Back to Journals » Clinical Interventions in Aging » Volume 17
Influence of Stent Length on Periprocedural Outcomes After Primary Percutaneous Coronary Intervention in Patients with ST Segment Elevation Myocardial Infarction
Authors Chen Y, Gao YF, Wang YF, Wang CJ, Du Y, Ding YH
Received 13 September 2022
Accepted for publication 19 November 2022
Published 28 November 2022 Volume 2022:17 Pages 1687—1695
DOI https://doi.org/10.2147/CIA.S389302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Yan Chen,1,2,* Ya-Fang Gao,3,* Yun-Fan Wang,2 Cheng-Jian Wang,1 Ying Du,1 Ya-Hui Ding1,2
1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Heart Center, Department of Cardiovascular Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, People’s Republic of China; 3Graduate Department, Bengbu Medical College, Bengbu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ya-Hui Ding, Heart Center, Department of Cardiovascular Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), No.158 Shangtang Road, Hangzhou, 310014, People’s Republic of China, Email [email protected]
Purpose: A longer stent is associated with adverse events after percutaneous coronary intervention (PCI). However, little information is available on the relationship between stent length and periprocedural prognosis in patients with ST segment elevation myocardial infarction (STEMI). We aimed to assess the target vessel stent length influence on angiographic outcomes and in-hospital major adverse cardiovascular event (MACE) during primary PCI in patients with STEMI.
Patients and Methods: This single-center retrospective observational study included 246 patients with STEMI admitted to the Zhejiang Provincial People’s Hospital between January 2019 and December 2021, who underwent primary PCI and successful stent implantation. The exclusion criteria included left main lesion, multiple diseased vessel-stenting, bleeding disorders, contrast allergy, and incomplete data. Patients were divided into two groups based on the median stents length: group A (≤ 29 mm, n=125) and group B (> 29mm, n=121). Periprocedural outcomes were slow flow/no-reflow (SF-NR) and in-hospital MACE, which included acute heart failure, malignant arrhythmia, cardiovascular death, non-fatal stroke, non-fatal myocardial infarction, and urgent revascularization. Multivariate logistic analyses were used to explore the correlation between stent length and SF-NR.
Results: A total of 246 patients (82.9% males) with a mean age of 59.9± 12.6 years were included in the analysis. The incidence of SF-NR was significantly higher in group B than in group A (36.4% vs 23.2%, p=0.024). However, the in-hospital MACE incidence rate was similar between the two groups (7.2% vs 7.4%, p=0.943). Multivariate logistic regression analysis showed that stent length and diameter, and peak troponin I level were independent risk factors for SF-NR.
Conclusion: Excessive stent length is an independent risk factor for SF-NR, without any significant influence on the risk of MACE during hospitalization.
Keywords: coronary stent, ST segment elevation myocardial infarction, primary percutaneous coronary intervention, slow flow/no-reflow, major adverse cardiovascular event
Introduction
Primary percutaneous coronary intervention (PCI) has become the preferred strategy for reperfusion therapy in patients with ST segment elevation myocardial infarction (STEMI) in recent years.1,2 However, after restoring the patency of the infarct-related artery with primary PCI in some patients with STEMI, coronary angiography has demonstrated significant slowing or loss of distal antegrade flow, resulting in inadequate perfusion of myocardial tissue. This is called the slow flow/no-reflow (SF-NR) phenomenon and is mainly associated with coronary microvascular dysfunction (MVD). Studies have shown that the SF-NR phenomenon can lead to progressive necrosis of cardiomyocytes and impaired left ventricular function, which is associated with adverse events, such as the development of heart failure (HF) as well as increased in-hospital mortality.3,4 The SF-NR phenomenon can also lead to an increase in infarct size after the intervention. Moreover, it predicts 5-year mortality and is associated with poor prognosis in patients with STEMI.5
The current preferred clinical PCI strategy for treating long coronary lesions or tandem lesions is to cover the lesion with a long stent or multiple interconnected short stents to avoid plaque or residual stenosis.6 In the current drug-eluting stent (DES) era, patients undergoing long stent implantation (total stent length >50 mm) are reportedly at a higher risk of target lesion revascularization.7 Study results in women undergoing PCI showed that stent length does not influence stent thrombosis (ST) risk, but increases the risk of major adverse cardiovascular event (MACE) at 3 years.8
Previous studies focused on patients with stable coronary artery disease. Research on whether increasing the stent length is associated with adverse prognosis in patients with STEMI is limited. This study combined angiography and in-hospital clinical outcomes to evaluate the influence of stent length on periprocedural outcomes in patients with STEMI.
Materials and Methods
Study Population
Patients with STEMI who presented to Zhejiang Provincial People’s Hospital between January 2019 and December 2021 were retrospectively analyzed. The inclusion criteria were patients with STEMI who underwent primary PCI and successful stent implantation.9 The exclusion criteria were left main lesion, multiple diseased vessel-stenting, bleeding disorders, contrast allergy, and incomplete data. A total of 246 patients with STEMI were included, and they were divided into two groups based on the median stent length: 1) group A, stent length ≤29 mm and 2) group B, stent length >29 mm. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (2022QT024).
