Back to Journals » Clinical Ophthalmology » Volume 16
Internal Limiting Membrane Peeling and Gas Tamponade For Full-Thickness Macular Holes of Different Etiology – Is It Still Relevant?
Authors Ruban A, Petrovski BÉ, Petrovski G , Lytvynchuk LM
Received 27 May 2022
Accepted for publication 10 August 2022
Published 13 October 2022 Volume 2022:16 Pages 3391—3404
DOI https://doi.org/10.2147/OPTH.S373675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video 1 of "ILM peeling and GT for FTMH" [ID 373675].
Views: 128
Andrii Ruban,1 Beáta Éva Petrovski,2 Goran Petrovski,2– 4 Lyubomyr M Lytvynchuk5,6
1Center of Clinical Ophthalmology, Kyiv, Ukraine; 2Department of Ophthalmology, Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; 3Center for Eye Research, Department of Ophthalmology, Oslo University Hospital, Oslo, Norway; 4Department of Ophthalmology, University of Split School of Medicine and University Hospital Centre, Split, Croatia; 5Department of Ophthalmology, Justus-Liebig-University Giessen, Eye Clinic, University Hospital Giessen and Marburg GmbH, Giessen, Germany; 6Karl Landsteiner Institute for Retinal Research and Imaging, Vienna, Austria
Correspondence: Lyubomyr M Lytvynchuk, Department of Ophthalmology, Justus-Liebig-University Giessen, Eye Clinic, University Hospital Giessen and Marburg GmbH, Campus Giessen, Friedrichstrasse 18, Giessen, 35392, Germany, Tel +49 64198543820, Fax +49 64198543809, Email [email protected]
Background: Despite the abundance of novel surgical approaches proposed for full thickness macular hole (FTMH) treatment, the choice of the optimal technique remains debatable Vitrectomy with «classic» internal limiting membrane peeling and gas tamponade remains the standard of FTMH surgery in many cases, but there are still very limited recent publications on the outcomes of such surgery.
Purpose: To investigate the anatomical and functional result and to analyze the significance of outcome-related risk factors of the classic 25-gauge pars plana vitrectomy (PPV) with ILM peeling and gas tamponade (GT) for treatment of FTMH of different etiology.
Patients and methods: Thirty-eight eyes of thirty-seven patients with FTMH who underwent 25-gauge PPV, ILM peeling and GT were recruited for this retrospective, consecutive, interventional study. Four eyes with persistent holes underwent a re-operation. Outcome-related factors were discussed.
Results: The primary closure rate was 89.5% (34/38). All eyes that underwent the repeated surgery (4 cases) obtained final closure. A hole size of > 500 μm has a statistically significant effect on the primary macular hole closure (F = 0.048; &phis; = 0.38; p ˂ 0.05). In the general group (N = 38), the duration of symptoms directly correlated with age (ρ = 0.34; p = 0.04), size of the hole (ρ = 0.66; p ˂ 0.001) and BCVA before surgery (ρ = 0.59; p ˂ 0.001), after 1 month (ρ = 0.36; p = 0.03), and after 3 months (ρ = 0.35; p = 0.03). Preoperative BCVA was better in initially closed cases (Group 1) (U = 26.0; p = 0.05). In the Group 2 with primary unclosed holes, 75% of the eyes (3/4) had an axial length (AL) > 26 mm, while in Group 1 such eyes were 12.5 times less (2/34) 5.9% (F = 0.004; &phis; = 0.63; р ˂ 0.01). The ELM recovery rate at 3 months was 92% (35/38 eyes) and the restoration of EZ at 3 months was 47% (18/38 eyes). Best-corrected visual acuity of all individuals improved significantly from 0.72 ± 0.35 (logMAR) (Me = 0.7; IQR: 0.5– 0.8) to 0.25± 0.14 (logMAR) (Me = 0.2; IQR: 0.2 – 0.3) at 1 month and 0.17 ± 0.13 (logMAR) (Me = 0.2; IQR: 0.1 – 0.2) at 3 months after surgery (P = 0.0001).
Conclusion: 25G PPV with ILM and GT for FTMH of different etiology provide satisfactory morphologic and functional outcomes. Elongated AL, large diameter of MH and long duration of symptoms are the risk factors for initial closure. Proper second surgery can obtain satisfactory outcomes for persistent holes.
Keywords: full-thickness macular hole, pars plana vitrectomy, internal limiting membrane peeling, gas tamponade, restoration of ELM/EZ, macular hole closure
A Letter to the Editor has been published for this article.
A Response to Letter by Professor Gonul has been published for this article.
Introduction
Macular holes (MH) are characterized by a full-thickness defect at the fovea due to abnormal vitreofoveal traction, which causes impaired central vision with scotoma and metamorphopsia. Full-thickness macular holes (FTMH) have an annual incidence of 8 per 100,000 individuals,1 and prevalence in the general population of 0.2 to 3.3 per 1000 individuals.2,3 This condition occurs more frequently in women and adults aged 70 years or older, and it is unilateral in around 80% of the cases.4,5
Although, MH have been known since the 19th century as an untreatable condition, the situation has changed after Kelly and Wendel have shown that pars plana vitrectomy (PPV) combined with vitreous cortex removal and fluid–gas exchange can result in MH closure and improve the vision.6
Internal limiting membrane (ILM) peeling in FTMH surgery was first reported by Eckardt et al7 and Park et al8 and gained widespread acceptance due to the improved closure rates. Therefore, most surgeons peel the ILM all around the MH. Randomized controlled trials also demonstrated higher anatomic closure and lower reoperation rates in patients who had macular ILM peeling compared to those who did not.9 The release of anteroposterior and tangential traction forces on an abnormally persistent vitreofoveal adhesion and the removal of the remaining macular cortical vitreous has been suggested to be the main mechanism behind FTMH closure after PPV with ILM peeling.10,11 ILM peeling also causes retinal displacement and mechanical trauma to the retina by tearing of the endfeet of the Müller cells, thus stimulating regeneration and reactive gliosis that can enhance the FTMH healing.12,13
Over the past two decades many innovative techniques have been proposed to improve the closure rate of FTMH surgery, and now these novel surgical approaches have become the most successful vitreoretinal procedures.14–19 Nevertheless, in order to assess the efficacy and safety of the new methods for FTMH surgery we should be aware and appreciate the capabilities of the classic technique consisting of PPV with ILM peeling and gas tamponade (GT). However, with the rapid development of the surgical systems, surgical instruments and minimally invasive approaches, there is still very scant recent literature on the outcomes of standard macular hole surgery for FTMH.
