Back to Journals » International Journal of Nanomedicine » Volume 18
Inorganic Nanoparticles-Based Systems in Biomedical Applications of Stem Cells: Opportunities and Challenges
Received 9 August 2022
Accepted for publication 9 December 2022
Published 7 January 2023 Volume 2023:18 Pages 143—182
DOI https://doi.org/10.2147/IJN.S384343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Xulu Ma,1,2 Zhao Luan,1,2 Jinming Li1– 3
1MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou, 510631, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou, 510631, People’s Republic of China; 3Guangzhou Key Laboratory of Spectral Analysis and Functional Probes, College of Biophotonics, South China Normal University, Guangzhou, 510631, People’s Republic of China
Correspondence: Jinming Li, Tel +86 20 85211438, Email [email protected]
Abstract: Stem cells (SC) are a kind of cells with self renewing ability and multipotent differentiation, which can differentiate into many types of cells such as osteoblast, chondrocyte, neurocyte to treat disease like osteoporosis, osteoarthritis and Alzheimer’s disease. Despite the development of novel methods for inducing cell differentiation, the inefficiency and complexity of controlling differentiation of stem cells remain a serious challenge, which necessary to develop a new and alternative approach for effectively controlling the direction of stem cell differentiation in vitro and in vivo in stem cells therapy. Recent advancement in nanotechnology for developing a new class of inorganic nanoparticles that exhibit unique chemical and physical properties holds promise for the treatment of stem cells. Over the last decade, inorganic nanoparticle-based approaches against stem cells have been directed toward developing nanoparticles with drug delivery, or utilizing nanoparticles for controlled cell behaviors, and applying nanoparticles for inducing cell differentiation directly. In addition, a strategy to functionalize inorganic nanoparticles as a nanoprobe towards enhanced penetration through near-infrared light or nuclear magnetic resonance has been receiving considerable interest by means of long-term tracking stem cell in vivo. This review summarizes and highlights the recent development of these inorganic nanoparticle-based approaches as potential therapeutics for controlling differentiation of stem cells and so on for stem cell therapy, along with current opportunities and challenges that need to be overcome for their successful clinical translation.
Keywords: inorganic nanoparticles-based systems, biomedical application, stem cells, opportunities and challenges
Introduction
Stem cells are cells that are known to have unlimited self-renewal and pluripotent differentiation. Under certain conditions, they can differentiate into multifunctional cells. According to the development stage of stem cells, they can be divided into embryonic stem cells (ES cells) and adult stem cells (somatic stem cells). According to the developmental potential of stem cells, they can be divided into three categories: totipotent stem cell (TSC), pluripotent stem cell and unipotent stem cell.1 Currently, Stem cells such as mesenchymal stem cells (MSCs) are already being widely used in clinical trials.2 MSCs can be isolated from a variety of tissues such as umbilical cord, endometrial polyps, menstrual blood, bone marrow, adipose tissue, etc., because of the large number of MSCs present in tissues, which makes MSCS excellent for future applications in clinical, regenerative medicine and experimental applications. Under specific in vivo and in vitro environment, MSCs can be induced to differentiate into a variety of tissue cells, such as nerve, heart, liver, bone, cartilage, tendon, fat, epithelium and so on (Figure 1).3 Since hematopoietic stem cells discovery by Ernest A. McCulloch and James E. Till in the bone marrow cells of mice,4 stem cells, specially MSCs have shown great therapeutic potential through the process of cell differentiation that produces a particular tissue to treat all kinds of disease including fracture, osteoporosis, osteoarthritis, Alzheimer’s disease and other nervous system diseases and diabetes, leukemia and other immune system diseases.5 Through differentiation into corresponding cells and tissues, the stem cells that transplanted into the body repair or replace damaged cells or tissues for the disease treatment. Thus, stem cells hold a great potentiality for clinical application to treat a wide range of diseases.6 Although stem cells have great potential as a novel treatment for major human diseases, one of the major challenges in stem cells therapy is how to direct the differentiation of stem cells in vivo, and simultaneously monitor transplanted stem cells or detect the differentiation of stem cells.7,8
 |
Figure 1 The differentiation direction of stem cells. Notes: Reprinted with permission from Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2010;8(3):301–316. Copyright (2010) Wiley-Blackwell.3 |
Inorganic nanoparticles in common sense mainly include various metals, semiconductor materials and metal oxide NPs, have been widely studied as therapeutic drugs for disease treatment in the biomedical field because of their controllable size and unique optical properties, electrical conductivity, magnetic properties, catalytic properties and good biocompatibility.9 These properties make inorganic nanoparticles are expected to have potential applications in elevating the quality of human life and improving mankind’s medical conditions, early detection, diagnosis, treatment and follow-up of various diseases.10 For example, various cancer treatment systems based on inorganic nanoparticles have been extensively developed over the past two decades, including photodynamic therapy, nanoprobes, hyperthermia, and drug delivery, indicating the potential of inorganic nanoparticles for the development of effective and multifunctional cancer therapies.11 Generally, the inorganic nanoparticles-based systems include lanthanide-based inorganic nanoparticles, semiconductor-based inorganic nanoparticles, iron-based inorganic nanoparticles, silicon-based inorganic nanoparticles, carbon-based inorganic nanoparticles, gold-based inorganic nanoparticles and so on.12
Because of the advantages and the successful application of inorganic nanoparticle-based systems in cancer therapy13,14 in recent years, more and more inorganic nanoparticles have been used in the field of stem cells biomedical application. Benefited from the following advantages of inorganic nanoparticles, one is the building of the physical and chemical microenvironment has good stability, and simulation of nano environment will affect stem cell function and behavior, can achieve long-term regulation and the fate of the stem cells in vivo tracking. Secondly, the morphology and structure of various inorganic nanomaterials are different. The contact between nanomaterials and cells provides a platform for spatially controlled stem cell differentiation and more strategies for directional regulation of stem cell fate. Thirdly, inorganic nanomaterials generally affect stem cells in contact with them through endocytosis or exocytosis, but have little impact on other cells, so they can have different functions and behaviors on stem cells in different positions. So far, there are many kinds of inorganic nanoparticles have been used for stem cell research and application, such as upconverting nanoparticles (UCNPs),13–24 quantum dots (QDs),25–38 magnetic nanoparticles (MNPs),39–58 mesoporous silica nanoparticles (MSNs),57–65 GO,65–75 gold nanoparticles (AuNPs),76–99 and carbon nanotubes (CNBS).100–113 Therefore, multifunctional inorganic nanoparticles-based systems have been developed and have great potential in the field of stem cell biomedical applications, which in turn may lead to therapeutic strategies for a better understanding of human diseases, as well as their prevention and treatment.
In this review, we described the selection of promising inorganic nanoparticles which were used for stem cell study such as monitoring the stem cells real-time, detecting the differentiation of stem cells, and controlling the cell behavior of adhesion, spreading and multi-differentiation by inorganic nanoparticles modified substrate, providing a summary of the different types of inorganic nanoparticles such as UCNPs, QDs, MNPs, MSNs, GO, AuNPs, and CNBS which are currently undergoing development in stem cells therapy. Finally, we briefly discuss the opportunities and challenges of current inorganic nanoparticle-based stem cell therapy systems.
Upconversion Nanoparticles (UCNPs)
Upconversion nanoparticles (UCNPs) can absorb NIR light into UV or visible light and are usually composed of trivalent lanthanide ions. Therefore, the NIR-to-UV/visible UCNPs have attracted much attention in biomedical applications (Figure 2).15,16
 |
Figure 2 All kinds of different biomedical application of UCNPs. Notes: Reprinted with permission from Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114(10):5161–5214. Copyright (2014) American Chemical Society, Open Access.15 |
Recently, the functional UCNPs were used as the nanocarriers to control the differentiation of stem cells. For example, Kang et al used NIR-to-visible UCNPs as the small molecule kartogenin (KGN) delivery system to control stem cells differentiation into chondrocyte or osteoblasts by NIR light (Figure 3).17 To control intracellular calcium levels in real time, they used NIR as a switch and UCNPs as nanocapsulers to trigger intracellular photorelease and KGN release. Because NIR can trigger intracellular calcium reduction and transfer KGN, Human enchymstem cells (hMSCs) can be induced to differentiate into chondrocytes by inhibiting hypertrophy. Therefore, the differentiation of hMSCs into osteoblasts can be further promoted by NIR control by increasing intracellular calcium and delivering KGN. By this way, they achieved the “light control cell differentiation of stem cells in vitro and in vivo through the multifunctional UCNPs system via NIR light.
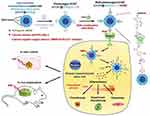 |
Figure 3 NIR light trigger of photo-uncaging and intracellular release of KGN or calcium by UCNPs nanocarriers to control the differentiation of stem cells in vitro and in vivo. Notes: Reprinted with permission from Kang H, Zhang K, Pan Q, et al. Remote Control of Intracellular Calcium Using Upconversion Nanotransducers Regulates Stem Cell Differentiation In Vivo. Adv Funct Mater. 2018;28(41):681–696. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.17 |
They have also developed some versatile UCNPs delivery systems that control stem cell differentiation through near-infrared light. In order to control the chondrogenic differentiation of hMSCs in vitro and in vivo, they synthesized mesoporous silica coated UCNPs (UCNP@mSiO2) loaded with drugs, and nanocarriers delivered KGN to hMSCs (Figure 4).18 The photoactivated small molecule ONA was used as a photocaged linker to conjugate the KGN on the surface of UCNP@mSiO2. Under NIR excitation, ONA photosensitive small molecules break down and UCNPs release KGN, thus inducing hMSCs chondrogenic differentiation in vitro and in vivo. Besides, the UCNPs were also used as nanoprobes to track the hMSCs in vitro and in vivo for a long-term. In addition, they also developed a siRNA delivery system that can stimulate UCNPs to release siRNA through near-infrared light to control the osteogenic differentiation of hMSCs.19 The siRNA that could silence the adipogenic related gene PPARγ was bind into the photoactivated small molecule DMNPB to form the photoactivatable caged compound (DMNPE/siRNA). The photoactivatable caged compound DMNPE/siRNA was encapsulated into the mesoporous silica coated UCNPs (UCNP@mSiO2) and targeted deliver into the hMSCs. In order to promote osteogenic differentiation of hMSCs, DMNPE/siRNA compounds in mesoporous silica were activated by near-infrared light, and siRNA was released from UCNPs to silence PPARγ gene. The MMP13 enzyme produced by osteogenic differentiation cleaved the MMP13-sensitive peptide and quenched the light from the release of UCNPs, thus achieving real-time monitoring of osteogenic differentiation in live stem cells.
 |
Figure 4 Schematic illustration of synthesis of UCNPs nanocarriers for tissue penetration of NIR-triggered release of KGN to induce the chondrogenic differentiation of stem cells in vitro and in vivo. (A) The synthesis of UCNP nanocarriers and NIR trigger release KGN of UCNP nanocarriers. (B) Near-infrared light penetrates the skin compared to ultraviolet light for trigger release KGN from UCNP nanocarriers. Notes: Reprinted from Biomaterials, Volume: 110(9), Li JM, Lee WYW, Wu TY, et al. Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Copyright (2016), with permission from Elsevier.18 |
In addition, NIR excited UCNP has many advantages, such as high photobleaching resistance, large anti-Stokes shift, sharp emission band, low toxicity in vitro to cells or animals and minimal fluorescence background signals.20 Therefore, UCNPs can be used as the multi-color nanoprobes for sensitive imaging and detection in stem cells research. For example, Cheng et al investigated a novel UCNP nanoprobe with superparamagnetic properties and upconversion luminescence (UCL) that could be used in stem cell research.21 UCNPs have the anti-stokes effect and are used for in vitro and in vivo cell imaging by excitation by near-infrared (NIR) light (808/980 nm) and emission of fluorescence at 550/650 nm (Er doping). In addition, when the UCNPs were doped with gadolinium (Gd), the UCNPs also can be used as a magnetic resonance (MR) probe. When using UCNPs and magnetic resonance (MR) for imaging at the same time, they found that UCNPs-labeled stem cells could be tracked in mice under near-infrared light or magnetic fields. In addition, Xu et al. Identification of human dry amniotic fluid (hAFS) cells labeled with dual polymer coated nanoparticles in a mouse model of acute lung injury (ALI).22 They found that fluorescent UCNPs could be used as the nanoprobes to label the hAFS cells with a high sensitive in vivo and observed the hAFS cells could migrate to the lung by the upconversion luminescence imaging. Moreover, hAFS cells labeled with UCNPs can maintain differentiation ability and activity. This imaging-guided hAFS cell-based therapy could be a promising role on ALI-damaged lung tissue repair. Besides, because the good fluorescence property of the UCNPs, the UCNPs also could be used as the luminescent biosensing nanoprobes to detect the differentiation of stem cells. For example, in order to reduce harmful reverse energy transfer, Rabie et al. A single-crystal core-shell-shell “sandwich” UCNP was developed as a nanoprobe to generate bright visible emission using UV excitation (Figure 5).23 Compared with traditional UCNP, this novel UCNP nanoprobe has a significantly stronger output luminescence than traditional UCNP, and it is subsequently used to detect dopamine release from stem cell derived dopaminergic neurons, which can be used as a novel ultra-sensitive. Thus, the luminescence of UCNP nanoprobes was enhanced when the stem cell differentiating into neuron cells because the neuron cells will produce the dopamine that could dissociate the GO (GO) from UCNPs to recover the luminescence of UCNPs for the fluorescence detection of cell differentiation.
