
Authors Sobol EK , Sakai Y, Wheelwright D, Wilkins CS, Norchi A, Fara MG, Kellner C , Chelnis J, Mocco J, Rosen RB, De Leacy RA , Lema GMC
Received 14 July 2020
Accepted for publication 18 August 2020
Published 16 February 2021 Volume 2021:15 Pages 601—608
DOI https://doi.org/10.2147/OPTH.S272126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ethan K Sobol,1,2 Yu Sakai,3 Danielle Wheelwright,3 Carl S Wilkins,1,2 Amanda Norchi,1 Michael G Fara,4 Christopher Kellner,3 James Chelnis,1 J Mocco,3 Richard B Rosen,1,2 Reade A De Leacy,3 Gareth MC Lema1,2
1Department of Ophthalmology, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA; 2Department of Ophthalmology, New York Eye and Ear Infirmary of Mount Sinai, New York, NY, 10003, USA; 3Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA; 4Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, 10029, USA
Correspondence: Gareth MC Lema
Department of Ophthalmology, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, New York, NY, 10029, USA
Tel +1 212-241-0939
Fax +1 212-824-2325
Email [email protected]
Purpose: To investigate the benefit of early intra-arterial tissue plasminogen activator (IAT) for treatment of central retinal artery occlusion (CRAO).
Patients and Methods: Fifteen eyes of 15 patients presenting with acute CRAO were included in this retrospective consecutive interventional case series. Patients were excluded if treatment with IAT was not initiated within 12 hours. The diagnosis was confirmed by an ophthalmologist. IAT was performed via a transfemoral arterial approach. Tissue plasminogen activator (tPA) was infused into the ophthalmic artery in aliquots up to 3mg to a maximum of 22mg. Paracentesis was done at the ophthalmologist’s discretion. The primary outcome measure was visual acuity after three weeks. Adverse events were recorded during treatment and follow-up visits.
Results: After treatment with IAT, there was a statistically significant improvement in visual acuity, with a mean change of − 0.76 (SD 0.91; range − 2.4 to 0.85) logMAR (p=0.006). Vision improved by 3 or more lines in 53%, and of these, the mean Snellen visual acuity improvement was > 6 lines. Notably, 4 patients (27%) improved from CF or worse to 20/80 or better. The mean dose of tPA used was 17mg and the mean time to treatment was 8.83 hours (range: 5.5 to 12 hours). There were no statistically significant differences based on time to treatment, dose of tPA, or use of a paracentesis. No major adverse events were recorded.
Conclusion: IAT was safe and showed significant visual improvement in this small uncontrolled study. Larger studies and efforts to decrease time to treatment should be initiated to optimize outcomes.
Keywords: CRAO, tPA, intervention, treatment, ophthalmic artery
Introduction
Acute non-arteritic central retinal artery occlusion (CRAO) presents as painless unilateral vision loss and is of embolic origin. The incidence of CRAO is approximately 1 in 100,000 patients, occurring more often in the elderly and in those with cardiovascular risk factors.1,2 Although CRAO is essentially a “stroke in the eye,” there is little consensus regarding the use of thrombolysis or a standardized approach to treatment.3,4 A recent survey found that while over half of academic medical centers in the United States offer intravenous fibrinolysis in select patients, few are routinely offering intra-arterial therapy.4 Prior studies have mixed outcomes and barriers in prompt diagnosis and early management remain significant.5 Current practices include the use of medical and procedural therapies that have little effect on visual outcomes.6
Previous studies have examined the outcomes of intravenous and endovascular intra-arterial thrombolysis for CRAO. Although some visual benefit has been shown with intravenous thrombolysis,7 proponents of local intra-arterial therapy (IAT) to the ophthalmic artery believe there is reduced risk of systemic complications and potential for greater benefit. Beginning in 1984, many studies ranging in size and methodology have examined the utility and safety of IAT using recombinant tissue plasminogen activator (tPA) for acute CRAO. Results have been mixed, with reported visual outcomes of no benefit to surpassing 50% of patients, and with adverse events ranging from minor (headache, pain or hematoma at the access site) to major (TIA, intracranial hemorrhage, and neurologic deficit).8,9
Only one randomized controlled trial8 of IAT in CRAO has been published thus far (the EAGLE trial), which found no difference between IAT and conservative management. Alternatively, retrospective trials have found visual acuity improvements with timely management.10 Critiques of prior studies, particularly the EAGLE trial, include a wide variety in time from onset to treatment, concern for selection bias, and interventional methodology.11
The purpose of this study was to investigate the potential for benefit of IAT for CRAO, and to determine whether this intervention can safely be implemented in a large hospital system with endovascular capabilities. With the degree of variability in prior studies, we sought to initiate a standardized protocol to evaluate visual outcomes, and to establish whether a larger, randomized study would be clinically beneficial in our patient population.