The aim of this study was to investigate the anatomical results (MH closure rate, recovery of the ELM line and EZ line) and functional result (postoperative BCVA) and to analyze the outcome-related risk factors and their significance regarding the anatomical and visual improvement of the classic 25G PPV with ILM peeling and gas tamponade (GT) for treatment of FTMH of different etiology (idiopathic, myopic and traumatic).
Methods
Ethical Statement
This retrospective, consecutive, interventional study was conducted at the Center of Clinical Ophthalmology (Kyiv, Ukraine) from December 2018 to August, 2020. The study was performed according to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the City of Kyiv (Ukraine) with protocol № 2018–11-032. All patients were informed about all risks and benefits of the surgical treatment and signed a written informed consent form. Also, a signed consent was obtained for the use of the image data presented in this study.
Patients and Clinical Examination
Thirty-eight eyes of 37 patients with diagnosis of idiopathic, myopic and traumatic FTMH, which were treated with PPV, ILM peeling and GT, were included in this study. All patients had a complete ophthalmic examination before and after surgery, including measurement of best-corrected visual acuity (BCVA, decimal value/logMAR), applanation tonometry, slit-lamp examination, fundus ophthalmoscopy and swept-source optical coherence tomography (SS-OCT) imaging.
Macular hole size was determined using a protocol previously described by the International Vitreomacular Traction Study Group.20 The minimum width of the MH was measured at the narrowest hole point in the mid retina of the foveal horizontal B-scan, using the OCT caliper function (Triton, TOPCON Corporation, Tokyo, Japan), as a line drawn parallel to the retinal pigmented epithelium.
Macular hole closure was defined by SS-OCT as the complete disappearance of the hole and absence of neurosensory defect over the fovea. Flat-open and elevated-open MHs were considered as surgical failures. The efficiency of the surgery was evaluated by the anatomical macular hole closure rate and BCVA at 1 and 3 months. The duration of the MH was based on the known duration of the symptoms.
Recovery of the ELM line and EZ line was determined by SS-OCT images through the fovea according to the method described by Iwasaki et al.21 When a complete continuous line could be confirmed in both the vertical and horizontal SD-OCT images, it was defined as recovered. When the line was disrupted by a gap or other tissue in the vertical or horizontal SD-OCT image, it was defined as unrecovered.
For the OCT protocol, the SS-OCT instrument (Triton, TOPCON Corporation, Tokyo, Japan) was used on postoperative Days 1, 2, 3, 7, and 14. We followed a previously reported protocol for imaging gas-filled eyes.22,23 The patient was in a sitting position looking straight ahead. The focus was then adjusted to around −20 diopters (D), until the best-focused image was obtained. If the image quality was not good enough, the patient was given a contact lens (with a maximum minus (-) diopters: range −15.0 - −20.0 D). Five 6-mm radical line scans including the macular center were obtained, making sure that either a foveal depression or an MH was included in the scanning area.
Patients were followed up at Day 1, Day 3, Week 1, Week 4, and then monthly up to 6 months after surgery. Postoperative complications related to the surgery were documented and analyzed as well.
In order to analyze the outcome-related risk factors and their significance regarding the anatomical and visual improvement all cases were subdivided into two groups: Group 1 - the group with primary MH closure after the first surgery (N = 34 eyes) and Group 2 - the group with persistent MHs after the first surgery, but with the complete MH closure after the second surgery (N = 4 eyes).
Surgical Procedure
All surgeries were performed by one experienced vitreoretinal surgeon (AR). A standard 25G PPV was performed under subtenon anesthesia in all patients using the Constellation® Vision System (Alcon, ForthWorth, TX, USA) under a non-contact viewing system (Topcon, Tokyo, Japan). The central core vitrectomy and posterior vitreous detachment were performed with triamcinolone-assisted visualization and the vitreous was cut to the peripheral vitreous base. Membrane Blue Dual (DORC, VC Zuidland, the Netherlands) was then injected to stain the ILM for approximately 30 seconds, followed by its removal. The ILM was then peeled in a circular manner 1.5–2.0 disk diameters (DD) around the MH. Fluid-air (FAX) exchange was facilitated with the backflush cannula and a maximum drainage of subretinal fluid was carefully performed through the macular hole. Retinal massage and stretch were gently applied in large holes with size >600 μm. Each surgery was completed by injection of 1.5 mL undiluted sulfur hexafluoride (SF6) gas (Al.chi.mi.a, Ponte San Nicolo PD, Italy). The postoperative protocol consisted of routine topical antibiotic and anti-inflammatory agents (dexamethasone and NSAID).
Postoperative Posturing
All the patients were ordered to strictly keep face-down posturing for 24 hours after surgery. SS-OCT examination was performed on postoperative Day 1. When closure of the FTMH was confirmed at Day 1, posturing was stopped. If not, posturing was continued for 2 days more. In cases where MH was still not closed on Day 3 confirmed by OCT, an additional surgery was offered to the patient.