 |
Figure 5 Schematic diagram of “sandwich” structure biosensor that based on UCNPs and the application of detecting dopamine in neural differentiation of stem cells (A), and the comparing energy migration mechanism of Yb/Er codoped UCNPs, Yb/Er@Yb “active-shell” UCNPs and novel Yb@Er@Yb “sandwich” UCNPs (B). Notes: Reprinted with permission from Rabie H, Zhang Y, Pasquale N, Lagos MJ, Batson PE, Lee KB. NIR Biosensing of Neurotransmitters in Stem Cell-Derived Neural Interface Using Advanced Core–Shell Upconversion Nanoparticles. Adv Mater. 2019;31(14):1806991–1806970. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.23 |
Finally, since the UCNPs possess the intrinsic properties to absorb and upconvert the NIR light to UV, visible and NIR irradiation, UCNPs modified substrates are used to control cell differentiation and adhesion in stem cells and to manipulate cell behavior on demand. For example, Yan et al developed a UCNP-based substrate by molecular engineering for regulating multidirectional differentiation of stem cells via adjusting NIR light (Figure 6).24 To preserve the cellular properties of the modified stem cells, the UCNPs substrate was attached with polyethylene glycol (P1) modified with 4-(hydroxymethyl)-3-nitrobenzoic acid. In order to alter the cell-matrix interaction, P1 was isolated from UCNPs by upconversion UV photocleavage under NIR irradiation. Thus, stem cells cultured on substrates can be specifically induced to differentiate into osteoblasts or adipocytes by modulating the power of the NIR spectrum. Our group also developed a UCNP-based substrate to control the multidirectional differentiation of stem cells by NIR in vivo (Figure 7).25 On the UCNPs substrate, the cell adhesion peptide RGD was modified on the surface of UCNPs to adhere cells, and the photocleavaged molecule ONA was linker with Asp of RGD. Under the NIR irradiation, the upconvert UV photocleavaged the ONA and explored the Asp of RGD to recover the activity of cell adhesion on the UCNPs substrate, which controlling the cell adhesion, spreading and multidirectional differentiation of stem cells by adjusting NIR power and irradiation time in vitro and in vivo. These UCNPs substrates will be a powerful tool to control cell adhesion, spreading and multidirectional differentiation of stem cells in vivo by NIR light.
 |
Figure 6 Schematic diagram of UCNPs substrate for controlling the multidirectional differentiation of stem cells by adjusting the power of NIR light. Notes: Reprinted with permission from Yan Z, Qin H, Ren J, Qu X. Photocontrolled Multidirectional Differentiation of Mesenchymal Stem Cells on an Upconversion Substrate. Angew Chem Int Ed. 2018;57(35):11182–11187. © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.24 |
 |
Figure 7 Schematic diagram of UCNPs substrate to control cell adhesion, spreading and multidirectional differentiation of stem cells by adjusting power of NIR light. (A) Synthetic procedure for UCNP@SiO2-RGD-ONA and NIR light-triggered cleavage of an ONA protective group. (B) The potential mechanism underlying the UCNP-substrate RGD photoactivated to control the adhesion, spreading, and differentiation of MSCs, governed by mechanosensing signaling. (C) NIR-triggered release of ONA to control cell adhesion, spreading, and multidifferentiation of MSCs in vivo on the UCNP-substrate by different powers of NIR irradiation. Notes: Reprinted with permission from Guo YJ, Yan R, Wang XC, Liang GH, Yang AL, Li JM. Near-Infrared Light-Controlled Activation of Adhesive Peptides Regulates Cell Adhesion and Multidifferentiation in Mesenchymal Stem Cells on an Up-Conversion Substrate. Nano Lett. 2022;22(6):2293–2302. Copyright (2022) American Chemical Society.25 |
Quantum Dots (QDs)
QDs are considered as the semiconductor materials with excellent fluorescence properties to be the imaging probes and universal platforms in the application of biomedicine with the advantages of easy surface chemistry and superior optical performance.26,27 QDs have great potential because of their small size, good water solubility, low toxicity, good biocompatibility and multifunctional surface chemistry, which can be used as nanoprobes or nanocarriers, which allow to conjugate some functional groups such as peptide or protein for cells labeling and drug loading/delivery. QDs have excellent optical properties to keep the fluorescence intensity and track cells in vitro and in vivo for a long time. The QD-based nanocarriers can be used for intracellular or systemic drug release therapy and real-time monitoring of nanocarriers.
The most application of QDs in stem cells is label and long-term tracking stem cells as the fluorescent nanoprobes in vitro and in vivo. QDs have unique photophysical properties and have great potential. Compared with traditional organic dyes, they can adjust the luminescence size, resist photobleaching and stimulate multiple fluorescence simultaneously with higher fluorescence (Figure 8).28,29
 |
Figure 8 The emission of QDs when compared with organic dyes. (A) Absorption and emission spectrum of six different QDs. (B) Absorption and emission spectrum of two organic dyes, Cy3 and Cy5. (C) Comparison of fluorescence photo graphs of six QD in (A) with CdSe core sizes. Notes: Reprinted with permission from Pisanic TR, Zhang Y, Wang TH. Quantum Dots in Diagnostics and Detection: Principles and Paradigms. Analyst. 2014;139(12):2968–2981. Copyright (2014) Royal Society of Chemistry.29 |
Shah et al used CGGRGD peptide modified ODs during hMSCs proliferation and osteogenic differentiation for long-term labeling and imaging in vitro.30 The RGD peptide conjugation gave QDs to bind with selected integrins on the membrane of hMSCs. HMSCs were effectively labeled by RGD-QDS during self-replication and differentiation into osteoblast lineages after overnight incubation. In addition, Muller-Borer et al used QDs to label rat bone marrow MSCs for toxic study.31 In order to use quantum dots as cell labeling probes in cardiomyocyte co-culture, QDs were used to label proliferation and cytotoxicity of rat bone marrow mesenchymal stem cells in vitro. They found that QDs had low cytotoxicity, and no changes in proliferation or DNA damage of QDS-labeled MSCs were observed in rat bone marrow at 24, 72 and 120 h. In addition, MSCS labeled by QDs dye diffusion in vitro were easily located and formed functional intercellular conjugation in cardiomyocyte co-culture. Lin et al used six different QDs as the cells tracker to track the embryonic stem (ES) cells in vivo.32 Their findings showed that, compared with unlabelled in control cells, QDs tag no adverse effect to the behavior of the ES cells, and injected in the animal model of back tag ES cells after, can be a specific imaging excitation wavelength. This study suggests that deep within the organization to track stem cell imaging, QDs has great potential. Another example of in vivo cell tracing via QDs is the potential of QDS-labeled bone marrow stem cells (BMstem cells) to track and treat laser-induced retinal injury.33 Early laser-induced apoptotic photoreceptors and late laser-induced quantum dot CNV by QDs mouse models were studied by examining the survival, migration, proliferation and differentiation of BMstem cells. Their findings showed that the cellular behavior of BMstem cells in the body was not significantly affected by quantum dots.
Furthermore, some special targeted labeling of QDs with stem cells also were reported. Zhao et al used phages conjugated QDs to targeted label embryonic stem cells (Estem cells) (Figure 9).34 They chose to clone the H178VGGEAWSSPTDL sequence of a phage with a higher binding affinity for hEstem cells. They combined ODs with H178 phage to specifically bind hEstem cells. The results suggest that there may be a specific interaction between peptides and the extracellular matrix (ECM) of stem cells. Our group used RGD peptide and β-cyclodextrin (β-CD) modified CdTe/ZnS core/shell QDs as a biocompatible nanoprobe for hMSCs labeling in vitro and in vivo for a long-term.35 The RGD peptide modification can make QDs targeted label hMSCs and the β-CD modification can reduce cytotoxicity and increase biocompatibility. Our results suggest that RGD-β-CD-QDs can effectively label hMSCs, and that RGD-β-CD-QDs can emit a stable fluorescence signal for real-time monitoring of in vitro cell uptake of QDs and long-term tracking of hMSCs in vivo. The results showed that RGD-β -CD-QDS could provide important information for determining the efficacy of hMSCs therapy.
 |
Figure 9 Schematic of targeted labeling hEstem cells by different modified QDs. (A) Phages that specifically bind to human ESCs were enriched in the phage pool by two rounds of bio-panning. (B) Illustration of chemical conjugation between the phage and QDs. (C) This enlarged view of (B) shows how the –NH2 groups on the phage are conjugated to the free –COOH groups on the surface of the QDs via EDC. Notes: Reprinted with permission from Zhao W, Jin L, Yuan H, et al. Targeting human embryonic stem cells with quantum dot-conjugated phages. Sci Rep. 2013;3:3134. Copyright (2013) Springer Nature. Open Access.34 |
Beside labeling for tracking stem cells, there were others special application of QDs in stem cells was reported. Dapkute et al reported the skin-derived mesenchymal stem cells (MSCs) as QD vehicles to tumors.36 They investigated the effect of nanoparticles on skin-derived MSCs using Invitrogen Qdot ® 625 ITK Carboxyl QDs to determine the optimal concentration of QDS within MSCs and the internalization kinetics of QDs in stem cells, nanoparticles intracellular localization, and QD-loaded skin-derived MSCs ability to migrate toward cancer cells in vitro and in vivo. Geng et al used nitrogen doped GO quantum dots (N-GQDs) to label rat bone MSCs (rbMSCs) for achieving stem cell imaging They found that the proliferation of rbMSCs was inhibited by high concentrations (100 μg/ mL) of N-GQDs labeling, and that rbMSCs were stably and uniformly labeled with N-GQDs in the absence of chemical substances. Importantly, N-GQDs can be used in regenerative medicine because it promotes osteogenic differentiation of stem cells. Guo et al reported a titanium carbide MXene QDs (Ti3C2 QDs)-based nanometer fluorescent probe for alkaline phosphatase (ALP) activity assay and embryonic stem cell (Estem cells) identification by taking advantage of inner filter effect (IFE).37 Ti3C2 MXenes quantum dots prepared by hydrothermal method have the following advantages: it has good salt tolerance, photobleaching resistance and dispersion stability in aqueous solution and cells. These good properties made the Ti3C2 QDs could be used as a probe to detect the ALP activity by quenching the fluorescence of Ti3C2 QDs through IFE with a low limit of detection (0.02 UL-1), as well as monitor the enzyme activity in real time. Thus, using ALP as the biomarker of Estem cells, Estem cells could be distinguished from other cells using this Ti3C2 QDs-based method. Our group used quantum dots as nanocarriers and probes to load drugs to promote the differentiation of hMSCs on the one hand and track hMSCs in vivo and in vitro for a long time on the other hand (Figure 10).38 Quantum dots (CD-QDs) can load small molecules of kartogenin and use cyclodextrin as “plugs” to induce chondrogenic differentiation of hMSCs, and the positive charge peptide modified of QDs could bind siRNA to reduce the side-effect of kartogenin. Furthermore, the fluorescent QDs also could used as nanoprobes to track the hMSCs in vitro and in vivo for a long-term to locate the cells with real-time.
 |
Figure 10 Schematic of RGD-CD-QDs as nanocarriers and nanoprobes to deliver small molecule and siRNA for controlling the chondrogenic differentiation of hMSCs and simultaneously long-term tracking hMSCs in vitro and in vivo. Notes: Reprinted with permission from Xu J, Li J, Lin S, et al. Nanocarrier-Mediated Codelivery of Small Molecular Drugs and siRNA to Enhance Chondrogenic Differentiation and Suppress Hypertrophy of Human Mesenchymal Stem Cells. Adv Funct Mater. 2016;26(15):2463–2472. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.38 |
Magnetic Nanoparticles (MNPs)
It can be used to track stem cells in and out of the body by local injection or intravenous administration.39 Over the past two decades, superparamagnetic nanoparticles (SPION) research has shifted to iron oxides and has been carried out in the field of nano-MNPs, which is a very promising candidate for cells tracking in vitro and in vivo.40 The MNPs, such as Fe2O3, Fe3O4, or Fe3O4@SiO2, is a nano MNP with a cubic spinel structure because of its magnetic properties that can be used for in vitro and in vivo NMR imaging.41
Base on the special physical properties of MNPs, the MNPs has been used to track stem cells over the past decade such as a promising way for in vivo localization after stem cells injection and transplantation. So far, the MNPs could be the inorganic nanoparticle that was the most widely used in stem cell research.42,43 Most applications revolve around labeling stem cells in vitro and in vivo by MNPs. Efficient delivery of MNPS-labeled stem cells can be demonstrated by using external magnets to enhance stem cell migration in vivo and in vitro44 Kolecka et al investigated the behavior of stem cells labeled with MNPs for magnetic resonance imaging (MRI).45 They used MNPs to co-incubated with stem cells for 24 h and found that the MNPs were a free cluster in the cytoplasm or in the lysozyme of stem cells. The results showed that osteogenic or adipogenic differentiation of stem cells was not affected by MNPs compared with control samples. In addition, there was no influence of MNPs labeling of stem cells by MTT-Test and wound healing assay on the proliferation. Therefore, the contrast agent of stem cells can be MNPs. Lin et al developed a novel biocompatible MNPs to label stem cells for in vivo tracking of therapeutic stem cells with MRI for better understanding the cell viability and migration of stem cells in vivo (Figure 11).46 They found that MCPs based on biodegradable amylose modification can be used as an efficient and safe new nano carrier for MRI imaging. As a result, a efficient tracking of transplanted stem cells by the biodegradable amylose modified MNPs in stroke was achieved by MRI up to 6 weeks, with the remarkably effective therapeutic of stem cells on treating stroke. Therefore, this novel MNP has the potential to be an efficient, rapid and safe marker for stem cell MRI imaging in regenerative medicine.
 |
Figure 11 Schematic diagram of synthesizing novel MNPs for stem cells labeling and MRI imaging in vivo. Notes: Reprinted with permission from Lin BL, Zhang JZ, Lu LJ, et al. Superparamagnetic Iron Oxide Nanoparticles-Complexed Cationic Amylose for In Vivo Magnetic Resonance Imaging Tracking of Transplanted Stem Cells in Stroke. Nanomaterials. 2017;7(5):107–124. Creative Commons CC BY.46 |
Besides, some study showed that MNPs not only could be a probe to label stem cells, also could be a nanocarrier simultaneously for the clinical therapy of stem cells. For example, Kim et al reported that the MNPs could be used as an efficient probe and nanocarrier of DNA to umbilical cord blood-derived MSCs.47 They used low molecular weight bPEI (1800 Da) to modified the surface of MNPs for better labeling the stem cells and binding pDNA to transfer pDNA into stem cells for the cardiovascular disease (CVD) therapy. Their result demonstrated that the PEI modified MNPs could label the blood-derived MSCs efficiently and simultaneously the PEI modified lead to an effective gene transfection into stem cells with the positive charge of PEI, which showed a great potential for tracking the stem cells with MRI in the stem cell therapy in future. Albukhaty et al loads brain-derived neurotrophic factor (BDNF) onto MNPs and transfers it as a carrier to neural stem cells (NSCs) with the beneficial effects of an external magnetic field.48 Their results showed that hygromycin B effectively transfected and stabilized NSC cultured with pSecTag2/Hygro B pro-BDNF modified MNPs vectors. The stem cells could be transfected with BDNF genes provided by the modified MNPs nanoraphernalia to treat neurodegenerative diseases.