Patients and Methods
This retrospective consecutive interventional case series was approved by the Institutional Review Board of The Mount Sinai Health System and the Icahn School of Medicine at Mount Sinai (New York, NY). Informed consent detailing the risks, potential benefits, and alternatives was obtained for the proposed treatment. The collection of patient information was subsequently attained in individuals who underwent intervention. The study was conducted in adherence to the Health Insurance Portability and Accountability Act and the Declaration of Helsinki. Patient identifying information was kept confidential.
Patients and Setting
Consecutive patients presenting with acute CRAO were enrolled at a single institutional system.
Study Protocol
Upon presentation to any site within the hospital system, patients were diagnosed with CRAO based on the following clinical presentation: (1) A history of acute, painless monocular vision loss, (2) evidence of retinal ischemia on fundoscopic exam by an ophthalmologist, including retinal whitening and/or the presence of a cherry red spot, and/or a relative afferent pupillary defect when relevant. Giant cell arteritis was ruled out in separate evaluations by the emergency medicine service, the stroke service, and the ophthalmology consultation. Non contrast computed tomography (CT) of the brain was acquired for all patients. After diagnosis, informed consent was obtained for the proposed intervention. At the discretion of the diagnosing ophthalmologist, some eyes were treated with anterior chamber paracentesis prior to IAT. Patients were treated only if IAT could be performed within 12 hours of symptom onset. Participants were excluded if there was evidence of any confounding retinal pathology, if there were any concomitant neurologic sequelae concerning for a cerebrovascular event, or there was concern for an arteritic CRAO (eg giant cell arteritis) based on symptomatology and/or erythrocyte sedimentation rate and C-reactive protein levels on routine laboratory investigation.
IAT was performed using biplane fluoroscopic guidance in the neuro-interventional suite, via a transfemoral arterial approach. All procedures were performed with anesthesiologist support under monitored anesthesia care (MAC). A standardized approach was used for all patients who underwent intervention. Guide catheter access to the target internal carotid artery was obtained and following that a microcatheter and micro-guidewire was used to cannulate the origin of the ipsilateral ophthalmic artery. Infusion of tPA was given in increments of up to 3mg/3mL in aliquots of normal saline over 3 to 5 minutes each up to a maximum total of 22mg. tPA infusion was stopped if visual acuity improved or newly observed patency of the central retinal artery was achieved as indicated by a retinal blush. Following this, final angiograms were performed, and all catheters were removed. All patients were admitted post-operatively to either a dedicated stroke unit or neurosurgical intensive care unit for monitoring.
The primary outcome measure was visual acuity at three-week follow-up. “Clinically meaningful improvement” in visual acuity was defined as a three-lines or more improvement in Snellen line acuity. If the Snellen line equivalent was either unchanged, improved by less than three lines, or declined by less than three lines, vision was considered “stable.” Vision was considered “worse” if acuity declined by three or more lines of Snellen equivalent. Secondary variables evaluated included age, gender, laterality, whether ocular paracentesis was performed, the total dose in milligrams of tPA administered, whether there was improvement in the retinal blush during IAT, and the time from symptom onset to IAT.