Statistical Methods
The BCVA was recorded as decimal value and converted to the logarithm of minimal angle of resolution (logMAR) and Snellen fraction for statistical analysis. The description used the mean ± standard deviation (M ± σ), median (interquartile range) (Me (IQR)), minimum - maximum (Range) values. Quantitative indicators were checked for compliance with the normal distribution law by the Kolmogorov–Smirnov criterion. When comparing independent groups, the Mann–Whitney test (U) was used, and when paired comparison, the Wilcoxon test (W) was used, the relationship between indicators was checked by the two-sided Spearman test (ρ). Qualitative indicators in the groups were compared using contingency tables (strength of connection (φ), presence of connection - Fisher’s exact test (F)). A P value of less than 0.05 was considered to be statistically significant. The data were processed using STATISTICA 10 software (StatSoft, Inc, Tulsa, OK, USA).
Results
Patients
Among all patients there were 9 males and 28 females. The mean postsurgical follow-up was 5.0 months. The mean age was 63.6 ± 11.5 (range: 15–84 years). The median minimum diameter of the macular hole was 391.4 ± 174.9 μm (range: 144–765 μm) (Figures 1A, 2A and 3A). Nineteen eyes (50%) had MH diameter >400 μm and 6 (15.8%) had MH > 600 μm. Five eyes (13.6%) had an axial length >26 mm. The median duration of reported symptoms was 5.7 ± 7.1 months (range: 1–36 months), and 8 eyes (21.05%) had symptoms duration >6 months. The median preoperative BCVA was 0.25 ± 0.18 (decimal) (Me = 0.2; IQR: 0.15–0.3), 0.72 ± 0.35 (logMAR) (Me = 0.7; IQR: 0.5–0.8).
The epidemiologic characteristics of the patients, as well as preoperative functional and anatomic conditions are summarized in Table 1.
 |
Table 1 Preoperative Characteristics of Patients with Full-Thickness Macular Holes Undergoing Vitrectomy with ILM Peeling |
Anatomical Results
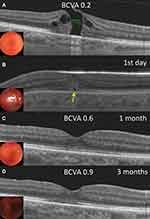
A combined procedure (phacoemulsification and intraocular lens implantation in combination with PPV) was performed in 31 eyes (81.5%). After the first vitrectomy MH closure was achieved in 89.5% (34/38) eyes (Figures 1B, 2B and 3B) (Table 2). Moreover, MH closure was observed in 84% (32/38) of the eyes on Day 1, 87% (33/38) on Day 2 and 89.5% (34/38) on Day 3. Macular hole closure was not achieved if the MH was still open at Day 3.
 |
Table 2 Anatomical and Functional Results in Patients with Full-Thickness Macular Holes Undergoing Vitrectomy with ILM Peeling |
Four eyes with persistent MHs required an additional surgery. In 3 of these eyes, the MH closed after the second surgery, and in one eye after the third surgery. In these cases, a combination of two techniques, namely subretinal application of BSS and pedicle ILM flap transposition technique were used (Supplementary Materials Video S1 and S2). There were no cases of reopened MH during the follow-up period. Specific details of persistent FTMH cases after the first surgery are depicted in Supplementary Material Table S1.
Primary closure rate was achieved in 96.5% for FTMHs <500 μm and in 70% for FTMHs >500μm. In patients with axial length <26.0 mm the primary closure rate was 97.4%, whereas in high myopic eyes (with axial length >26.0 mm) – only 40%. Additionally, FTMHs were closed after the first surgery in 93.4% cases with duration of the symptoms <6 months and in 75% eyes with duration of the symptoms >6 months.
Additional detailed information about functional and anatomical results in the entire group can be found in Supplemental Material; Table S1.
Functional Outcomes
Mean BCVA significantly improved from 0.25 ± 0.18 (decimal), (Me = 0.2; IQR: 0.15–0.3) or 0.72 ± 0.35 (logMAR) (Me = 0.7; IQR: 0.5–0.8) to 0.6 ± 0.2 (decimal) (Me = 0.6; IQR: 0.5 – 0.7) or 0.25±0.14 (logMAR) (Me = 0.2; IQR: 0.2 – 0.3) at 1 month, and 0.73 ± 0.2 (decimal) (Me = 0.7; IQR: 0.6 – 0.9) or 0.17 ± 0.13 (logMAR) (Me = 0.2; IQR: 0.1 – 0.2) at 3-months after surgery (P = 0.0001) (Table 2).
Thirty-two eyes (84.2%) showed a visual improvement of at least two lines at 1 month and 34 eyes (89.5%) at the 3-months follow-up. Twenty-four eyes (63.1%) achieved a postoperative BCVA > 0.5 decimal at the 1 month- and 30 eyes (78.9%) at the 3-months follow-up. In 6 eyes (15.8%), the visual acuity (VA) reached 1.0 decimal at 3-months after surgery. The average increase in the BCVA (decimal) at 1 month was 0.35 (IQR: 0.07–0.7) and 0.48 (IQR: 0.1–0.8) at 3-months, respectively.
The comparison of microperimetry (SLO) examination before and after the surgery demonstrated the involution of paracentral scotoma and restoration central retinal sensitivity (Figures 1G and 2E) in case with primary MH closure.
Analysis of SS-OCT Imaging
The ELM recovery rate at 3 months was 92% (35/38 eyes) and the restoration of EZ at 3 months was 47% (18/38 eyes). In all cases, the EZ recovery always preceded ELM recovery (Figures 1D–F, 2D and 3C). During the entire follow-up period we have not seen any case of the recurrency of integrity failure of ELM or EZ lines, which recovered after the surgery.