Furthermore, cell delivery of stem cells loaded with MNPs by magnetic field has been reported.49 Stem cells loaded with MNP can be magnetically induced by magnetic fields and transported to specific sites for disease treatment. For instance, Yanai et al targeted delivered stem cells by magnetic field to the upper hemisphere of the rodent retina to demonstrate feasibility in neurological tissue.50 They labeled the MNPs with the rat MSCs and found that the MNPs labeling have no effect of the stem cells viable and differentiation capabilities in vitro. In vivo experiments, MSCs labeled with MNPs were injected into the S334TER-4 transgenic rat model of retinal degeneration in vivo or caudal vein. Meanwhile, gold-plated neodymium disk magnets were placed in the orbit to set up the control group. Retinal planar stents and cryosection imaging showed that MSCs were localized to a fixed area in the retina after injection at the position of the orbital magnet and increased tenfold compared with magnetic MSC delivered backward to the retina, while retinal localization was achieved. Their findings suggested that MNPs labeled stem cells could be targeted delivered to the key areas of specific organs by magnetic field, which had therapeutic potential for the retina. Vandergriff et al showed how to enhance stem cells retention and engraftment by magnetic field for solving the problem of“wash-out”of stem cells from coronary blood flow and heart contraction after injecting the stem cells to the key areas of treatment position such as knee joint cavity.51 Cardiac globule derived stem cells were fDA-approved ferumoxytol nanoparticles Feraheme (®) (F) with heparin (H) and protamine (P) labeled cardiac globule derived dry fine. Cardiac retention of mnPs-labeled stem cells on magnetic resonance imaging, fluorescence imaging, and quantitative PCR showed an increase in magnetic targeting. After 3 weeks, histology showed enhanced cardiac cell implantation and angiogenesis in the magnetic field group. Kodama et al developed a system to deliver MNPs labeled mesenchymal stromal cells (MSCs) using an in vitro magnetic device for the treatment of nonunion fractures.52 Ferucarbotran-labeled luciferase positive bone marrow-derived MSCS were transplanted into a rat model of femur fracture and placed in a magnetic field. They tracked mnPs-labeled MSCs through bioluminescence imaging and found that fluorescence from MSCs increased during the first three days and decreased three days after injection. On days three to four weeks, the imaging showed a large concentration of magnetic cells at the fracture site.
In addition, the MNPs were used to modify on the surface of various substrates to control cell adhesion, spreading and differentiation of stem cells. For example, our group used the silica coated MNPs to modified the class substrate for controlling the cell adhesion, spreading and differentiation of MSCs in vitro (Figure 12).53 Using glass as a substrate, MNPs containing RGD can be significantly reduced by applying magnetic attraction to MNPs by using high molecular weight (PEG, average molecular weight:2000) and polyethylene glycol (ethylene glycol). Our results showed that hMSCs cultured in RGD tethers on glass plates showed better adhesion, diffusion and osteogenic differentiation due to shorter PEG linker (MW: 200) or magnetic actuation. However, high RGD tether mobility affected the early adhesion and diffusion of hMSCs, and reduced the later osteogenic differentiation. Thus, this kind of MNPs modified substrate provide a novel method to control cell adhesion, spreading and differentiation of stem cells by magnetic field.
 |
Figure 12 Schematic diagram of the silica coated MNPs modified glass substrate controlled cell adhesion, spreading and differentiation of hMSCs by magnetic field. Notes: Reprinted with permission from Wong D, Li J, Yan X, et al. Magnetically Tuning Tether Mobility of Integrin Ligand Regulates Adhesion, Spreading, and Differentiation of Stem Cells. Nano Lett. 2017;17(3):1685–1695. Copyright (2017) American Chemical Society.53 |
Moreover, Wong et al used the MNPs modified soft polymeric matrix hydrogel to inhibit/enhance the cell adhesion and mechanosensing-dependent differentiation in hMSCs in vitro (Figure 13).54 They developed a magnetic MNP material with RGD (RGD-MnP, Fe3O4@SiO2-RGD) combined with a soft hydrogel substrate to form a magnetically driven dynamic cell culture platform. The movement of RGD-MnP in the soft hydrogel matrix is controlled by magnetic attraction provided by an external magnetic field, thereby inhibiting/enhancing cell adhesion and mechanical-sensing dependent differentiation. At the same time, the external magnetic field can promote the migration of cells on the hydrogel. Therefore, magnetically driven dynamic cell culture platform can be effectively enhanced the differentiation potential of hMSCs.
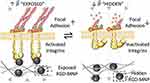 |
Figure 13 The RGD peptide conjugated MNPs modified a soft hydrogel substrate to inhibit/enhance the cell adhesion and mechanosensing-dependent differentiation of hMSCs by magnetic field. Notes: Reprinted with permission from Wong S, Wong W, Lai C, Oh J, Bian L. Soft Polymeric Matrix as a Macroscopic Cage for Magnetically Modulating Reversible Nanoscale Ligand Presentation. Nano Lett. 2020;20(5):3207–3216. Copyright (2020) American Chemical Society.54 |
Similarly, Kang et al reported a strategy for regulating stem cell adhesion and differentiation in vivo by adjusting the frequency of oscillating magnetic fields (Figure 14).55 They built SPION binding to RGD ligand via polyethylene glycol on a glass-based substrate, allowing the oscillating motion of the ligand to be magnetically tuned. They found that ligand oscillations promoted integrin-ligand binding and the formation and maturation of adhesive plaques, with substrate adhesion of stem cells promoted at low oscillation frequencies (0.1 Hz) of the magnetic field, while integrin binding and stem cell adhesion were inhibited at high frequencies. As a result, multimodal ligand oscillations, which can switch between low and high modes, reversibly modulate stem cell adhesion and subsequently induce stem cell behavior. Their findings not only provide additional insights into the design of biomaterials that control cell adhesion in vivo, but also shed light on the fundamentals of dynamic integrin ligand binding that regulates adhesion, differentiation, and regulates the cellular behavior of stem cells.
 |
Figure 14 Remote control of multimodal ligand oscillations was used to regulate the adhesion and differentiation of stem cells by altering the oscillation frequency of the magnetic field. Notes: Reprinted with permission from Kang H, Wong D, Yan X, et al. Remote Control of Multimodal Nanoscale Ligand Oscillations Regulates Stem Cell Adhesion and Differentiation. ACS Nano. 2017;11(10):9636–9649. Copyright (2017) American Chemical Society.55 |
Finally, Kang et al also developed a hybrid system that was consisting of MNPs coupled to an underlying RGD-coated gold nanoparticles (AuNPs) via a long flexible linker (Figure 15).56 Through the external magnetic field, MNPs can move relative to AuNPs to achieve reversible control of RGD opening and caging, and at the same time regulate the physical accessibility of RGD to integrin binding, thus establishing a new system for controlling stem cell adhesion in vitro and in vivo. Reversible RGD decoating of magnetic nanomatrix can temporarily regulate stem cell adhesion, diffusion and differentiation by mechanical induction. Therefore, this MNPs/AuNPs hybrid nanosubstrate holds unprecedented promise for physical and reversible RGD unlocking using external magnetic fields, so that cell behavior can be controlled remotely.
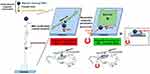 |
Figure 15 Schematic diagram of MNPs/AuNPs hybrid system nano-substrate was remotely controlled to regulate cell adhesion, spreading and differentiation of stem cells by reversibly manipulating RGD ligand cage and uncaging utilizing magnetic field. Notes: Reprinted with permission from Kang H, Jung HJ, Wong DSH. Remote Control of Heterodimeric Magnetic Nanoswitch Regulates the Adhesion and Differentiation of Stem Cells. J Am Chem Soc. 2018;140(18):5909–5913. Copyright (2018) American Chemical Society.56 |
Mesoporous Silica Nanoparticles (MSNs)
Over the last decades, mesoporous silica nanoparticles (MSNs) have had many advantages, such as large specific surface area, high loading capacity of mesoporous structure, and biocompatibility as nanocarriers for drug delivery.57 MSNs have been reported many times for delivering different biomolecules against several diseases enhancing the efficacy of the treatment such as cancer. MSNs also were used to deliver cargo into the stem cells or as a nanoprobe to track stem cells in vitro and in vivo in past studies. For example, Huang et al synthesized the MSNs to label the stem cell for a long-term tracking in vitro (Figure 16).58 Stem cells can be conjugated with fluorescein isothiocyanate to form FITC-MONOdisperse silica microspheres. In addition, cell viability, growth, and differentiation were not affected by internalized FITC-SNP. At the same time, They found that clusterin-mediated endocytosis was important in cellular uptake of FITC-MSN, leading to greater uptake and longer retention of FITC-MSNs by hMSCs compared to other cells. Fitc-msi absorbed by cells can escape from endocytic vesicles and resist lysosomal degradation.
 |
Figure 16 Schematic diagram of intracellular internalization and endolysosomal escape of FITC-MSNs in hMSCs. Notes: Reprinted with permission from Huang DM, Hung Y, Ko BS, et al. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. FASEB J. 2005;19(14): 2014–2016. Copyright (2005) © FASEB.58 |
Hsiao et al developed a novel fluorescent and paramagnetic GD-fluorescein isothiocyanate mesoporous silica nanoprobe (Gd-Dye@MSN) to track hMSCs as an effective nanoprobe.59 HMSCs can be labeled by Gd-Dye@MSN by endophagocytosis, without affecting their activity, proliferation and differentiation ability, while the cell behavior can be detected by MRI. Therefore, they showed MRI images observed in the brains of mice injected with Gd-Dye@MSN tagged MSC and tagged MSC. Furthermore, Kempen et al reported a theranostic MSNs that can improve stem cell survival through both diagnostic and therapeutic approaches (Figure 17).60 Monodisperse silica microspheres were loaded with insulin-like growth factor (IGF) as a model for promoting survival, which as a nanocarrier to deliver theranostic cargo for stem cell therapy. Thus, parallel ultrasound/magnetic resonance imaging can be image-guided delivery and quantification and provide higher resolution, while monodisperse silica nanoloaded drugs can be used for stem cell therapy, and the MSNs successfully deliver drug into stem cell and release drug to improved the survival rate of stem cells. Therefore, the treatment of cardiac stem cells and abdominal regenerative medicine can be treated with MSN.
 |
Figure 17 Schematic diagram of theranostic MSNs and characteristic of MSNs. (A) MSNs have impedance mismatch to backscatter ultrasound, MRI signal via Gd3+ and optical signal from fluo rescein. (B) TEM image of MSNs. (C) Enlarged TEM image of MSNs with 4.1 nm pores. (D) Red box in B indicates area imaged at higher magnification in C, line in C is representative of profile used to construct D. (E) DLS of MSNs. (F) EDS of MSNs shows expected peaks for silicon and oxygen as well as gadolinium from the secondary tag. (G) Histogram of MSN sizes from the TEM data in nm. Notes: Reprinted with permission from Kempen PJ, Sarah G, Parker KA, et al. Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics. 2015;5(6):631–642. Copyright (2005) Ivyspring International Publisher, Open Access.60 |
Huang et al created a multifunctional MSCs platform targeting glioblastoma in situ and this multifunctional platform has the advantage of multimodal imaging by MSNs (Figure 18).61 They first studied the uptake, retention time and stability of monodisperse silica by stem cells to establish a multifunctional platform based on efficient stem cells. They validated tumor-targeted delivery of MSCs by multimodal imaging in vivo in an in-situ U87MG glioblastoma model, showing higher tumor uptake than MSCs-free particles. Therefore, the Msc-based mDISPERsible silica microsphere platform can be used as a carrier and probe, which has great potential in stem cell therapy.