The presence of any major adverse events related to IAT treatment (such as intracranial hemorrhage, transient or permanent ischemic events, access site hematoma or hemorrhage, and any neurologic deficits) were recorded at the time of intervention and at the three-week follow up visit.
All patient identifiers were removed on completion of the data collection. Data were then saved on a password-protected Microsoft Excel file and subsequently uploaded to statistical software for analyses. All statistical analysis was conducted using Stata 13 (StataCorp LP, College Station, TX).
Statistical Analysis
All Snellen visual acuity data was converted to logMAR for the purposes of statistical analysis. The results and discussion are discussed in terms or Snellen acuity for clinical relatability. No light perception, light perception, hand motion, and count fingers vision were approximated by logMAR values of 3.0, 2.7, 2.28, and 1.85, respectively.12,13 A two-sided paired t-test was used to compare visual acuity outcomes before IAT and at three-week follow up. An unpaired two-sided t-test was used for comparison of visual acuity changes between patients treated before and after eight hours. Individual simple linear regression analyses were used to assess for a relationship between visual acuity outcomes and time to IAT, the dose of tPA used, and the relationship between pre and post IAT visual acuity. A two-tailed Fischer’s exact test was used to compare categorical variables. A cutoff of p<0.05 for statistical significance was used for all measures of association.
Results
Fifteen patients (15 eyes; 11 right, 4 left) were included in this study. Patients were an average of 60 years old (range 28 to 84); four (27%) were male and 11 (73%) female. Ocular paracentesis was performed in six (40%) of patients. Mean dose of tPA used was 16.87 mg (SD 7.19; range 3 to 22) and mean time from symptom onset to treatment initiation was 8.83 hours (SD 2.37; range 5.5 to 12). Final visual acuity at three weeks after IAT improved by three or more Snellen lines in eight (53%) eyes, remained stable or unchanged (within three Snellen lines) in 6 (40%) eyes, and was worse (by three or more lines) in one (7%) eye (Figure 1). Table 1 lists the individual patient characteristics, visual acuity, and IAT treatment data.
 |
Table 1 Patients with CRAO Treated with Intra-Arterial tPA |
Prior to treatment, logMAR visual acuity was on average 2.18 (SD 0.82; range 0.6 to 3). In comparison, mean logMAR visual acuity three weeks after IAT was 1.42 (SD 0.99; range 0.1 to 3). This demonstrated a statistically significant improvement of 0.76 (SD 0.91; range −2.4 to 0.85) in logMAR visual acuity (p=0.0061). In those patients with a clinically significant improvement in visual acuity after IAT (n=8), there was an average improvement of 1.37 logMAR visual acuity (SD 0.72; range −2.4 to −0.42). These changes after intervention are depicted graphically in Figure 2. Overall, there was an average of 3.43 lines of improvement in Snellen visual acuity (SD 3.99; range −3 to 11). In those patients with a clinically significant improvement in visual acuity, the average improvement was 6.44 lines of Snellen visual acuity (SD 2.80, range 3 to 11).
On average, the visual acuity before and then three weeks after treatment was correlated in a linear fashion (p=0.054, Figure 3). Average change in BCVA in patients who received IAT within 8 hours or less from symptom onset was −0.94 logMAR (95% CI: −1.42 to 0.21) versus −0.60 (95% CI: −1.74 to −0.13) in those who received treatment between 8 and 12 hours, with no statistically significant difference between the two groups (2-sided t-test, p=0.498). Of the patients who received IAT within 8 hours or less, 3 (20%) had a clinically significant visual improvement while 4 (27%) had stable or worse visual acuity at follow-up. A linear regression analysis of the degree of change in logMAR BCVA as a function of time to IAT did not demonstrate a statistically significant relationship (p=0.284). Similarly, a linear regression of change in BCVA as a function of dose of tPA was not statistically significant (p=0.856).