Correlation Between Pre- and Postoperative Variables
The difference in variables between the groups with primary closed (Group 1) and persistent MH cases (Group 2) after the first surgery is demonstrated in Table 3.
 |
Table 3 Differences in Variables Between the Groups with Primary Closed and Persistent MH Cases After the First Surgery |
Comparing the characteristics between these two groups, it was noted that the preoperative BCVA was better in Group 1 (U = 26.0; p = 0.05), and there were no significant differences in the macular hole size (U = 45.0; p = 0.29), axial length (U = 32.0; p = 0.09), duration of symptoms (U = 45.5; p = 0.29) and age (U = 44.0; p = 0.28) between the two groups. At the same time, in the Group 2 with primary unclosed holes, 75% of the eyes (3/4) had an axial length >26 mm, while in Group 1 such eyes were 12.5 times less prevalent (2/34) 5.9% (F = 0.004; φ = 0.63; р ˂ 0.01). In the Group 1, patients with axial length >26 mm had statistically significant smaller hole sizes (179.5 ± 26.2 µm) (U = 4.0; p = 0.03), while for axial length <26 mm, the average hole size was 391.35 ± 162.3 µm (Me = 388.5; IQR: 260.3–482.8 µm).
In Group 1 with primary closure, there were 20.6% eyes (7/34) with a macular hole size >500 μm, whereas in Group 2, there were 75.0% (3/4) of such patients. A hole size >500 μm had a statistically significant effect upon the primary macular hole closure (F = 0.048; φ = 0.38; p ˂ 0.05).
VA in the groups, both before surgery and after 1, 3 months showed a statistically significant difference. Only in Group 2, the BCVA at 3 months was not statistically different from the preoperative values (W = 1.8; p = 0.07).
In the general or combined group (N = 38), the duration of symptoms directly correlated with age (ρ = 0.34; p = 0.04), size of the hole (ρ = 0.66; p ˂ 0.001) and BCVA before surgery (ρ = 0.59; p ˂ 0.001), after 1 month (ρ = 0.36; p = 0.03), and after 3 months (ρ = 0.35; p = 0.03).
The visual acuity (BCVA LogMAR) before surgery and at 1 month after surgery was also statistically significantly correlated with the macular hole size (ρ = 0.53; p = 0.001) (ρ = 0.41; p = 0.01), while, at 3 months, the BCVA in the general or combined group was not dependent on the macular hole size (ρ = 0.30; p = 0.07).
The dynamics of the visual restoration (BCVA at 1 and 3 months) depended with a high degree of correlation on the preoperative VA (ρ = 0.62; p = 0.001) (ρ = 0.64; p = 0.001).
Postoperative Complications
One phakic patient developed cataract during the follow-up (2.6%). Postoperative complications included one case of transitory intraocular hypotension (2.6%), which resolved spontaneously, and 4 cases of intraocular hypertension in four patients (10.5%), which resolved after administration of topical medications. In all cases, there were no serious local or systemic complications noted.
Discussion
Idiopathic, traumatic and myopic full-thickness macular holes remain to be among the most vision threatening diseases. The management of FTMH ranges from classic to different novel surgical techniques and there is no consensus, which approach is more efficient for the treatment of MH of different etiology.
This study defined the anatomical (MH closure rate and integrity of ELM/EZ) and functional (BCVA) outcomes in patients undergoing classic PPV with ILM peeling and gas tamponade, as well as it assessed the factors predicting MH closure. Our primary anatomical success rate of 89.5% met the generally accepted rate in recent reports (87.7%,24 89.6%,25 89.92%,26 92.5%,27 77.8%28). The final MH closure rate in the entire cohort after repeated surgeries in four cases was 100% and there were no cases where the MH would reopen during the follow-up period. Our surgical procedure had a consistently high success rate >90% for MHs the size of <500 μm. For MHs with the size of >500 μm the success rate dropped to <70%. This corresponds to the report of Steel et al29 where the authors found in a large database study of 1483 primary MH operations treated by PPV, ILM peeling and gas/air tamponade that a minimum linear diameter of ~500 μm was the threshold for the success rate, which in cases with larger MHs started to decline.
A number of studies demonstrated the similar correlation between the MH size and success rate. Ch’ng et al26 analyzed 258 eyes after MH surgery with conventional ILM peeling and found the closure rate to e 98% for size 400 to 477 µm, 91% for 478 to 558 µm, 94% for 559 to 649 µm, and 76% for 650 to 1416 µm. Wong et al15 used 650 µm as the “cutoff” to describe large MHs, with no given reason as to why they chose that threshold. The study of Yu et al25 on 135 eyes after MH surgery with conventional ILM peeling and air tamponade provided a “cutoff” value of 677 mm to predict success of the initial surgery, with closure rate of 97.94% for smaller, and 68.42% for larger holes from that threshold. They further suggested that MHs <650 mm in diameter are highly possible to get satisfactory outcomes after primary surgery, whereas those >700 mm should be treated cautiously.
Our data support the opinion of these other studies that a consensus on an updated anatomic classification system of FTMH would be needed and the definition of large MH probably should be changed to ≥500 μm. Standard MH surgery has very high success rate up to 500 μm and an alternative surgical technique such as ILM flaps or hydraulic retinal relocation should be reserved for larger holes.
It is generally accepted that axial length is an important predictor of macular closure.30–35 We hereby argue that axial length of the eye can be a more significant prognostic factor of the anatomical outcome compared to the MH size. The size or the “cut-off” for the MHs that were closed in high myopic eyes after the first operation was significantly smaller compared to holes in non-myopic eyes (Me = 388.5 µm; IQR: 260.3–482.8 µm).