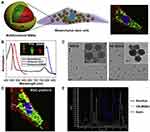 |
Figure 18 Schematic illustration and characterization of the stem cell-based multifunctional MSN-based platform for targeting delivery. (A) Schematic of the structure of the MSC-platform showing the internal and external layer. (B) The fluorescence properties of the MSNs. (C) TEM images of MSNs before and after HA coating. (D) and (E) 3D co-localization imaging of MSC-platform by confocal microscopy. The signal intensity (white line) of actin, particles and nucleus were quantified (E). Notes: Reprinted from Biomaterials. Volume: 34(7), Huang X, Fan Z, Wang H, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34(7):1772–1780. Copyright (2013), with permission from Elsevier.61 |
Zhou et al established a drug delivery system for osteogenic differentiation of bone mesenchymal stem cells (BMSCs) using BMP-2 peptide-coupled monodisperse silica (MSNs-pep) as a nanocarrier loaded with dexamethasone (DEX@MSNs-pep, Figure 19).62 The cytotoxicity of MSNs-pep was tested with different concentrations of BMSCs. Further studies showed that MSNs-pep functionalized by BMP-2 peptide conjugation had better cell uptake efficiency than bare MSNs. Furthermore, the alkaline phosphatase (ALP) activity, calcium deposition and bone-related protein expression of BMSCs could be increased by MSNs-pep. In addition, the addition of dexamethasone to MSNs-pep could further promote osteogenic differentiation of BMSCs. On the surface in vivo, computed tomography (CT) images and histological examination of rat muscle after DEX@MSNs-pep 3 weeks showed enhanced osteogenic differentiation and bone regeneration. In conclusion, DEX@MSNs-pep system can promote the osteogenic differentiation of bone marrow mesenchymal stem cells, which has potential application value in the treatment of osteoporosis therapy.
 |
Figure 19 Schematic illustration of synthesis of the DEX@MSNs-pep nanocomplexes and the size distribution of MSNs and MSNs-pep by TEM and DLS. (A) Schematic illustration for the preparation of DEX@MSNs-pep. (B) TEM images of MSNs (inset is the enlarged image). (C) MSNs-pep. (D) Size distribution of MSNs and MSNs-pep. Notes: Reprinted with permission from Zhou X, Feng W, Qiu K, et al. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl Mater Interfaces. 2015;7(29):15777–15789. Copyright (2015) American Chemical Society.62 |
Shi et al reported a mesoporous silica (MS) -hydroxyapatite (HA) composite mediated by polylactic acid-glycolic acid (PLGA) microspheres (Figure 20).63 The microspherical sustained release system was based on MSN, and the HA nanoparticles were hybridized with alendronate (AL) and assembled into the mesoporous of MSN to construct the HA-Al-MS structure (MSH-AL). This novel PLGA/MSH-AL microphere drug deliver/release system displayed exclusively chondrogenesis, which demonstrated a strong osteogenic differentiation with the cell experiment results of ALP activity assay, calcium secretion was measured for immunohistochemical analysis and real-time PCR. Therefore, the PLGA/MSH-AL controlled release system will provide a powerful tool for bone repair and regeneration through stem cell therapy.
 |
Figure 20 Schematic illustration of PLGA/MSH-AL’s three-level structure. Notes: Reprinted from Biomaterials, Volume: 30(23-24), Shi X, Wang Y, Varshney RR, Li R, Feng Z, Wang DA. In-vitro osteogenesis of synovium stem cells induced by controlled release of bisphosphate additives from microspherical mesoporous silica composite. 3996–4005. Copyright (2009), with permission from Elsevier.63 |
Finally, in order to induce osteogenic differentiation of human bone marrow stromal cells (hBMSCs), Shi et al prepared monodisperse silica microspheres with spherical size of 90 nm and mesoporous size of 2.7 nm, which could activate ALP activity and release silicon ions (Si), bone-related gene and protein (OCN, RUNX2 and OPN) expression.64 In addition, the hypoxia induction therapy drug DMOG (Dimethyloxaloylglycine DMOG) was loaded into the mesopore of monodispersed silica, which could sustainably release stable HIF-1A and further stimulate the vascular differentiation of hBMSCs by enhancing protein expression and VEGF secretion. Therefore, the easy-to-handle MSS can combine to promote the osteogenic and angiogenic activities of hBMSC, which has great potential in stem cell therapy.
Graphene Oxide (GO)
In recent years, GO-based nanomaterials can be fully used in regenerative medicine and stem cell therapy due to their excellent stability and biocompatibility.65 Since the separation of multilayer GO (FLG) flakes from graphite in 2004, it has been repeatedly used in the fields of materials, physics, chemistry and biology. It is a two-dimensional honeycomb lattice sheet composed of SP2 hybrid carbon atoms.66 In the past nearly 20 years, GO and its derivatives in the application of biomedicine such as imaging, regenerative medicine, cancer therapy, drug delivery, stem cell and tissue engineering, biosensing, protein interaction, diagnostics, etc. Especially in stem cell biomedical application, for example, Lee et al used GO to enhance the cardiomyogenic differentiation of human embryonic stem cells (hESCs).67 They prepared a large area of GO coated with vitronectin (VN) on a glass substrate to ensure high activity of hEMCs. Studies have shown that hESCs grown on GO substrates have a higher survival rate and differentiated gene expression than hESCs grown on glass or substrate only. In fact, GO enhanced gene expression during the gradual differentiation into mesoderm, endoderm cell lines, and subsequent myocardial differentiation. Therefore, GO-based stem cell culture substrates may provide a new approach for developing stem cell therapy for ischemic heart disease by enhancing the expression of myocardial differentiation genes in hESCs.
Talukdar et al studied the cytotoxicity and the effect on differentiation of GO in stem cells.68 They used 2D GO nanostructures: effects of GO nanoplatelets (GONPs), GO nanoonions (GNOs) and GO nanoribbons (GONRs) on the differentiation and viability of hMSCs. The cytotoxicity of 2D GO nanostructures was found with concentration dependent, not time dependent, and no significant differences when the concentration of GO was less than 50 μg/mL, compared to control. In addition, by changing the concentration of the three GOs, it was found that the differentiation potential of hMSCs towards lipids and osteogenesis was not significantly changed. This results display a foundation of using GO at potentially safe label and application for stem cell-based imaging and therapy in vivo. Shah et al used GO-nanofiber hybrid scaffolds to induce stem cell differentiation into oligodendrocytes (Figure 21).69 The next step was to change the amount of GO coating on the nanofibers so that the cells differentiated into oligodendrocytes or stem cells in high concentrations of GO because the neural genes varied depending on the concentration of GO. They further found that the GO coating on the nanofiber scaffold, without the addition of differentiation inducing media, can promote the differentiation of oligodendrocytes by integrin-associated intracellular signaling molecules during normal development. Controlling the differentiation of hMSCs by modifying the concentration of GO-nanofiber hybrid scaffides offers a novel therapeutic strategy that could be used to supplement or eliminate the effects of differentiation inducers such as small molecule drugs, viral gene vectors and growth factors. To explore the influence of different conditions on stem cell differentiation, Lee et al developed multifunctional GO-gold (Au) hybrid nanoelectrode arrays (NEAs) to characterize stem cell differentiation and improve stem cell differentiation efficiency in real time (Figure 22).70 They found that the GO-Au hybrid NEAs facilitate highly enhanced adhesion and spreading of stem cells. In addition, by changing the size of GO-gold hybrid NEAs, intrafocal adhesion signals are increased and stem cell differentiation efficiency can be increased. At the same time, the GO-gold hybrid NEAs system was able to monitor osteogenic differentiation of stem cells in real time by electrochemistry, indicating significant progress in the field of stem cell therapy and regenerative medicine application of multifunctional GO-gold hybrid NEAs. This kind of system will be a powerful tool for regeneration engineering in OA therapy.
 |
Figure 21 Schematic diagram of fabrication and application of GO-based nanofiber hybrid scaffolds for enhancing the differentiation of mature oligodendrocytes in stem cells. Notes: Reprinted with permission from Shah S, Yin PT, Uehara TM, Chueng S, Yang L, Lee KB. Guiding Stem Cell Differentiation into Oligodendrocytes Using GO-Nanofiber Hybrid Scaffolds. Adv Mater. 2014;26(22):3673–3680. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.69 |
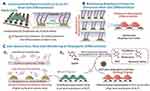 |
Figure 22 Schematic illustration of multifunctional GO-Au hybrid nanoelectrode arrays (NEAs) and its application for enhancing the differentiation of stem cells and monitoring the osteogenic differentiation of stem cell. (A) Investigation of the combinatorial effects of physicochemical cues on stem cell. (B) Identification of optimal biophysical cues for stem cell differentiation. (C) Enhanced electrochemical signal for monitoring osteogenic differentiation. Notes: Reprinted with permission from Lee JH, Choi HK, Yang L, Chueng SD, Choi JW, Lee KB. Nondestructive Real-Time Monitoring of Enhanced Stem Cell Differentiation Using a GO-Au Hybrid Nanoelectrode Array. Adv Mater. 2018;30(39):1802762–1802770. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.70 |
Park et al reported a GO modified substrate that could promote the cell adhesion of human neural stem cell (hNSCs) and enhance the differentiation into neurons of stem cells (Figure 23).71 They modified the GO on the coverglass and culture the hNSCs on the laminin-coated GO glass substrate. The hNSCs could live well on the laminin-coated GO glass substrate and the microarray studies were performed to explore a plausible explanation for promoting the cell adhesion of hNSCs. In addition, GO as a transparent electrode has been shown to electrically stimulate cells differentiated from hNSCs. Therefore, GO can promote the differentiation of hNSCs into neurons due to its unique surface properties. This research is full of potential in neuroscience and regenerative medicine, as well as stem cell treatment of nervous diseases.
 |
Figure 23 Schematic illustration of the GO modified substrate and its application of promoting the cell adhesion of hNSCs and enhancing the differentiation into neurons by GO. Notes: Reprinted with permission from Park SY, Park J, Sim SH, et al. Enhanced Differentiation of Human Neural Stem Cells into Neurons on GO. Adv Mater. 2011;23(36):263–267. Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.71 |
Solanki et al synthesized a axonal alignment GO-nanoparticle hybrid structure to enhanced the neuronal differentiation of neural stem cells (Figure 24).72 They created an array of GO-nanoparticles hybrid structures by attaching positively charged silica nanoparticles to a GO substrate. GO nanosheets with oxygen functional groups can be easily attached and modified so that they can be covered on the surface of silica nanoparticles (SiO2) to form multifunctional GO-silica nanoparticle hybrids (SINP-GO). Sinp-GO was then treated with ECM protein laminin for the cell activity of hNSCs. The neuronal differentiation of hNSCs 14 days after differentiation was detected by immunocytochemistry and quantitative PCR, and the results showed that SiO2-GO mixed substrate could effectively induce neuronal differentiation of hNSCs. Sinp-GO substrates have been shown to enhance the adhesion, growth and differentiation of hNSCs, showing great potential. This kind of system will provide a new idea to treat neurological diseases by stem cell.
 |
Figure 24 Schematic illustration of the SiO2 modified, GO modified and SiO2-GO modified substrates and their application of inducing the neuronal differentiation and axonal alignment of hNSCs. (A) Different control and experimental conditions for differentiating hNSCs into neurons. (B) hNSCs cultured and differentiated on Substrate D having a monolayer of NPs coated with GO show enhanced neuronal differentiation and axonal alignment. Notes: Reprinted with permission from Solanki A, Chueng S T D, Yin P T, et al. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv Mater. 2013;25(38):5477–5482. Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.72 |
On the other hand, in order to improve cell adhesion dependent biological function, Noh et al induced osteoblastic differentiation of human adipose-derived stem cells (hADSCs) by modifying PEG-linked GO on hydrogels, which enhanced osteogenic differentiation of stem cells.73 The incorporation of GO into three-dimensional PEG hydrogel networks improved cell attachment, activated the local adhesion kinase (FAK) signal of hydrogel-coated hADSCs and participated in local adhesion. The hADSCs were shown to have significantly improved cell adhesion, viability and survival rate with GO-modified PEG hydrogels compared to conventional peg hydrogels. When cultured with osteogenic differentiation medium, the hADSCs that were cultured with PEG-GO hydrogel showed more significant osteogenic differentiation and stimulated osteogenic phenotypes in vitro. Furthermore, Elkhenany et al evaluated the effect of GO on growth and differentiation of goat adult MSCs in vitro.74 Next, control variables were used to compare the effects of polystyrene coated and GO-coated tissue culture plates on the proliferation and differentiation of MESenchymal stem cells. The results showed that hMSCs cultured on GO films underwent osteogenic differentiation in media without any glucocorticoids or differentiation factors. These studies demonstrate GO’s potential as a novel bone inducer and stem cell carrier, and suggest that the combination of GO with goat mesenchymal stem cells has great potential in the field of regenerative medicine. Moreover, Yang et al enhanced the osteogenic differentiation of stem cells on phase-engineered GO (Figure 25).75 They induced a phase transition in GO, demonstrating that graphene can both preserve oxygen content and benefit stem cell culture and differentiation. The unique oxygen atoms on GO can improve its ability to stick to cells and lead to the development of hMSCs towards osteogenic differentiation. Their findings demonstrated the potential of GO for stem cell applications while maintaining its oxygen content for enhancing the osteogenic differentiation of stem cells.
 |
Figure 25 The annealed-GO modified substrate exhibited a higher amount of molecular adsorption and peptide-grafted content, enabling the osteogenic differentiation of hMSCs toward oxygen content. Notes: Reprinted with permission from Yang JW, Hsieh KY, Kumar PV, et al. Enhanced Osteogenic Differentiation of Stem Cells on PhaseEngineered GO. ACS Appl Mater Inter. 2018;10(15):12497–12503. Copyright (2018) American Chemistry Society.75 |
Gold Nanoparticles (AuNPs)
Gold nanoparticles (AuNPs) are the most stable metal nanoparticles with good biocompatibility and easy surface modification. They are ideal for stem cell research.76 AuNPs have been widely used in the study of biomedical research that based on their unique properties and multiple surface functionalities (Figure 26).77 Spherical AuNPs possess unique physical and chemical properties, such as photoelectric properties, large surface-to-volume ratios, and excellent biocompatibility. In bionanotechnology, AuNPs are widely used because of their special properties including surface plasmon resonance (SPR) and fluorescence quenching capabilities. Thus, the AuNPs are especially useful for therapeutic strategies in biosensor of detection, nanocarrier of drug delivery, and so on.78
 |
Figure 26 The application of AuNPs in biomedical field. Notes: Reprinted with permission from Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1871–1870. Copyright (2012) Royal Society of Chemistry.77 |
For stem cells, there are many reports of the application of AuNPs in stem cells, showing the AuNPs have a good application potential in stem cells. For example, Li et al synthesized the functional AuNPs with amine (AuNP-NH2), AuNPs-COOH and AuNPs-OH functional groups with different positive and negative surface charges were phagocytosed by human bone marrow-derived mesenchymal cells after incubation stem cells (hMSCs) to test the cell viability and osteogenic differentiation ability.79 HMSCs can phagocytose the functionalized AuNPs without significant cytotoxicity, among which the positively charged AuNPs showed higher cell uptake. Osteogenic differentiation of hMSCs was not affected by surface functionalized AuNPs, but ALP activity and calcium deposition were significantly reduced under AUNP-COOH treatment. In addition, PCR results showed that the expression of TGF-β and FGF-2 in AuNPs, HMSC was up-regulated, and surface treatment promoted cell proliferation rather than osteogenic differentiation. Studies have shown that surface functionalized AuNPs affect the behavior of hMSCs, so the material has great potential for regenerative medicine and stem cell therapy. Encabo-Berzosa et al synthesized the PEGylated hollow AuNPs and incubated with MSCs to study the cell migration and proliferation.80 They found that the AuNPs functionalized with PEG could promote the cell migration, successful scaffold colonization and regeneration of MSCs. Peg-modified AuNPs are easily internalized by mesenchymal stem cells with increased actin and tubulin expression, leading to increased cell mobility. Furthermore, the MSCs containing functional AuNPs were able to successfully colonize fibrin and PCL-based scaffolds, which enhancing the osteogenic, cells loaded with nanoparticles differentiated mesenchymal stem cells better than untreated cells.