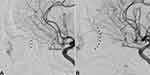
An improved retinal blush (example, Figure 4) was present in 6 (75%) of the 8 cases where visual acuity improved by three or more Snellen lines, compared with 2 (29%) of the remaining 7 without significant visual improvement (Fischer’s exact test, p=0.13).
At the time of IAT intervention, no major adverse events took place. At three-week follow-up, no late effects or neurologic deficits were present in any patient.
Discussion
More than half of the patients in this study had a clinically significant improvement in visual acuity of greater than or equal to 3 Snellen lines. Notably, 75% of those who responded to treatment showed a substantial change in visual acuity, with improvement of at least five lines. A subset of patients (4/15, 27%) improved from count fingers or worse to 20/80 or better. Thus, patients may gain a considerable functional improvement from this intervention. As a point of reference, the cutoff for a conditional driver’s license in New York State is 20/70 or better in at least one eye. Our results are consistent with past reports10 and demonstrate better outcomes compared to natural history.14,15
The most extensive and definitive studies of natural history have been done by Hayreh and colleagues.15,16 In cases of non-arteritic CRAO without cilioretinal artery sparing, they found that 22% showed any improvement. In cases of non-arteritic CRAO with cilioretinal artery sparing the rate of any improvement was 67%.15 In our study, 67% of patients showed any visual improvement and 53% showed improvement of 3 lines or more. Since we conducted a retrospective review of a small sample of consecutive patients treated on the stroke service, most did not have either photographs or fluorescein angiography. Therefore, we were not able to differentiate between patients with or without cilioretinal artery sparing. For our statistics to resemble the natural history studies, all of them would have been expected to have cilioretinal artery sparing, which are more likely to result in visual improvement. This is unlikely because a cilioretinal artery is found in only about 10–32% of eyes.17,18 Although transient CRAO with visual recovery was possible in a subset of our cohort, overall the expected change in visual acuity in these patients treated with IAT was significantly different than what would be expected by natural history alone.
In 2015, Schrag, et al7 performed a comprehensive meta-analysis of studies using intravenous fibrinolysis compared to natural history studies. The authors considered clinically significant visual acuity an improvement from worse than logMAR 1.0 to better than logMAR 0.7. This corresponds to a starting visual acuity worse than 20/200 with improvement to 20/100 or better. They identified the rate of improvement to be 17.7% (range: 13.9–21.4%). The percentage of patients that improved with intravenous fibrinolysis was 31.8% (range: 24.3–39.3%). Applying this analysis to our data, 26.7% of patients showed improvement, which is within the range of their treatment group. Additionally, this analysis would exclude patients who improved from NLP to count fingers and from LP to 20/800. While these patients did not improve to better than legal blindness, we consider their visual benefit clinically significant.
In the eyes that responded, six of eight showed improvement of at least five lines. Initial vision appeared to correlate with final visual acuity, and time from symptom onset to IAT did not correlate with visual improvement. In eyes that responded, the retinal blush was more likely to have improved during IAT. However, this result was not statistically significant compared with eyes that did not respond, most likely due to the small sample size of this study.
In a large study of visual acuity outcomes of CRAO by Hayreh and Zimmerman,14 61% of patients were found to have a final VA of 20/400 or worse. In our study, this was similarly the case in 9 (60%) of the patients at the final three-week follow up. Considering that 8 (53%) of the eyes in this study showed clinically relevant visual improvement after intervention compared with the 30–35% of eyes with spontaneous improvement reported by Hayreh and Zimmerman, these results appear to differ from the natural course of the disease. Given that standard therapy is of little help in altering the visual outcomes in CRAO,19,20 and noting the absence of serious adverse outcomes in our study, IAT may be a reasonable first line therapy for these patients.