Previous reports have shown better closure rates and better final visual acuity outcome when the duration of symptoms is less than 6 months.4,36 Our data indicated that a MH, which has been present for more than 2 to 3 years, could be surgically closed, yet the success rate was lower (63%), and the VA outcome was worse than for MH of shorter duration.37–39 Yu et al25 reported 73 eyes, which had symptomatic durations less than 6 months and 83.56% of them were closed after the initial surgical intervention. This study concluded that proper surgical intervention and sufficient gas tamponade work efficiently in such cases. In our study, anatomical success was achieved in 93.4% eyes after first vitrectomy with symptoms duration <6 months, and in 75% eyes with symptoms duration >6 months.
One of the probable reasons for failed surgical outcome in chronic MH surgery may be due to a tight adhesion of the photoreceptor layer of the retina to the underlying RPE that was observed in our preliminary study as well as by C. Yun et al40,41 Meyer at al.42 advocated that remaining retinal adhesion in long-standing MH can explain the failure rate after surgery for persistent MHs with application of subretinal fluid.
Though accurate determination of the known duration of symptoms is problematic, our data confirm its correlation with the MH size, preoperative BCVA and patient’s age, as well as confirm the need for an earlier operation in order to obtain a higher VA.
It is quite difficult to correctly compare the results of different studies due to the lack of the uniform method for determining the integrity of the outer retinal layers after MH vitrectomy. We used the method described by Iwasaki et al21 in which the ELM recovery rate and period in the ILM peeling group was 70.0%, and 3.4 months, respectively, while the EZ recovery rate in this group was 30.0%. Baumann et al24 assessed the integrity of the ELM and EZ for large MH surgery with ILM peeling. At 3 months, the EZ was fully and partially restored in 10% and 70% of patients, respectively. The ELM was fully and partially restored in 78% and 15% of patients, respectively. The integrity of the EZ also improved significantly between 3, 6, and 12 months. Caprani et al43 classified the layers as either present or absent with no mention disruptions after MH surgery with ILM peeling: they also observed the ELM was the first layer to be restored in the healing process, while the integrity of the EZ was present in 53.5% of the patients at 3 months and in 73.91% at 6 months. We observed the ELM recovery at 3 months in 92% eyes and the restoration of EZ in 47% eyes. In all cases ELM recovery preceded EZ recovery, and in no cases were the recovered ELM or EZ lines torn again. This is in line with the results obtained by others that the integrity of the ELM is critical for achieving a normal IS/OS postoperatively.44,45
It may be hypothesized that the main predisposing factor for closing a MH by “primary intention” is the minimum size of the foveal defect. Kawashima et al45 found that eyes with a longer preop OS and ELM gap length had a significantly worse postoperative VA, more severe glial cell proliferation and poor photoreceptor layer status than did eyes with a smaller defect. In other words: the larger the defect we leave at the end of the surgery, the greater the chance of healing by “secondary intention” and the worse the VA after surgery. That is why intraoperative MH size reduction through subretinal fluid aspiration and convergence of hole’s edges during the fluid–gas exchange can be considered highly desirable. Moreover, this maneuver allows induction of centripetal movement of foveal tissue that according to Hillenkamp et al46 is a key point in closing the MH. We do not drain the MH if its diameter is less than 250 μm and in cases with foveolar flap confirmed by SS-OCT.
The BCVA of all eyes in this study was improved after surgery, with a significant preoperative-to-postoperative difference in the BCVA at 1 and 3 months after surgery. It corresponds with the results of conventional MH surgery with ILM peeling reported by Shiono et al27 where the authors were able to achieve a mean postoperative BCVA 0.11 ± 0.17 LogMAR, but with a much smaller mean macular hole size (240 μm). Meanwhile, our results are somewhat higher than published data of recent studies on MH surgery with ILM peeling, where mean postoperative BCVA did not exceed 0.3 logMAR, although the average MH size in these studies was much larger and exceeded 500 μm. 21,24–26,28 Functional success rate in our study (defined as VA of 0.30 or better logMAR) was achieved in 63.1% eyes at 1 month, and in 78.9% eyes at 3 months, which is slightly better than in Ch’ng study,26 where only 19.8% eyes achieved 0.3 logMAR (6/12 Snellen equivalent) or 20% eyes in another study by Gupta et al.47 The better visual outcome in our study could be related to a greater proportion of ELM and EZ recoveries at 3 months, which goes in support of the peeling technique which allows better improvement in VA due to complete restoration of ELM and EZ.21,24,44
Postoperative positioning after MH surgery remains controversial. Strong evidence is not available on the optimal duration of the prone position to achieve MH closure. Facedown posturing (FDP) is recommended because the gas bubble with superficial tension forces could support the apposition of the MH edges for a certain period of time and also providing a scaffold for the migration of glial cells and blocking fluid entry into the hole.48,49 The force of the gas bubble is greatest at the apex of the arc of contact to the retinal surface, which diminishes to near zero where the lower meniscus of the bubble contacts the retina.50 Nevertheless, prone positioning is considered uncomfortable for the patients and may lead to pressure sores or neuropathy.51,52 Ye et al53 in a meta-analysis of five randomized controlled trials compared MH surgeries with ILM peeling with postoperative FDP versus those with non-supine posturing (NSP). The MH closure rate was higher in the FDP group, while a significant difference in the closure rate for MH with size >400 μm, but not for those sized <400 μm was found. Smaller MHs may require only gas tamponade without FDP to achieve successful anatomic healing. All the patients in our cohort followed a strict FDP for 1 day after surgery until the next morning. Further recommendations for FDP were determined by the control OCT at Days 1 and 3. Eckardt et al54 and others55–57 considered it necessary to end the FDP as soon as OCT confirmed closure; non-closure at Day 3 would mean an additional surgery performed Days 5 and 6 to achieve better surgical outcomes.
In all failed to close (FTC) cases in this study, we used the novel technique recently described by us:58 a combination of SR-fluid application with centripetal displacement of the macula using silicone-tipped cannula, combined with inverted ILM flap technique: inverted pedicle ILM flap technique was used in 3 cases and free ILM flap in 1 case (Supplementary Video S2).