In addition, it has been shown that stem cells can be directly induced to osteogenic differentiation by functional AuNPs and this biotechnology approach could serve to develop reliable and potent therapies to treat bone defects, arthritis, and bone fractures.81 Recent studies have reported that AuNPs can deregulate and induce stem cell differentiation toward osteogenesis through a variety of channels. Yi et al showed that the AuNPs promoted osteogenic differentiation of MSCs through p38/MAPK Pathway.82 They first studied the molecular mechanism and cellular effects of AuNPs in differentiated mesenchymal stem cells. The results showed that hMSCs could be induced by AuNPs to enhance osteogenic transcription profile and attenuated lipid transcription profile to promote differentiation. MSCs can be mechanically stressed by the interaction of AuNPs on cell membrane and protein binding in cytoplasm, which activates the P38 mitogen-activated protein kinase pathway (MAPK) signaling pathway and regulates the expression of related genes, leading to the development of osteogenesis. Choi et al found that the AuNPs promoted the osteogenic differentiation in human adipose-derived MSCs through the Wnt/β-Catenin signaling pathway.83 Human adipose-derived mesenchymal stem cells were promoted osteogenic differentiation by chitosan-coupled AuNPs using alizarin red staining and quantitative PCR. Studies have shown that human adipose-derived mesenchymal stem cells can be enhanced by chitosan-coupled AuNPs at non-toxic concentrations to increase calcium content and expression of marker genes related to osteogenic differentiation. Immunofluorescence and Western blot results showed that chitosan coupled AuNPs promoted osteogenic differentiation of hMSC through Wnt/β-catenin signaling pathway. Beside, the size and shape of AuNPs also could influence the osteogenic differentiation of stem cells (Figure 27).84 Li et al synthesized a series of Au nanospheres, Au nanostars, and Au nanorods of different structures coated with bovine serum protein (BSA) with a diameter of 40, 70 and 110 nm, respectively, to investigate the effect of AuNPs size and shape on osteogenic differentiation of hMSCs. At the concentration studied, the proliferation of hMSCs was not affected by AuNPsThe results showed that by changing the size and shape of aunp, the alkaline phosphatase (ALP) activity and calcium deposition content of the cells could be significantly increased to promote osteogenic differentiation, while ROD-40 decreased ALP activity and calcium deposition to reduce osteogenic differentiation. PCR result revealed that the expression of osteogenic marker genes was down-regulated after incubation with rod-40. However, up-regulation of osteogenic genes was found in SPHER-40, SPHER-70 and RoD-70 treatments. The activation of YAP (YAP) is regulated by changing the size and shape of AuNPs, leading to osteogenic differentiation of hMSCs.
 |
Figure 27 The different size and shape of AuNPs affect the osteogenic differentiation response of hMSCs. Notes: Reprinted with permission from Li J, Li J, Zhang J, Wang X, Kawazoe N, Chen G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016;8(15):7992–8007. Copyright (2016) Royal Society of Chemistry.84 |
On the other hand, although MSCs have the potential to treat various disease such as osteoporosis, osteoarthritis, cardiovascular disease and so on, it remains a challenge to monitor these cells in an effort to determine their regenerative abilities, retention, and bio-distribution post-transplantation. Due to their unique physical, chemical and biological properties, AuNPs are used as nanoprobes to track cells in vitro and in vivo for long periods of time. Betzer et al developed a AuNPs-based CT imaging technique for longitudinal MSCs tracking within the brain in a rat model for depression.85 Studies have shown that MSCs therapy is a potential treatment for neuropsychiatric disorders, however aunPs-labeled MSCs can be tracked from 24 hours to 1 month, suggesting that MSCs can specifically navigate and target different depression-related brain regions. Ricles et al synthesized a dual AuNPs system that uses photoacoustic imaging to monitor the transfer of stem cells and infiltrating macrophages. (Figure 28).86 They found that the two kinds of stem cell were preferential labeling with the respective AuNPs in vitro and the AuNPs labeling did not effect the viability and the function of stem cells. In addition, aunPs-based drug delivery system can monitor stem cells and differentiate and quantify macrophage infiltration in a rat hind limb ischemia model. The efficiency of modified AuNPs tracing in animal models is high, which is of great significance for stem cell tracing and stem cell therapy using modified AuNPs.
 |
Figure 28 Schematic illustration of a two-nanoparticle system for labeling mesenchymal stem cells with gold nanorods and macrophages with gold nanospheres. Notes: Reprinted with permission from Ricles LM, Nam SY, Treviño EA, Emelianov SY, Suggs LJ. A dual gold nanoparticle system for mesenchymal stem cell tracking. J Mater Chem B. 2014;2(46):8220–8230. Copyright (2014) Royal Society of Chemistry.86 |
Furthermore, in order to improve biocompatibility and enhance the uptake of BMSCS, Wan et al designed a novel AuNPs with a surface modified silica layer and A DNA-transfected protein 3000 (TS) for dual-energy computed tomography (DECT) tracking of BMSCS. The results showed that bone marrow mesenchymal stem cells were effectively labeled by AuNPs@SiO2-TS, and the endocytosis of A cells was up to ~255 pg/ cell after 1 day, but did not decrease significantly after 14 days. Meanwhile, the cell viability, cell cycle and the ability to differentiate into osteoblasts, chondroblasts and adipocytes were not affected by AuNPs@SiO2-TS. In the rabbit model of bone defect, DECT images showed that the volume of BMSCS remained unchanged and migrated to the bone cortical defect. The study showed that AuNPs@SiO2-TS could be used as a novel nanoprobe for real-time tracking of BMSCS with clinical CT or DECT for stem cell therapy in vivo. Moreover, Kim et al developed the gold-poly-L-lysine nanocomplexes to label the hMSCs for micro-CT imaging in vivo.87 AuNPs modified with polylysine (PLL) and rhodamine B isothiocyanate (RITC) could be used to label hMSCs. Aunp-pll-ritc did not affect the cell viability, osteogenic and adipogenic differentiation of hMSCs. Labeled hMSCs can be tracked in vitro and in vivo with a miniature CT scanner. The unit value of the dog field could be calculated as the intracellular Au content per pg by inductively coupled plasma mass spectrometry, and the cell concentration was linear within a certain range. Therefore, aunP-PLL-RitC nanocomplexes under CT can be used for stem cell tracking and subsequent application in vivo visualization of injected stem cell therapy.
Furthermore, AuNPs have been widely used in drug delivery systems due to their good stability, dispersion, adjustability, functional flexibility, non-cytotoxicity and biocompatibility in aqueous solution.88 And the application of AuNPs as drug nanocarriers in stem cells has been reported. Our group developed a multifunctional AuNPs to control and detect the osteogenic differentiation in hMSCs in real time (Figure 29).89 Firstly, polyvinyl imine (PEI)(AU-PEI) was coated on the gold surface, and then the multifunctional gold nanoparticles (AuNP-PEI-Peptipe-FITC) were synthesized by coupling matrix metalloproteinase-13 (MMP13) sensitive peptide-FITC group (EGPLGVRG-FITC) with PEI. SiRNA and PEI can be bound by electrostatic interaction. On the other hand, PEI on gold surface can be electrostatic adsorbed with siRNA to form AuNP-PEI-Peptipe-FITC/siRNA nanocomplex. After being phagocytosed by hMSCs, siRNA can be efficiently transferred in hMSCs. AuNP-PEI-Peptipe-FITC/siRNA can effectively silence the peroxidase proliferator-activated receptor γ(PPARγ) gene associated with adipogenesis, thus enhancing the osteogenic differentiation of hMSCs. After osteogenic differentiation was induced, the expression level of MMP13 increased, and the MMP13 enzyme activity generated by osteogenic differentiation was detected by corresponding enzyme digestion and FITC fluorescence recovery as a probe, leading to real-time detection of hMSCs cell differentiation. Therefore, AuNP-PEI-Peptipe-FITC/siRNA is a novel fluorescent probe to control cell differentiation and detect cell differentiation in real time, which is promising for regenerative medicine.
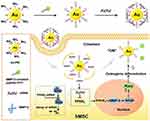 |
Figure 29 AuNPs (AuNP-PEI-peptide-FITC) and siRNA can silence PPARg gene to control osteogenic differentiation, and detect the cell differentiation level in hMSCs in real time. Notes: Reprinted with permission from Wu Q, Wang K, Wang X, Liang G, Li J. Delivering siRNA to control osteogenic differentiation and real-time detection of cell differentiation in human mesenchymal stem cells using multifunctional gold nanoparticles. J Mater Chem B. 2020;8(15):3016–3027. Copyright (2020) Royal Society of Chemistry.89 |
Moreover, Das et al used PEI-coated AuNPs efficiently delivered C/EBP beta gene into hMSCs for enhancing the adipogenic differentiation.90 They synthesized two functional AuNPs: 25 kDa branched PEI-entrapped AuNPs (AuPEINPs) and covalently bound PEI-AuNPs (AuMUAPEINPs) as nanocarriers for efficient gene delivery into hMSCs. Lipofectamine 2000 was 52.3% and 40.7% less efficient than AuPEINPs and AuumuapeinPs. The results of oil red O staining and mRNA expression analysis showed that after introducing exogenous C/EBP beta gene into hMSCs, the exogenous C/EBP Beta was overexpressed, and THE hMSCs differentiated to the adipogenic direction. Therefore, AuPEINPs and AuMUAPEINPs as vectors can be used to control the adipogenic differentiation of hMSCs, which has great potential in the field of regenerative medicine. Kong et al reported a system for trapping AuNPs (Au DENPs) using dendritic molecules modified with arg-glycine-aspartate (Arg-Gly-Asp, RGD) peptide for specific gene delivery to stem cells, which was shown to be more efficient.91 They captured AuNPs by RGD modification using 5th generation poly (amidoamine) dendritic macromolecules via polyethylene glycol (PEG) spacers and PEG monomethyl ether as templates. The functional Au DENPs could transfect with plasmid DNA (pDNA) into hMSCs efficientlyGene transfer efficiency was further studied by ALP activity, osteocalcin secretion, calcium deposition and von Kossa staining to detect the concentration of TRANSFected BMP-2 and osteogenic differentiation of hMSCs. Studies have shown that the composition and surface modification of dendritic molecular vectors can affect the gene delivery efficiency of hMSCs. The main reason is that the coexistence of RGD and AuNPs makes the designed dendritic vector have higher stem cell binding efficiency. By binding the integrin receptor on the cell surface, the gene delivery efficiency and specificity of stem cells are more efficient and specific.