In animal models of CRAO, irreversible retinal ischemia took place after a mean of 105 minutes.16 The total occlusion induced in these models may not fully replicate the embolic etiology causing the majority of CRAO in real world conditions.
Given the safety profile reported here, potential for severe vision loss without treatment, and the inability to identify constitution of thrombi in-vivo, the benefit we saw with treatment may outweigh the nearly assured severe visual loss in untreated eyes. Although one patient experienced a meaningful visual decline at follow-up, it is difficult to assess whether this was related to IAT failure or a treatment related adverse event.
Although our study was not powered to show greater improvement with decreased time to treatment, recent clinical evidence has suggested this as a possibility.7,10,21 We expect the greatest clinical benefit to be within 6 hours or less, although we did observe one case of significant visual improvement even 11 hours after the onset of symptoms. There were also two patients with considerable improvement at 10 hours with presenting visual acuities of 20/100 and 20/80. Although we are unable to determine whether a degree of collateralization or reperfusion had taken place, these results suggest that future studies may benefit from implementing a pre-operative visual acuity cutoff. There are also inherent limitations to initiating even earlier IAT (eg less than 4.5 hours), mostly related to the time it takes to present to an emergency department, time from presentation to diagnosis, and initiation of a treatment protocol.
There has been only one randomized controlled trial examining IAT for CRAO.8 Despite a sufficiently powered study, no significant difference was found between IAT and conservative therapy. This study has been criticized for including patients too long after symptom onset.11 Patients were treated as long as 20 hours from last known well and fewer than 20% were treated within 6 hours. A post hoc analysis of this trial showed a benefit to earlier treatment21 which indicates that a more streamlined protocol for earlier treatment initiation with stricter cutoffs may prove beneficial.
A large retrospective study of IAT and recent meta-analysis of IAT have shown more promising results for fibrinolysis of CRAO. Aldrich, et al10 found that 66% of patients treated with IAT showed visual improvement, versus 33% in the standard therapy group. Our procedural protocol mirrored that employed by Aldrich et al and has provided similar results.
In a recent meta-analysis by Page et al,9 50.4% of eyes treated with IAT had an improvement in visual acuity compared with 31.8% in eyes managed with standard or conservative therapy. These recent positive results, and the visual improvement achieved in several of our patients, indicate that treatment of retinal artery occlusions with IAT may lead to meaningful improvement in visual acuity for most patients.
Since all patients were treated consecutively and then reviewed retrospectively, there was no randomization to IAT versus standard therapy. The intention was to treat patients with the best-known intervention, thereby precluding use of a control group. This is a limitation, but it also decreased the possibility of selection bias in this retrospective review. Retinal imaging, including OCT and fluorescein angiography, were not available in the emergency room setting. Finally, this was a relatively small sample size. This was due in part to the low incidence of CRAO and the tendency for patients to present outside of the clinical window to perform IAT. More efficient screening protocols and streamlined system-wide coordination of care will help limit logistical barriers to treatment.