In our study, the final closure rate after reoperations was 100% and ≥2-line BCVA improvement at 3-months in 100% eyes (4 of 4 eyes), while 75% (3 of 4 eyes) of FTC eyes achieved BCVA ≥0.5 decimal. These results are higher than in one recent systematic review and meta-analysis59 in which it was shown that FTC idiopathic FTMH after the first surgery achieved only BCVA ≥0.5 decimal in 15% of cases, with a pooled estimated probability of ≥2-line BCVA improvement being 58% in the FTC group. Additionally, the interval between the first and second operations in three cases was relatively short (1 week), and between the second and the third surgery in one case – it was not >2 weeks. It has been suggested that an increased time between first and second surgeries may contribute to poorer visual and anatomical outcomes.60–62
There are currently no prescribed parameters for the optimal extent of ILM to be peeled during MH surgery. Modi et al63 performed a prospective study of 50 patients undergoing surgery with ILM peel radii of 1 and 1.5 diameter disks (DD), and found no significant difference in hole closure rates, but better visual outcomes in the smaller peel radii group with less retinal nerve fiber layer thinning, particularly temporally. In our case series, the ILM was peeled in a circular manner 1.5–2.0 disk diameters (DD) around the MH.
This study has several limitations. First, it is retrospective with a minimum follow-up of 3 months, which means the true functional results may be underestimated, since vision is likely to improve further in successfully closed FTMH.64 Furthermore, the exact duration of symptoms was not clearly documented in most patients, which may influence the FTMH closure rate and post-operative visual result. Finally, we have used minimum linear diameter (MLD) (obtained only by horizontal scan) to define FTMH and have not included other parameters such as base diameter, vertical diameter and macular hole indexes. Radial macular scans which were carried out in our study may not be a true determinant of horizontal minimum MH size in some cases due to the extrafoveal patient’s fixation, and should be replaced with raster scans. We argue that measuring the MH only by horizontal diameter does not demonstrate the actual hole’s size. MH largest diameter is not always horizontal, it could be vertical or lie in an oblique axis. Moreover, this method is manual and subjective, therefore, its reproducibility and precision depend on the observer’s experience. We also believe that a new objective method for determining not only MLD, but the surface area of the MH is needed.
Overall, the standard PPV with ILM peeling and air/gas endotamponade is considered to be one of the choices to treat FTMH. Multimodal pre- and postoperative examination techniques including multifocal retinogram and OCT angiography could deliver additional knowledge about the efficacy of different surgical techniques with regard to anatomical and functional postoperative results.65 Moreover, the utilization of intraoperative OCT during pars plana vitrectomy showed to be useful in controlling the surgical maneuvers and avoiding intrasurgical iatrogenic trauma, which could also influence postoperative results.66 Nevertheless, the treatment of complicated cases of FTMH, such as postoperative MH or MH associated with high myopia and macular schisis remains challenging.67,68
Conclusions
Classic vitrectomy with ILM peeling and gas tamponade seems to be an effective and safe procedure which could obtain satisfactory anatomical and functional results for primary FTMH of different etiology (idiopathic, myopic and traumatic). Though accurate determination of the known duration of symptoms is problematic, our data confirm its correlation with the MH size, preoperative BCVA and patient’s age, as well as confirm the need for an earlier operation in order to obtain a higher vision. A more comprehensive classification system for FTMH is highly needed, based on the most prognostically important preoperative factors that determine the anatomical and functional outcomes of surgery. Further prospective studies are needed to compare anatomic and especially functional outcomes of standard macular hole surgery with alternative techniques, such as ILM flap, retinal detachment technique and autologous platelet-rich plasma concentrate for primary FTMH.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; they all took part in the drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work..
References
1. McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology. 2009;116(7):1366–1369. doi:10.1016/j.ophtha.2009.01.052
2. Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The blue mountains eye study, Australia. Ophthalmology. 1997;104(6):1033–1040. doi:10.1016/S0161-6420(97)30190-0
3. Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore eye survey. Ophthalmology. 1996;103(11):1721–1726. doi:10.1016/S0161-6420(96)30435-1
4. Kang H, Chang AA, Beaumont PE. The macular hole: report of an Australian surgical series and meta-analysis of the literature. Clin Experiment Ophthalmol. 2000;28(4):298–308. doi:10.1046/j.1442-9071.2000.00329.x
5. Johnson MW, Van Newkirk MR, Meyer KA. Perifoveal vitreous detachment is the primary pathogenic event in idiopathic macular hole formation. Arch Ophthalmol. 2001;119(2):215–222.
6. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–659. doi:10.1001/archopht.1991.01080050068031
7. Eckardt C, Eckardt U, Groos S, Luciano L, Reale E. Entfernung der membrana limitans interna bei makulalöchern. Klinische und morphologische befunde [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings]. Ophthalmologe. 1997;94(8):545–551. German. doi:10.1007/s003470050156
8. Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal limiting membrane peeling and intravitreous air. Ophthalmology. 1999;106(7):1392–1398. doi:10.1016/S0161-6420(99)00730-7
9. Lois N, Burr J, Norrie J, et al.; Full-thickness Macular Hole and Internal Limiting Membrane Peeling Study (FILMS) Group. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci. 2011;52(3):1586–1592. doi:10.1167/iovs.10-6287
10. Steel D, Lotery A. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye. 2013;27:1–21. doi:10.1038/eye.2013.212
11. Smiddy WE, Flynn HW
12. Hauck SM, von Toerne C, Ueffing M. The neuroprotective potential of retinal Müller glial cells. Adv Exp Med Biol. 2014;801:381–387.
13. Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi:10.1016/j.devcel.2011.11.020
14. Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–2025. doi:10.1016/j.ophtha.2010.02.011
15. Wong R, Howard C, Orobona GD. Retina expansion technique for macular hole apposition report 2. Efficacy, closure rate and risks of a macular detachment technique to close large full-thickness macular holes. Retina. 2018;38:660–663. doi:10.1097/IAE.0000000000001705
16. Ho TC, Yang CM, Huang JS, et al. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole. Graefes Arch Clin Exp Ophthalmol. 2014;252:1553–1560. doi:10.1007/s00417-014-2613-7
17. Mahajan VB, Chin EK, Tarantola RM, et al. Macular hole closure with internal limiting membrane abrasion technique. JAMA Ophthalmol. 2015;133(6):635–641. doi:10.1001/jamaophthalmol.2015.204
18. Chakrabarti M, Benjamin P, Chakrabarti K, Chakrabarti A. Closing macular holes with “macular plug” without gas tamponade and postoperative posturing. Retina. 2017;37(3):451–459. doi:10.1097/IAE.0000000000001206
19. Tian T, Chen C, Peng J, Jin H, Zhang L, Zhao P. Novel surgical technique of peeled internal limiting membrane reposition for idiopathic macular holes. Retina. 2019;39(1):218–222. doi:10.1097/IAE.0000000000001745
20. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi:10.1016/j.ophtha.2013.07.042
21. Iwasaki M, Kinoshita T, Miyamoto H, Imaizumi H. Influence of inverted internal limiting membrane flap technique on the outer retinal layer structures after a large macular hole surgery. Retina. 2019;39:1470–1477. doi:10.1097/IAE.0000000000002209
22. Sano M, Inoue M, Itoh Y, et al. Duration of prone positioning after macular hole surgery determined by swept-source optical coherence tomography. Retina. 2017;37(8):1483–1491. doi:10.1097/IAE.0000000000001394
23. Kikushima W, Imai A, Toriyama Y, Hirano T, Murata T, Ishibashi T. Dynamics of macular hole closure in gas-filled eyes within 24 h of surgery observed with swept source optical coherence tomography. Ophthalmic Res. 2015;53(1):48–54. doi:10.1159/000368437
24. Baumann C, Kaye S, Iannetta D, Sultan Z, Dwivedi R, Pearce I. Effect of inverted internal limiting membrane flap on closure rate, postoperative visual acuity, and restoration of outer retinal layers in primary idiopathic macular hole surgery. Retina. 2020;40(10):1955–1963. doi:10.1097/IAE.0000000000002707
25. Yu Y, Liang X, Wang Z, et al. Internal limiting membrane peeling and air tamponade for stage iii and stage iv idiopathic macular hole. Retina. 2020;40(1):66–74. doi:10.1097/IAE.0000000000002340
26. Ch’ng SW, Patton N, Ahmed M, et al. The Manchester large macular hole study: is it time to reclassify large macular holes? Am J Ophthalmol. 2018;195:36–42. doi:10.1016/j.ajo.2018.07.027
27. Shiono A, Kogo J, Sasaki H, et al. Hemi-temporal internal limiting membrane peeling is as effective and safe as conventional full peeling for macular hole surgery. Retina. 2019;39(9):1779–1785. doi:10.1097/IAE.0000000000002215
28. Narayanan R, Singh SR, Taylor S, et al. Surgical outcomes after inverted internal limiting membrane flap versus conventional peeling for very large macular holes. Retina. 2019;39(8):1465–1469. doi:10.1097/IAE.0000000000002186
29. Steel DH, Donachie PHJ, Aylward GW, Laidlaw DA, Williamson TH, Yorston D. BEAVRS Macular hole outcome group. Factors affecting anatomical and visual outcome after macular hole surgery: findings from a large prospective UK cohort. Eye. 2021;35(1):316–325. doi:10.1038/s41433-020-0844-x
30. Rizzo S, Tartaro R, Barca F, Caporossi T, Bacherini D, Giansanti F. Internal limiting membrane peeling versus inverted flap technique for treatment of full-thickness macular holes: a comparative study in a large series of patients. Retina. 2018;38(1):S73–S78. doi:10.1097/IAE.0000000000001985
31. Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156(1):125–131.e1. doi:10.1016/j.ajo.2013.02.014
32. Sulkes DJ, Smiddy WE, Flynn HW, Feuer W. Outcomes of macular hole surgery in severely myopic eyes: a case-control study. Am J Ophthalmol. 2000;130:335–339. doi:10.1016/S0002-9394(00)00489-X
33. Patel SC, Loo RH, Thompson JT, Sjaarda RN. Macular hole surgery in high myopia. Ophthalmology. 2001;108:377–380. doi:10.1016/S0161-6420(00)00532-7
34. García-Arumí J, Martínez V, Puig J, Corcóstegui B. The role of vitreoretinal surgery in the management of myopic macular hole without retinal detachment. Retina. 2001;21:332–338. doi:10.1097/00006982-200108000-00006
35. Kobayashi H, Kobayashi K, Okinami S. Macular hole and myopic refraction. Br J Ophthalmol. 2002;86:1269–1273. doi:10.1136/bjo.86.11.1269
36. Thompson JT, Sjaarda RN, Lansing MB. The results of vitreous surgery for chronic macular holes. Retina. 1997;17(6):493–501. doi:10.1097/00006982-199711000-00002
37. Scott RA, Ezra E, West JF, Gregor ZJ. Visual and anatomical results of surgery for long standing macular holes. Br J Ophthalmol. 2000;84(2):150–153. doi:10.1136/bjo.84.2.150
38. Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120(1):29–35. doi:10.1001/archopht.120.1.29
39. Brooks HL
40. Lytvynchuk L, Ruban A, Stieger K. Strong adhesion of RPE to photoreceptors explains in part the failure of macular detachment technique for large macular hole closure: iOCT findings. Invest Ophthalmol VisSci. 2020;61(9):B0034.