Beside, the AuNPs also used to modified on the surface of substrate such as glass to control the cell adhesion, spreading and differentiation of stem cell. For example, Choi et al developed a AuNPs modified substrate to investigate the effects of varied coupling strength between the RGD peptide and the substrate on stem cell adhesion and pull (Figure 30).92 Negatively charged citrate top AuNPs (cito-AuNPs) were desimmobilized with (3-aminopropyl) triethoxysilane (APTES) by electrostatic interaction. Citi - AUNPs is linked to thiorGD peptide by a sulfur gold bond. Meanwhile, 0.5% and 12.5% APTES were used to prepare the surface with low coupling strength, whereas 25% and 50% of APTES are used to produced high-coupling-strength surfaces. The cell fluorescence imaging showed that hMSCs spread well and form stable actin filamentous structure on high-coupling-strength surfaces but not on low-coupling-strength surfaces. Moreover, hMSCs exhibit enhanced osteogenesis on high-coupling-strength surfaces, which was governed by Yes-associated protein.
 |
Figure 30 The AuNPs modified RGD-coupled substrate and its application of controlling cell adhesion, spreading and differentiation of stem cell by different coupling strength. Notes: Reprinted with permission from Choi C, Xu YJ, Wang B, Zhu M, Zhang L, Bian L. Substrate Coupling Strength of Integrin-Binding Ligands Modulates Adhesion, Spreading, and Differentiation of Human Mesenchymal Stem Cells. Nano Lett. 2015;15(10):6592–6600. Copyright (2015) American Chemical Society.92 |
Furthermore, Ye et al used the RGD peptide conjugated AuNPs to modified the PEG hydrogel as the matrix for controlling the special cell adhesion and differentiation of rat MSCs (Figure 31).93 The rat MSCs were cultured on four different substrates and incubated in the mixed osteogenic and adipogenic medium. Their results suggest that matrix stiffness can be used to regulate stem cells. At the same time, the RGD nanodistance on the surface of AuNPs can affect the spread and differentiation of rat MSCS, so the matrix stiffness and the nanoscale spatial organization of cell adhesion ligands can directly induce the differentiation behavior of stem cells. Thus, the four different AuNPs modified substrate hydrogels could investigate the effects of stiffness of matrix and organization of cell-adhesive ligands on behaviors of rat MSCs, which have great potential for stem cell therapy and regenerative medicine applications.
 |
Figure 31 Schematic of four modified AuNPs modified matrices to study the effect of matrix stiffness and organization of cell adhesion ligands on adhesion and differentiation of rat mesenchymal stem cells. Notes: Reprinted with permission from Ye K, Wang X, Cao L, et al. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015;15(7):4720–4729. Copyright (2015) American Chemical Society.93 |
In the same research group, Wang et al developed a visible RGD-AuNPs modified micro/nanopattern by combining block copolymer micelle nanolithography, photolithography, HF etching, and transfer lithography (Figure 32).94 They designed a series of RGD-AUNPs modified micro/nano patterns to decoupled the effects of RGD nanocrystal spacing and cell diffusion size, and the micro/nano patterns help reveal cell-substance interactions. Using the RGD-AuNPs modified micro/nanopatterning technique, smaller RGD spacing leads to stronger battery tension in same case. And the visible RGD-AuNPs modified micro/nanopattern could control the cell spreading size and cell differentiation of stem cells by the different RGD/AuNPs modification on the micro/nanopattern. Therefore, RGD nano-spacing can be used to regulate stem cell differentiation in tissue repair.
 |
Figure 32 Schematic representation of the RGD-AuNPs modified micro/nanopatterns with different RGD nanospacings to control the cell spreading size and cell differentiation of stem cells. Notes: Reprinted with permission from Wang X, Li S, Yan C, Liu P, Ding J. Fabrication of RGD Micro/Nanopattern and Corresponding Study of Stem Cell Differentiation. Nano Lett. 2015;15(3):1457–1467. Copyright (2015) American Chemical Society.94 |
In addition, Wong et al decoupled the anisotropy of cells at the nanoscale by using gold nanorods (AuNRs) with different transverse to longitudinal ratios (ARs, from 1 to 7), cells were cultured on substrate (glass) and adhesive RGD peptides on cell adhesion and differentiation (Figure 33).95 Compared with AuNRs with small ARs, Because of the larger surface area and more RGD surface modifications, the arrangement of cytoskeletal structures and the significant attachment of nanofeet, AuNRs of larger ARs can promote cell spreading. In addition, large ARs can recruit -β3 and -β1 class integrins into AuNRs, facilitating the development of focal adhesion to fibrillar adhesion. In contrast, small ARs AuNRs can impede the recruitment of integrins and the development of cell adhesion structures, thereby promoting the osteogenic differentiation of stem cells. Their findings showed the potential use of well-controlled synthetic nanoplatforms based on AuNRs modification to unravel the fundamental mechanisms of cell adhesion and differentiation for regeneration medicine.
 |
Figure 33 Schematic summary and characterization of the array of RGD-bearing gold nanorods (AuNRs) modified glass with various aspect ratios (ARs, 1, 2, 4, and 7) for enhanced cell adhesion and osteogenic differentiation in stem cells. (A–C) Schematic illustration of fabrication of immobilized RGD-conjugated AuNRs substrate blocked by nonfouling poly(ethylene) glycol. (D) UV–vis absorbance of AuNRs with different aspect ratios. (E–H) TEM images of AuNRs with different aspect ratios. (I–L) SEM images of AuNRs with different aspect ratios. (M–P) Schematic illustration postulation of how AuNRs with various ARs regulate cell adhesion structures, the differential recruitment and spatial organization of different integer. Notes: Reprinted with permission from Wong SHD, Yin B, Yang B, et al. Anisotropic Nanoscale Presentation of Cell Adhesion Ligand Enhances the Recruitment of Diverse Integrins in Adhesion Structures and Mechanosensing Dependent Differentiation of Stem Cells. Adv Funct Mater. 2019;29(8):1806812–1806822. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.95 |
Finally, the AuNPs were also used as a nanoprobe to detect the differentiation of stem cells. To identify specific miRNA targets, PDA coats were mounted with fluorescently labeled hairpin DNA strands (hpDNAs). At the same time, the fluorescence of immobilized hpDNAs can be quenched by gold core and PDA shell, bind to hpDNAs after the appearance of target miRNAs, and Au@PDA NPs are separated, leading to the recovery of fluorescence signal for real-time monitoring (Figure 34). The Au@PDA NPs nanoprobe could entry the hMSCs efficiently and after the cell uptake of the Au@PDA NPs nanoprobe, the Au@PDA NPs nanoprobe showed the intense and miR-29b and miR-31, two important osteogenic markers, were expressed in osteogenic differentiated hMSCs or osteoblasts with time-dependent fluorescence responses. Importantly, the Au@PDA NPs nanoprobe could long-term tracking of miRNAs in the differentiating hMSCs of more than 5 days, without the need of continuously replenishing cell culture medium with fresh nanoprobes. Thus, their Au@PDA NPs nanoprobe showed the capability of monitoring the differentiation of hMSCs via the detection of specific miRNAs in living stem cells, which is a powerful tool for the investigation of the long-term tracking of stem cell differentiation, identification and isolation of specific cell types, and high-throughput drug screening.
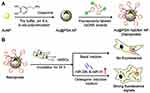 |
Figure 34 Schematic representation of the preparation of Au@PDA NPs nanoprobe and its application of real-time detecting the osteogenic differentiation in living hMSCs by intracellular detection of miRNAs. (A) Preparation of the Polydopamine-Coated Gold Nanoparticles (Au@PDA NPs) and Hairpin-DNA-Based (hpDNA) Nanoprobes. (B) Intracellular Detection of miRNAs in Living Human Mesenchymal Stem Cells. Notes: Reprinted with permission from Choi C, Li J, Wei K, et al. A Gold@Polydopamine Core–Shell Nanoprobe for Long-Term Intracellular Detection of MicroRNAs in Differentiating Stem Cells. J Am Chem Soc. 2015;137(23):7337–7346. Copyright (2015) American Chemical Society.96 |
Sathuluri et al developed a Raman spectroscopy method using aunps-based surface matrix to detect differentiation of mouse embryonic stem cells (mES).97 AuNPs were sent to different stages of stem cell differentiation without affecting cell activity and proliferation. AuNPs can be located by biological transmission electron microscopy (TEM) in the cells and the bio-TME result displayed the AuNPs were inside the following cell organelles: mitochondria, secondary lysosome, and endoplasmic reticulum. Therefore, AuNPs and nanoaggregates at different stages of embryonic stem cell differentiation scatter in both bright and dark field imaging. In addition, SERS peaks (amide I, amide II and Amide III peaks) related to mitochondrial metabolic activity and protein translation were observed in both early and terminally differentiated cardiomyocytes, but rarely in undifferentiated embryonic stem cells. In addition, Kim et al reported a 3D GO-based AuNPs that could detect the differentiation of SERS on neural stem cells (NSCs) (Figure 35).98 First, they synthesized the 3D GO-encapsulated AuNPs, which could induce the double enhancement effect of GO and AuNPs on SERS signals for undifferentiated NSCs. The results showed that the Raman spectra of undifferentiated NSCs were 3.5 times higher than those of normal metal structures, which was significantly different from differentiated cells. In addition, the differentiation state of NSC can also be effectively distinguished by 3D GO-loaded AuNPs substrates in electrochemical and electrical techniques. Although GO on the gold nanoparticles also has C=C bonds, the major SERS signals was from gold nanoparticles when the gold nanoparticles directly contact stem cells membrane and the stem cells grow and differentiate on gold nanoparticles. Therefore, GO-encapsulated AuNPs substrates can serve as a powerful real-time monitoring tool for detecting the state of stem cell differentiation.
 |
Figure 35 Detection strategy to monitor differentiation of mNSCs by the GO-encapsulated AuNPs substrate through the change of SERS signals. Notes: Reprinted from Biomaterials, Volume: 34(34), Kim TH, Lee KB, Choi JW. 3D GO-encapsulated gold nanoparticles to detect neural stem cell differentiation. 8660–8670. Copyright (2013), with permission from Elsevier.98 |
Carbon Nanotubes (CNBS)
CNBS has unique physical and chemical properties. It has a one-dimensional hollow nanostructure, have become an important new multifunctional carrier and new probe, which can be used as super-strong structural materials for electronics, stem cell therapy and biomedical engineering.99 Specially, because neuroprosthesis devices need good electrical conductivity, corrosion resistance, strength and biocompatibility, CNB was specially used to control the differentiation of neural stem cells (NSCs). For example, Jan et al demonstrated that mouse embryonic neural stem cells (NSCs) of the cortex can differentiate into neurons, astrocytes, and oligodendrocytes.100 Due to the unique physical and chemical properties of SWNT composite, SWNT composite is expected to become a new material for fabrication of neural prosthesis devices. Their results showed that there were no adverse affect on the differentiation of NSCs by the SWNT and the biocompatibility, neurite outgrowth, and similar to the use of polornithine (PLO) on the expression of differentiated neural markers.
Mooney et al studied the impact of CNT on hMSCs behavior of biocompatibility, proliferation and differentiation.101 In order to promote future approaches to tissue repair/regeneration, they focused on the effects of carbon nanotubes on hMSCs cell activity. The intracellular movement of CNT through the cytoplasmic to nuclear position has been shown to have no adverse effect on the biocompatibility, proliferation or differentiation of stem cells. Mooney et al also reported that electrically stimulated carbon nanotubes could be used to provide a simulated cardiac signal to hMSCs.102 It was found that CNT could promote the differentiation of hMSCs into cardiomyocyte lineage. So they cultured hMSCs on polylactic acid scaffolds based on carbon nanotubes and electrically stimulated them in electrophysiological bioreactors. The results showed that the hMSCs morphology after stimulation was pulled up and oriented perpendicular to the current. PCR and Western blot analysis showed that a series of cardiac markers were up-regulated, and differentiation into cardiac progenitor cells was more obvious. In addition, C43 staining patterns showed increased cell interaction after electrical stimulation. The results suggest that a paradigm of biomimetic cues at the nanoscale can be established for other electrically active tissue repair applications.
Furthermore, Nayak et al developed the thin films of functionalized multiwalled CNT as a suitable scaffold for stem cells proliferation, differentiation and bone formation.103 The proliferation, morphology and osteogenic differentiation of hMSCs could be affected by peG-mwCNTS spray drying in preheated coating. They found that the functionalized carbon nanotubes layer is non-toxic to cells and creates a viable microenvironment, and accelerates cell differentiation faster than carboxylated nanotubes or uncoated coatings. Studies have shown that cell differentiation can occur in media without biochemical inducers, as evidenced by multiple independent criteria of transcription, protein expression, and functional levels. Therefore, functional carbon nanotubes can be selected for the selective differentiation of stem cells into bone scaffolds. Huang et al promoted the differentiation of neural stem cells (NSCs) into mature neurons through the combined effect of CNT rope and electrical stimulation.104 They used a new type of CNT rope matrix to electrically stimulate NSCs in culture medium. By analyzing the axon growth direction, phenotype, maturity degree of NSCs, as well as quantitative PCR and immunostaining to analyze neuronal gene and protein expression, the results showed that compared with traditional tissue culture plate, NSCs induce differentiation on the CNT cord matrix and advance towards differentiated neurons at an early stage. In addition, the maturation of neurons and the growth rate of neurites can be promoted by electrical stimulation parameters. Therefore, conductive CNT cord matrix and electrical stimulation can jointly promote neuronal elongation, differentiation into mature neurons, and nerve regeneration of neural stem cells. Namgung et al controlled the growth and differentiation of hMSCs by the arrangement of individual CNT (Figure 36).105 They report that hMSCs can be grown in the carbon nanotube network, and the growth direction and differentiation trend of the upper hMSCs can be further controlled by identifying the arrangement of individual carbon nanotubes, thus achieving directional growth of hMSCs along the arrangement direction of individual CNTs. Interestingly, hMSCs on the directed CNT network showed stronger proliferation and osteogenic differentiation than those on the undirected CNT network. Therefore, the study shows that the ability of cells to recognize nanostructures provides new insights into the potential of carbon nanotube networks for stem cell therapy and regenerative medicine.
 |
Figure 36 By controlling the arrangement of carbon nanotubes, hMSCs can grow and differentiate in the carbon nanotube network. (A) Schematic diagram showing the experimental procedure. (B) Plausible model to explain the hMSC responses to the aligned and the randomly oriented CNT networks. Notes: Reprinted with permission from Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5(9):7383–7390. Copyright (2011) American Chemical Society.105 |
By labeling stem cells with protamine-modified single-wall CNT, Wang et al achieved in vivo Raman/magnetic resonance/photoacoustic trimodal imaging.106 They found that hMSCs can engulf protamine-bound carbon nanotubes modified with polyethylene glycol (PEG) without significant cytotoxicity. Due to the strong intrinsic resonance Raman scattering of CNT, the labeled hMSCs can be used for Raman imaging in vivo and in vitro, and can be used to detect about 500 hMSCs in mice. In addition, hMSCs can be labeled with metal catalyst nanoparticles attached to carbon nanotubes for magnetic resonance imaging (MR) and in vivo photoacoustic imaging. Therefore, functional CNT can be used as a multifunctional nanoprobe for stem cell labeling and multimodal tracking in vivo, which has great potential in stem cell therapy. Tay et al investigated the effects of hMSCs cell adhesion, diffusion, and cell line combinations of single-walled carbon nanotubes (SWCNTs) functionalized with carboxyl groups. HMSCs were cultured on a fine reticular layer of SWCNTs at less than 100 nm and the cell adhesion and spreading of hMSCs was significantly effected by the SWCNT film. The hMSCs showed better diffusion on SWCNT membranes and larger cell area and cell boundary filamentous feet compared with cap slide. PCR and Western blot showed that neurogenic markers such as nistin, glial fibrillary acidic protein and microtubule-associated protein 2 genes were upregulated during the first week, while osteogenic genes remained at normal levels. Studies have shown that cell behavior and early stages of stem cell lineages in the absence of inducible cytokines can be modulated by nanoroughness. In addition, Baik et al developed a CNT monolayer cue for osteogenesis of hMSCs without any chemical treatments.107 They found that CNTs treated with oxygen plasma surface enhanced cell adhesion and differentiation of hMSCs. It is currently thought that stress due to enhanced cell diffusion on CNTs may enhance osteogenesis. Venkatesan and Kim prepared several SWCNT grafted with different molecular weight polysaccharides: Bone marrow MSCS were induced osteogenic differentiation by SWCNT-glucosamine, SWCNT-chito-oligosaccharide (<1 KDa), SWCNT-chito-oligosaccharide (1–3 KDa), SWCNT-chitosan (310 KDa) and SWCNT-chitosan (510 KDa) in vitro. It can be used in bone tissue engineering and regenerative medicine.108 Their results showed that SWcnT-glucosamine was soluble in cultured cells and had lower cytotoxicity, higher ALP activity and enhanced mineralization than glucosamine alone. Therefore, SWcnT-polysaccharide derivatives have great potential in bone tissue engineering and regenerative medicine.