Conclusion
Significant visual morbidity may be prevented by timely treatment for CRAO by IAT administered within the first 12 hours of symptom onset. The safety profile reported in this cohort and past studies suggests a low risk of adverse events. Considering the significant visual improvement gained by several patients in this study it is important to continue to evaluate this treatment for CRAO. Future efforts should focus on improving education of patients and clinicians about the emergent nature of CRAO while also streamlining the clinical evaluation of the patient to shorten the time to treatment.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Dr J Mocco is consultant/investor/stockholder for Endostream, Viseon, Imperative Care, RIST, Synchron, Viz.ai, Perflow, CV Aid, Cerebrotech, Echovate, Rebound, Blink TBI, Serenity, Cardinal Consulting, and NTI; research support for Stryker, Microvention, and Penumbra, outside the submitted work. Dr Richard B Rosen reports personal fees, non-financial support for intellectual property from Optovue; personal fees/grants from Boehringer-Ingelheim, Astellas, Regeneron, Genentech-Roche, NanoRetina, CellView, Bayer, and Teva; non-financial support from OD-OS and Diopsys, personal financial interest from Guardian Health and Opticology, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820–823. doi:10.1016/j.ajo.2011.05.005
2. Park SJ, Choi NK, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015;122(11):2336–2343. doi:10.1016/j.ophtha.2015.07.018
3. Limaye K, Wall M, Uwaydat S, et al. Is management of central retinal artery occlusion the next frontier in cerebrovascular diseases? J Stroke Cerebrovasc Dis. 2018;27(10):2781–2791. doi:10.1016/j.jstrokecerebrovasdis.2018.06.006
4. Youn TS, Lavin P, Patrylo M, et al. Current treatment of central retinal artery occlusion: a national survey. J Neurol. 2018;265(2):330–335. doi:10.1007/s00415-017-8702-x
5. Plant GT, Landua K. Thrombolysis for central retinal artery occlusion. J Neurol Neurosurg Psychiatry. 2005;76(2):160–161. doi:10.1136/jnnp.2004.045583
6. Atkins EJ, Bruce BB, Newman NJ, Biousse V. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol. 2009;148(1):172–173. doi:10.1016/j.ajo.2009.03.020
7. Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous fibrinolytic therapy in central retinal artery occlusion: a patient-level meta-analysis. JAMA Neurol. 2015;72(10):1148–1154. doi:10.1001/jamaneurol.2015.1578
8. Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117(7):1367–1375. doi:10.1016/j.ophtha.2010.03.061
9. Page PS, Khattar NK, White AC, et al. Intra-arterial thrombolysis for acute central retinal artery occlusion: a systematic review and meta-analysis. Front Neurol. 2018;9:76. doi:10.3389/fneur.2018.00076
10. Aldrich EM, Lee AW, Chen CS, et al. Local intraarterial fibrinolysis administered in aliquots for the treatment of central retinal artery occlusion: the Johns Hopkins Hospital experience. Stroke. 2008;39(6):1746–1750. doi:10.1161/STROKEAHA.107.505404
11. Hayreh SS. Comment re: multicenter study of the European assessment group for lysis in the eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. Graefes Arch Clin Exp Ophthalmol. 2007;245(3):454–466. doi:10.1007/s00417-006-0473-5
12. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
13. Bach M, Schulze-Bonsel K, Feltgen N, Burau H, Hansen L. Author response: numerical imputation for low vision states. Invest Ophthalmol Vis Sci. 2007.
14. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–391. doi:10.1016/j.ajo.2005.03.038
15. Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25. doi:10.1016/j.preteyeres.2014.04.001
16. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75–78. doi:10.1016/S0161-6420(80)35283-4
17. Justice J, Lehmann R. Cilioretinal arteries: a study based on review of stereo fundus photographs and fluorescein angiographic findings. Arch Ophthalmol. 1976;94(8):1355–1358. doi:10.1001/archopht.1976.03910040227015
18. Awan KJ. Arterial vascular anomalies of the retina. Arch Ophthalmol. 1977;95(7):1197–1202. doi:10.1001/archopht.1977.04450070095007
19. Rudkin AK, Lee AW, Aldrich E, Miller NR, Chen CS. Clinical characteristics and outcome of current standard management of central retinal artery occlusion. Clin Exp Ophthalmol. 2010;38(5):496–501. doi:10.1111/j.1442-9071.2010.02280.x
20. Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev. 2009;21(1):CD001989.
21. Pielen A, Pantenberg S, Schmoor C, et al. Predictors of prognosis and treatment outcome in central retinal artery occlusion: local intra-arterial fibrinolysis vs. conservative treatment. Neuroradiology. 2015;57(10):1055–1062. doi:10.1007/s00234-015-1588-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.