41. Yun C, Oh J, Hwang SY, Togloom A, Kim SW, Huh K. Morphologic characteristics of chronic macular hole on optical coherence tomography. Retina. 2012;32(10):2077–2084. doi:10.1097/IAE.0b013e31825620ba
42. Meyer CH, Szurman P, Haritoglou C, et al. Application of subretinal fluid to close refractory full thickness macular holes: treatment strategies and primary outcome: APOSTEL study. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2151–2161. doi:10.1007/s00417-020-04735-3
43. Caprani SM, Donati S, Bartalena L. Macular hole surgery: the healing process of outer retinal layers to visual acuity recovery. Eur J Ophthalmol. 2017;27(2):235–239. doi:10.5301/ejo.5000905
44. Hayashi H, Kuriyama S. Foveal microstructure in macular holes surgically closed by inverted internal limiting membrane flap technique. Retina. 2014;34:2444–2450. doi:10.1097/IAE.0000000000000252
45. Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117(9):1815–1824. doi:10.1016/j.ophtha.2010.01.017
46. Hillenkamp J, Kraus J, Framme C, et al. Retreatment of full-thickness macular hole: predictive value of optical coherence tomography. Br J Ophthalmol. 2007;91(11):1445–1449. doi:10.1136/bjo.2007.115642
47. Gupta B, Laidlaw DA, Williamson TH, Shah SP, Wong R, Wren S. Predicting visual success in macular hole surgery. Br J Ophthalmol. 2009;93:1488–1491. doi:10.1136/bjo.2008.153189
48. Reppucci V. Mechanism of macular hole closure. Acta Ophthalmol. 2013;91:252. doi:10.1111/j.1755-3768.2013.1417.x
49. Hasler PW, Prunte C. Early foveal recovery after macular hole surgery. Br J Ophthalmol. 2008;92:645–649. doi:10.1136/bjo.2007.130971
50. Ryan S, Chang S. Retina (Elsevier); 2007:2165-21–77. Chapter 127 Volume III
51. Ciulla TA, Frederick AR
52. Treister G, Wygnanski T. Pressure sore in a patient who underwent repair of a retinal tear with gas injection. Graefes Arch Clin Exp Ophthalmol. 1996;234(10):657–658. doi:10.1007/BF00185301
53. Ye T, Yu JG, Liao L, Liu L, Xia T, Yang LL. Macular hole surgery recovery with and without face-down posturing: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2019;19(1):265. doi:10.1186/s12886-019-1272-1
54. Eckardt C, Eckert T, Eckardt U, Porkert U, Gesser C. Macular hole surgery with air tamponade and optical coherence tomography-based duration of face-down positioning. Retina. 2008;28(8):1087–1096. doi:10.1097/IAE.0b013e318185fb5f
55. Hejsek L, Stepanov A, Dusova J, et al. Microincision 25G pars plana vitrectomy with peeling of the inner limiting membrane and air tamponade in idiopathic macular hole. Eur J Ophthalmol. 2017;27:93–97. doi:10.5301/ejo.5000815
56. Liu M, Guo JL, Zhang H. Clinical observation on vitreous surgery in treating idiopathic macular hole. Int Eye Sci. 2013;13:2456–2458.
57. Tao M, Li Y, Zhang WF. Clinical observation of vitrectomy combined with room air-filled for idiopathic macular hole. Int Eye Sci. 2015;15:2006–2008.
58. Lytvynchuk LM, Ruban A, Meyer C, Stieger K, Grzybowski A, Richard G. Combination of Inverted ILM Flap technique and subretinal fluid application technique for treatment of chronic, persistent and large macular holes. Ophthalmol Ther. 2021;10(3):643–658. doi:10.1007/s40123-021-00361-2
59. Reid GA, McDonagh N, Wright DM, Yek JTO, Essex RW, Lois N. First failed macular hole surgery or reopening of a previously closed hole: do we gain by reoperating?-A systematic review and meta-analysis. Retina. 2020;40(1):1–15. doi:10.1097/IAE.0000000000002564
60. D’Souza MJ, Chaudhary V, Devenyi R, Kertes PJ, Lam WC. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br J Ophthalmol. 2011;95(11):1564–1567. doi:10.1136/bjo.2010.195826
61. Patel R, Gopalakrishnan M, Giridhar A. Timing and outcome of surgery for persistent macular hole. Retina. 2017;39:314–318. doi:10.1097/IAE.0000000000001939
62. Moisseiev E, Fabian ID, Moisseiev J, Barak A. Outcomes of repeated pars plana vitrectomy for persistent macular holes. Retina. 2013;33:1137–1143. doi:10.1097/IAE.0b013e31828076c5
63. Modi A, Giridhar A, Gopalakrishnan M. Comparative analysis of outcomes with variable diameter internal limiting membrane peeling in surgery for idiopathic macular hole repair. Retina. 2017;37:265–273. doi:10.1097/IAE.0000000000001123
64. Leonard RE, Smiddy WE, Flynn HW
65. Li J, Wang W, Zhang X, et al. Morphological and functional features in patients with idiopathic macular hole treatment. J Gen Med. 2022;15:4505–4511.
66. Lytvynchuk LM, Falkner-Radler CI, Krepler K, et al. Dynamic intraoperative optical coherence tomography for inverted internal limiting membrane flap technique in large macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1649–1659. doi:10.1007/s00417-019-04364-5
67. Okonkwo ON, Akanbi T, Agweye CT. Secondary macular holes post pars plana vitrectomy. Med Case Rep J. 2022;15:141–155.
68. Lee S, Gallemore RP. Macular hole surgery in dome-shaped maculopathy. Int Med Case Rep J. 2021;14:493–496. doi:10.2147/IMCRJ.S282118
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.