Furthermore, Yan et al studied the GO/single-walled carbon nanotube (G/SWCNT) hybrids to promote the osteogenic differentiation of MSCs by activating p38 signaling pathway.109 They investigated the interactions between G/SWCNT hybrids and rat MSCs on the proliferation and differentiation. They found that the cell viability and morphology were good when were cultured on the G/SWCNT hybrids. By detecting ALP activity, RMSCS treated with G/SWCNT hybrid showed higher osteogenic differentiation and enhanced mineralized matrix nodule formation. In addition, PCR results showed that the expression levels of osteogenic related genes were up-regulated, and the expression levels of adipocyte specific markers were down-regulated, indicating that the cells developed toward osteogenesis. The P38 signaling pathway is activated and the extracellular signal-regulated kinase 1/2 pathway is inhibited, so G/SWCNT hybrids promote osteogenic differentiation of RMSCS. Therefore, G/SWCNT hybrids provide a promising platform for regenerative medicine and bone tissue engineering. Liu et al found that MSCS could be carboxylated single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) to inhibit cellular behavior such as proliferation, differentiation, and mineralization.110 Studies have shown that the cytotoxicity of carboxylated CNTS is independent of reactive oxygen species (ROS). The expression of osteoblast genes and adipocyte differentiation specific genes was significantly reduced by Q-PCR. TEM images showed that proteins located in the cell membrane or cytoplasm may interact with CNTs to influence cellular signaling pathways for proliferation and differentiation. Thus, SMADdependent bone morphogenetic protein (BMP) signaling can be regulated by functionalized CNTs. Chen and Hsiue directly control the neural differentiation of human bone marrow mesenchymal stem cells (hBMMSCs) by the MWCNTs (Figure 37).111 They found that neural differentiation of hBMSCs could be induced and maintained by MWCNTs in the absence of exogenous growth factors, as evidenced by the protein expression. Although the carboxylated MWCNTs showed a low cytotoxicity for hBMMSCs by a proliferation assay, Q-PCR results showed that bone-related genes were inhibited but nerve-related genes were promoted when cells were cultured with carboxylated MWCNTs. In addition, carboxylated MWCNTs can provide a suitable environment for long-term neural differentiation and promote the neural differentiation of hBMMSCs through nerve growth factors. Therefore, it can be used as a low cytotoxicity method for spontaneous neural differentiation after transplantation in the field of regenerative medicine.
 |
Figure 37 Schematic representation of using carboxylated MWCNTs to regulated the neural gene expression in hBMMSCs in the basal medium. Notes: Reprinted from Biomaterials, Volume: 34(21), Chen YS, Hsiue GH. Directing neural differentiation of mesenchymal stem cells by carboxylated multiwalled carbon nanotubes. 4936–4944. Copyright (2013), with permission from Elsevier.111 |
Zhao et al studied the effect of PEGylated multi-walled carbon nanotubes (PEG-CNT) membrane on the differentiation of hMSCs into skeletal muscle.112 Polyethylene glycol carbon nanotube films are widely used for their unique physical and chemical properties. Studies have shown that PEG-CNT membranes can guide skeletal musculogenic differentiation of hMSCs without exogenous inducers, and RT-PCR results showed that general musculogenic markers and skeletal musculogenic markers of hMSCs on uninduced PEG-CNT membranes were also significantly increased. Therefore, the directed differentiation of treated hMSCs into skeletal muscle-derived lineages and peG-CARBON nanotube films could be a promising material for skeletal muscle injury repair in the field of regenerative medicine. Du et al investigated the osteogenic ability of treated multiwalled carbon nanotubes (MCNTs) and nano-hydroxyapatite (nHA), the major inorganic mineral component of native bone, in the same group.113 In vitro culture of human adipose-derived mesenchymal stem cells (HASCs) on MCNTs and nHA showed that cell adhesion strength and proliferation were better on MCNTs. And the osteogenic differentiation tendency of HASCs induced by MCNTs is greater than that of nHA, which may be due to the fact that MCNTs can accumulate more proteins to activate Notch specific bone inducible proteins and other signaling pathways. In addition, studies have shown that MCNTs can induce ectopic bone formation in vivo, while nHA cannot. The mechanism may be that MCNTs can accumulate more proteins and stimulate more inducing cells in tissues to form induced bone, including the specific bone-inducing proteins secreted by M2 macrophages.
Conclusion
Despite the development of novel agents to control cell differentiation, the efficiency and complexity of directing cell differentiation in vivo remain a serious challenge, which necessitates the development of new and alternative approaches for an effective method for clinical treatment. As reviewed here, recent advances in the development and application of a new class of nanoparticles with unique chemical and physical properties are full of potential as an alternative to conventional antibiotic treatment for controlling cell differentiation.
In the past two decades, stem cells such as bone marrow mesenchymal stem cells (BMSCS) have attracted much attention in the treatment of diseases due to their diverse differentiation potential and low immune response. How to control the direct differentiation of stem cells and detect the cell differentiation, or tracking the transplanted stem cells in vivo, is still the limit of using stem cells to treat disease in clinical practice. In recent years, the research on multifunctional inorganic particles for the application of stem cells has made a rapid progress. In order to improve the efficacy and specificity of inducing stem cell differentiation and further effective cell therapy, multifunctional inorganic particles can be customized when used as nanocarriers to achieve encapsulation or binding of small molecules, controlled release and surface functionalization. Inorganic nanoparticles can also be used as cell culture substrates to induce cell differentiation directly. Or it could be used as a nanoprobe to track stem cells in vivo and in vitro in real time over long periods of time. In this review, we provide a latest bird’s-eye view of the latest study situation of inorganic nanoparticle-based formulations in stem cells, with a particular emphasis on UCNPs, QDs, MNPs, MSNs, GO, AuNPs, and CNBS-based nanosystems, and the current challenges of clinical treatment (Figure 38).
 |
Figure 38 A summary of the biomedical applications map of inorganic nanoparticles, including the current research progresses in laboratories and challenges. |
However, there are still many challenges and barriers that must be overcome when using inorganic nanoparticles for the stem cells therapy in clinical treatment to achieve the ideal formulation in terms of selectivity, efficacy, safety, batch production. For example, the major limitation of inorganic nanoparticles for clinical application is the problem of in vivo degradation. At present, no inorganic nanoparticles have been approved by the FDA for use in stem cell therapy, and there are currently no clinical trials for the treatment of diseases Future research will focus on the in vivo safety of various inorganic nanoparticle systems for stem cell therapy and applications, including adverse immune stimulation, cytotoxicity and in vivo degradation. Future prospective is critical to develop safe, biocompatible, and biodegradable inorganic nanoparticle-based systems for the clinical application of stem cells therapy. But we believe that material nanostructures that fuse stem cells and related biological substances will have a major impact on the science and technology of regenerative medicine and clinical applications. Despite many obstacles and obstacles, nanoarchitecture may have the ability to advance both cell-based and stem cell-based therapies in the near future, and stem cell nanotechnology is expected to be used to treat degenerative diseases in the near future.
Acknowledgments
This work is supported by the Research Initiation Fund for Young Top Talents of South China Normal University (8S1043) and the National Natural Science Foundation of China (31971267).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68–90. doi:10.1186/s13287-019-1165-5
2. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147.
3. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2010;8(3):301–316.
4. Steensma DP, Kyle RA. James Till and Ernest McCulloch: hematopoietic stem cell discoverers. Mayo Clin Proc. 2021;96(3):830–831.
5. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689.
6. Mayhall EA, Paffett-Lugassy N, Zon LI. The clinical potential of stem cells. Curr Opin Cell Biol. 2004;16(6):713–720.
7. Haack‐Sorensen M, Friis T, Bindslev L, et al. Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scand J Clin Lab Invest. 2008;68(3):192–203.
8. Segers V, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942.
9. Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17(5):1041–1054.
10. Wang X, Zhong X, Li J, Liu Z, Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem Soc Rev. 2021;50(15):8669–8742. doi:10.1039/d0cs00461h
11. Wang F, Li C, Cheng J, Yuan Z. Recent advances on inorganic nanoparticle-based cancer therapeutic agents. Int J Environ Res Public Health. 2016;13(12):1182–1197. doi:10.3390/ijerph13121182
12. Huang H, Feng W, Chen Y, Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. 2020;35:100972–100995. doi:10.1016/j.nantod.2020.100972
13. Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1–2):104–111. doi:10.1016/j.ijpharm.2018.01.034
14. Bayda S, Hadla M, Palazzolo S, et al. Inorganic nanoparticles for cancer therapy: a transition from lab to clinic. Curr Med Chem. 2018;25(34):4269–4303. doi:10.2174/0929867325666171229141156
15. Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114(10):5161–5214.
16. Wang F, Banerjee D, Liu Y, Chen X, Liu X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst. 2010;135(8):1839–1854.
17. Kang H, Zhang K, Pan Q, et al. Remote control of intracellular calcium using upconversion nanotransducers regulates stem cell differentiation in vivo. Adv Funct Mater. 2018;28(41):681–696.
18. Li J, Lee WY, Wu T, et al. Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Biomaterials. 2016;110(9):1–10.
19. Li J, Leung CWT, Wong DS, et al. Photocontrolled siRNA delivery and biomarker-triggered luminogens of aggregation-Induced emission by up-conversion NaYF4: Yb(3+)Tm(3+)@SiO2nanoparticles for Inducing and monitoring stem-cell differentiation. ACS Appl Mater Interfaces. 2019;11(25):22074–22084.
20. Cao T, Yang Y, Gao Y, et al. High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging. Biomaterials. 2011;32(11):2959–2968.
21. Cheng L, Wang C, Ma X, et al. Multifunctional upconversion nanoparticles for dual-modal imaging-guided stem cell therapy under remote magnetic control. Adv Funct Mater. 2013;23(3):272–280.
22. Xu Y, Xiang J, Zhao H, et al. Human amniotic fluid stem cells labeled with up-conversion nanoparticles for imaging-monitored repairing of acute lung injury. Biomaterials. 2016;100(5):91–100.
23. Rabie H, Zhang Y, Pasquale N, et al. NIR biosensing of neurotransmitters in stem cell-derived neural interface using advanced core–shell upconversion nanoparticles. Adv Mater. 2019;31(14):1806990–1806991.
24. Yan Z, Qin H, Ren J, Qu X. Photocontrolled multidirectional differentiation of mesenchymal stem cells on an upconversion substrate. Angew Chem Int Ed Engl. 2018;57(35):11182–11187.
25. Guo Y, Yan R, Wang X. Near-Infrared light-controlled activation of adhesive peptides regulates cell adhesion and multidifferentiation in mesenchymal stem cells on an up-conversion substrate. Nano Lett. 2022;22(6):2293–2302.
26. Chan W, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281(5385):2016–2018.
27. Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4(6):435–446.
28. Zhao MX, Zeng EZ. Application of functional quantum dot nanoparticles as fluorescence probes in cell labeling and tumor diagnostic imaging. Nanoscale Res Lett. 2015;10(1):1–9.
29. Pisanic TR, Zhang Y, Wang TH. Quantum dots in diagnostics and detection: principles and paradigms. Analyst. 2014;139(12):2968–2981.
30. Shah B, Clark P, Stroscio M, Mao J. Labeling and imaging of human mesenchymal stem cells with quantum dot bioconjugates during proliferation and osteogenic differentiation in long term. Conf Proc IEEE Eng Med Biol Soc. 2006;1470–1473. doi:10.1109/IEMBS.2006.260082
31. Muller-Borer BJ, Collins MC, Gunst PR, Cascio WE, Kypson AP. Quantum dot labeling of mesenchymal stem cells. J Nanobiotechnology. 2007;5(1):1–9.
32. Lin S, Xie X, Patel MR, et al. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007;7(1):1–10.
33. Wang HC, Brown J, Alayon H, Stuck BE. Transplantation of quantum dot-labelled bone marrow-derived stem cells into the vitreous of mice with laser-induced retinal injury: survival, integration and differentiation. Vision Res. 2010;50(7):665–673.
34. Zhao W, Jin L, Yuan H, et al. Targeting human embryonic stem cells with quantum dot-conjugated phages. Sci Rep. 2013;3(1):1–9.
35. Li J, Lee WY, Wu T, et al. Multifunctional quantum dot nanoparticles for effective differentiation and long‐term tracking of human mesenchymal stem cells in vitro and in vivo. Adv Healthc Mater. 2016;5(9):1049–1057.
36. Dapkute D, Steponkiene S, Bulotiene D, et al. Skin-derived mesenchymal stem cells as quantum dot vehicles to tumors. Int J Nanomedicine. 2017;12:8129–8142.
37. Guo Z, Zhu X, Wang S, et al. Fluorescent Ti3C2 MXene quantum dots for an alkaline phosphatase assay and embryonic stem cell identification based on the inner filter effect. Nanoscale. 2018;10(41):19579–19585.
38. Xu J, Li J, Lin S, et al. Nanocarrier‐Mediated codelivery of small molecular drugs and siRNA to enhance chondrogenic differentiation and suppress hypertrophy of human mesenchymal stem cells. Adv Funct Mater. 2016;26(15):2463–2472.
39. Yoneyama R, Chemaly ER, Hajjar RJ. Tracking stem cells in vivo. Ernst Schering Res Found Workshop. 2006;60:99–109.
40. Lu AH, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46(8):1222–1244.
41. Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications - ScienceDirect. Biomaterials. 2005;26(18):3995–4021.
42. de Souza GT, Louzada RA, Rosado-de-Castro PH, Mendez-Otero R, de Carvalho AC. Tracking stem cells with superparamagnetic iron oxide nanoparticles: perspectives and considerations. Int J Nanomedicine. 2017;12:779–793.
43. Barrow M, Taylor A, Murray P, Rosseinsky MJ, Adams DJ. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem Soc Rev. 2015;44(19):6733–6748.
44. Ahn YJ, Kong TH, Choi JS, et al. Strategies to enhance efficacy of SPION-labeled stem cell homing by magnetic attraction: a systemic review with meta-analysis. Int J Nanomedicine. 2019;14:4849–4866.
45. Kolecka MA, Arnhold S, Schmidt M, et al. Behaviour of adipose-derived canine mesenchymal stem cells after superparamagnetic iron oxide nanoparticles labelling for magnetic resonance imaging. BMC Vet Res. 2016;13(1):1–11.
46. Lin BL, Zhang JZ, Lu LJ, et al. Superparamagnetic iron oxide nanoparticles-complexed cationic amylose for in vivo magnetic resonance imaging tracking of transplanted stem cells in stroke. Nanomaterials. 2017;7(5):107–124.
47. Kim YS, Park IK, Kim WJ, et al. SPION nanoparticles as an efficient probe and carrier of DNA to umbilical cord blood-derived mesenchymal stem cells. J Nanosci Nanotechnol. 2011;11(2):1507–1510.
48. Albukhaty S, Naderi-Manesh H, Tiraihi T, Jabir MS. Poly-l-lysine-coated superparamagnetic nanoparticles: a novel method for the transfection of pro-BDNF into neural stem cells. Artif Cells Nanomed Biotechnol. 2018;46(sup3):1–8.
49. Kubinová Š. Biomaterials and magnetic stem cell delivery in the treatment of spinal cord injury. Neurochem Res. 2020;45(1):171–179.
50. Yanai A, Häfeli UO, Metcalfe AL, et al. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012;21(6):1137–1148.
51. Vandergriff AC, Hensley TM, Henry ET, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials. 2014;35(30):8528–8539.
52. Kodama A, Kamei N, Kamei G, et al. In vivo bioluminescence imaging of transplanted bone marrow mesenchymal stromal cells using a magnetic delivery system in a rat fracture model. J Bone Joint Surg Br. 2012;94(7):998–1006.
53. Wong D, Li J, Yan X, et al. Magnetically tuning tether mobility of integrin ligand regulates adhesion, spreading, and differentiation of stem cells. Nano Lett. 2017;17(3):1685–1695.
54. Wong SHD, Wong WKR, Lai CHN, et al. Soft polymeric matrix as a macroscopic cage for magnetically modulating reversible nanoscale ligand presentation. Nano Lett. 2020;20(5):3207–3216.
55. Kang H, Wong D, Yan X, et al. Remote control of multimodal nanoscale ligand oscillations regulates stem cell adhesion and differentiation. Acs Nano. 2017;11(10):9636–9649.
56. Li G, Bian L, Zhang L, et al. Remote control of heterodimeric magnetic nanoswitch regulates the adhesion and differentiation of stem cells. J Am Chem Soc. 2018;140(18):5909–5913.
57. Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–1534.
58. Huang DM, Hung Y, Ko BS, et al. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. FASEB J. 2005;19(14):2014–2016.
59. Hsiao JK, Tsai CP, Chung TH, et al. Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small. 2010;4(9):1445–1452.
60. Kempen PJ, Sarah G, Parker KA, et al. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics. 2015;5(6):631–642.
61. Huang X, Fan Z, Wang H, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34(7):1772–1780.
62. Zhou X, Fen W, Qiu K, et al. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. Acs Appl Mater Interfaces. 2015;7(29):15777–15789.
63. Shi X, Wang Y, Varshney RR, et al. In-vitro osteogenesis of synovium stem cells induced by controlled release of bisphosphate additives from microspherical mesoporous silica composite. Biomaterials. 2009;30(23–24):3996–4005.
64. Shi M, Zhou Y, Shao J, et al. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015;21:178–189.
65. Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2017;155:236–250.
66. Mao HY, Laurent S, Chen W, et al. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem Rev. 2013;113(5):3407–3424.
67. Lee TJ, Park S, Bhang SH, et al. Graphene enhances the cardiomyogenic differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2014;452(1):174–180.
68. Talukdar Y, Rashkow J, Lalwani G, Kanakia S, Sitharaman B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials. 2014;35(18):4863–4877.
69. Shah S, Yin PT, Uehara TM, et al. Graphene: guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv Mater. 2014;26(22):3673–3680.
70. Lee JH, Choi HK, Yang L, et al. Nondestructive real-Time monitoring of enhanced stem cell differentiation using a graphene-Au hybrid nanoelectrode array. Adv Mater. 2018;30(39):1802762–1802776.
71. Park SY, Park J, Sim SH, et al. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv Mater. 2011;23(36):H263.
72. Solanki A, Chueng STD, Yin PT, et al. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv Mater. 2013;25(38):5477–5482.
73. Noh M, Kim SH, Kim J, et al. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adipose-derived stem cells. RSC Adv. 2017;7(34):20779–20788.
74. Elkhenany H, Amelse L, Lafont A, et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: potential for bone tissue engineering. J Appl Toxicol. 2015;35(4):367–374.
75. Yang JW, Hsieh KY, Kumar PV, et al. Enhanced osteogenic differentiation of stem cells on phase-engineered graphene oxide. ACS Appl Mater Interfaces. 2018;10(15):12497–12503.
76. Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346.
77. Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1870–1871.
78. Gil PR, Zhang F, Zanella M, Parak WJ, Sperling RA. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37(9):1896–1908.
79. Kawazoe N, Chen G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials. 2015;54:226–236.
80. Encabo-Berzosa MDM, Sancho-Albero M, Crespo A, et al. The effect of PEGylated hollow gold nanoparticles on stem cell migration: potential application in tissue regeneration. Nanoscale. 2017;9(28):9848–9858.
81. Xiang Z, Wang K, Zhang W, et al. Gold nanoparticles inducing osteogenic differentiation of stem cells: a review. J Clust Sci. 2018;29(1):1–7.
82. Yi C, Liu D, Fong CC, Zhang J, Yang M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. Acs Nano. 2010;4(11):6439–6448.
83. Choi SY, Song MS, Ryu PD, et al. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int J Nanomed. 2015;10:4383–4392.
84. Li J, Li J, Zhang J, et al. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016;8(15):7992–8007.
85. Betzer O, Shwartz A, Motiei M, et al. Nanoparticle-Based CT imaging technique for longitudinal and quantitative stem cell tracking within the brain: application in neuropsychiatric disorders. ACS Nano. 2014;8(9):9274–9285.
86. Ricles LM, Nam SY, Treviño EA, Emelianov SY, Suggs LJ. A dual gold nanoparticle system for mesenchymal stem cell tracking. J Mater Chem B. 2014;2(46):8220–8230.
87. Kim T, Lee N, Arifin DR, et al. In vivo Micro‐CT imaging of human mesenchymal stem cells labeled with Gold‐Poly‐l‐Lysine nanocomplexes. Adv Funct Mater. 2017;27(3):1604213–1604218.
88. Ghosh P, Gang H, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307–1315.
89. Wu Q, Wang K, Wang X, Liang G, Li J. Delivering siRNA to control osteogenic differentiation and real-time detection of cell differentiation in human mesenchymal stem cells using multifunctional gold nanoparticles. J Mater Chem B. 2020;8(15):3016–3027.
90. Das J, Choi YJ, Yasuda H, et al. Efficient delivery of C/EBP beta gene into human mesenchymal stem cells via polyethylenimine-coated gold nanoparticles enhances adipogenic differentiation. Sci Rep. 2016;6(1):1–17.
91. Kong L, Alves CS, Hou W, et al. RGD peptide-modified dendrimer-entrapped gold nanoparticles enable highly efficient and specific gene delivery to stem cells. Acs Appl Mater Interfaces. 2015;7(8):4833–4843.
92. Choi C, Xu YJ, Wang B, et al. Substrate coupling strength of integrin-binding ligands modulates adhesion, spreading, and differentiation of human mesenchymal stem cells. Nano Lett. 2015;15(10):6592–6600.
93. Ye K, Wang X, Cao L, et al. Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. Nano Lett. 2015;15(7):4720–4729.
94. Wang X, Li S, Yan C, Liu P, Ding J. Fabrication of RGD micro/nanopattern and corresponding study of stem cell differentiation. Nano Lett. 2015;15(3):1457.
95. Wong SHD, Yin B, Yang B, et al. Anisotropic nanoscale presentation of cell adhesion ligand enhances the recruitment of diverse integrins in adhesion structures and mechanosensing‐dependent differentiation of stem cells. Adv Funct Mater. 2019;29(8):1806822–1806834.
96. Choi C, Li J, Wei K, et al. A Gold@Polydopamine core–shell nanoprobe for long-term intracellular detection of microRNAs in differentiating stem cells. J Am Chem Soc. 2015;137(23):7337–7346.
97. Sathuluri RR, Yoshikawa H, Shimizu E, et al. Gold nanoparticle-based surface-enhanced Raman scattering for noninvasive molecular probing of embryonic stem cell differentiation. PLoS One. 2011;6(8):e22802.
98. Kim TH, Lee KB, Choi JW. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials. 2013;34(34):8660–8670.
99. Volder MD, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339(6119):535–539.
100. Jan E, Kotov NA. Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 2007;7(5):1123–1128. doi:10.1021/nl0620132
101. Mooney E, Dockery P, Greiser U, Murphy M, Barron V. Carbon nanotubes and mesenchymal stem cells: biocompatibility, proliferation and differentiation. Nano Lett. 2008;8(8):2137–2143. doi:10.1021/nl073300o
102. Mooney E, Mackle JN, Blond DJ-P, et al. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33(26):6132–6139. doi:10.1016/j.biomaterials.2012.05.032
103. Nayak TR, Jian L, Phua LC, et al. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. Acs Nano. 2010;4(12):7717–7725. doi:10.1021/nn102738c
104. Huang Y, Wu H, Tai N, Wang T. Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small. 2012;8(18):2869–2877. doi:10.1002/smll.201200715
105. Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. Acs Nano. 2011;5(9):7383–7390. doi:10.1021/nn2023057
106. Wang C, Ma X, Ye S, et al. Protamine functionalized single-walled carbon nanotubes for stem cell labeling and in vivo Raman/Magnetic resonance/photoacoustic triple-modal imaging. Adv Funct Mater. 2012;22(11):2363–2375. doi:10.1002/adfm.201200133
107. Baik KY, Park SY, Heo K, Lee K-B, Hong S. Carbon nanotube monolayer cues for osteogenesis of mesenchymal stem cells. Small. 2011;7(6):741–745. doi:10.1002/smll.201001930
108. Venkatesan J, Kim SK. Stimulation of minerals by carbon nanotube grafted glucosamine in mouse mesenchymal stem cells for bone tissue engineering. J Biomed Nanotechnol. 2012;8(4):676–685. doi:10.1166/jbn.2012.1410
109. Yan X, Yang W, Shao Z, Yang S, Liu X. Graphene/single-walled carbon nanotube hybrids promoting osteogenic differentiation of mesenchymal stem cells by activating p38 signaling pathway. Int J Nanomedicine. 2016;11:5473–5484. doi:10.2147/IJN.S115468
110. Liu D, Yi C, Zhang D, Zhang J, Yang M. Inhibition of proliferation and differentiation of mesenchymal stem cells by carboxylated carbon nanotubes. Acs Nano. 2010;4(4):2185–2195. doi:10.1021/nn901479w
111. Chen Y-S, Hsiue G-H. Directing neural differentiation of mesenchymal stem cells by carboxylated multiwalled carbon nanotubes. Biomaterials. 2013;34(21):4936–4944. doi:10.1016/j.biomaterials.2013.03.063
112. Zhao C, Andersen H, Ozyilmaz B, Ramaprabhu S, Pastorin G, Ho HK. Spontaneous and specific myogenic differentiation of human mesenchymal stem cells on polyethylene glycol-linked multi-walled carbon nanotube films for skeletal muscle engineering. Nanoscale. 2015;7(43):18239–18249. doi:10.1039/C5NR04303D
113. Du Z, Feng X, Cao G, et al. The effect of carbon nanotubes on osteogenic functions of adipose-derived mesenchymal stem cells in vitro and bone formation in vivo compared with that of nano-hydroxyapatite and the possible mechanism. Bioact Mater. 2021;6(2):333–345. doi:10.1016/j.bioactmat.2020.08.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