Operation Method and Used Drugs
Prior to primary PCI, the patients chewed 300 mg of aspirin, 300/600 mg of clopidogrel, or 180 mg of ticagrelor. All PCI procedures followed the PCI guidelines with radial or femoral artery access.10 At least three consecutive cardiac cycles were collected in each position. If no contraindications were found during the perioperative period, statin, β-blockers and angiotensin-converting enzyme inhibitors were routinely administered.
Data Collection
Clinical Baseline Data
Patient characteristics included age, sex, family history, and comorbidities. Indications for baseline laboratory testing included white blood cell count, hypersensitive C-reactive protein, creatinine, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, type B natriuretic peptide, troponin I, and left ventricular ejection fraction. These data were collected from electronic medical records at Zhejiang Provincial People’s Hospital.
Coronary Angiography Data
This study involved two experienced cardiac interventionalists who were blinded to the study’s purpose in recording and interpreting the coronary angiography images. We measured and recorded the preoperative and postoperative thrombolysis in myocardial infarction (TIMI) grade and corrected the TIMI frame count (CTFC) immediately after the procedure. TIMI grade flow is a scoring system from 0 to 3, with TIMI grade <3 being defined as SF-NR.11 CTFC refers to the number of frames required to count coronary vessels from the start of contrast shading to the standardized distal marker. In this study, the CTFC proposed by Gibson et al was used to record the number of contrast frames filling each coronary artery, and all TIMI frames were corrected at 30 frames/s.12 The first frame is where the contrast agent reaches the coronary artery and fills the proximal transverse diameter. In the last frame, the contrast agent fills the target coronary artery and reaches the distal marker. The anatomical landmarks of each coronary artery are as follows: the left anterior descending coronary artery (LAD) is the apical “figure-of-eight” bifurcation; the left circumflex artery is the distal most obtuse marginal branch bifurcation; and the right coronary artery is the first branch of the posterior left ventricular branch artery. As the LAD is longer, the frame count is divided by 1.7 to obtain a corrected frame rate. CTFC was defined as a slow flow that was greater than two standard deviations from the regular coronary TIMI flow rate.
Definition of Total Stent Length
The total stent length was the sum of all the stents implanted at the lesion site. If multiple stents were connected and overlapped, the total length of the stents was calculated and subtracted from the length of the overlapping portions.
Definition of Study Endpoints and MACE
The primary study endpoint was the comparison of SF-NR incidence at the end of the procedure in both groups. All patients were assessed for coronary flow using CTFC immediately at the end of the interventional procedure to determine whether SF-NR occurred.
The secondary study endpoint was the comparison of the prevalence of in-hospital MACE between the two groups. MACE is defined as acute HF, malignant arrhythmia, cardiovascular death, non-fatal stroke, non-fatal myocardial infarction, and urgent revascularization.13–15
Statistical Analysis
Data were statistically analyzed using SPSS 25.0 (IBM, Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation or median with interquartile range. Student’s t-test or Mann–Whitney U-test was used to compare differences between groups. Categorical variables were expressed as percentages and compared using the chi-square test. Multivariate logistic analysis was used to analyze the risk factors affecting the SF-NR. Statistical significance was set at p<0.05, and all probabilities were two-tailed.
Results
Clinical Baseline
The study included 246 patients with a mean age of 59.9±12.6 years, and 82.9% were males. Of these, 209 (85%) had symptoms of pre-infarction angina. Clinical baseline and demographic data are shown in Table 1. No statistically significant differences in patient characteristics, such as age, sex, incidence of risk factors, comorbid diseases, and pre-PCI Killip class, were found between the two groups. The laboratory indications including white blood cell count, hypersensitive C-reactive protein, creatinine, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, Peak type B natriuretic peptide, Peak troponin I, and left ventricular ejection fraction were almost similar between the two groups (Table 2).
 |
Table 1 Clinical Characteristics of the Patients |
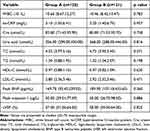 |
Table 2 Laboratory Indications of the Patients |
Angiographic Characteristics
The lesions and procedural characteristics are shown in Table 3. During primary PCI, patients in group B had a higher rate of right coronary artery lesions (27.2% vs 47.9%, p=0.004) and a higher rate of multiple vessel disease (41.6% vs 56.2%, p=0.022). No significant differences in other coronary lesion characteristics were found between the two groups (p>0.05).
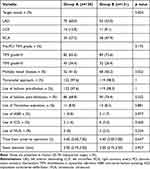 |
Table 3 Angiographic Characteristics of the Patients |
Periprocedural Outcomes
The impact of stent length on the periprocedural outcomes is shown in Table 4. The incidence of MACE, including acute HF, malignant arrhythmia, cardiovascular death, non-fatal stroke, non-fatal myocardial infarction, and urgent revascularization, was considerably similar between groups A and B (7.2% vs 7.4%, p=0.943). However, the incidence of SF-NR was significantly higher in group B than in group A (36.4% vs 23.2%, p=0.024).
 |
Table 4 Periprocedural Outcomes |
Multivariate Analysis
Univariate and multivariate analyses were conducted to identify independent predictors of SF-NR (Figure 1). A multivariate regression analysis was performed on variables with p<0.05 on a univariate basis, showing that stent length, stent diameter, and peak troponin I were independent predictors of SF-NR.
 |
Figure 1 Predictors of slow flow/no-reflow. |
Discussion
In the study, we report the effect of stent length on in-hospital clinical and angiographic outcomes in patients with STEMI. Our research found that gradual stent length increase was associated with an increased risk of SF-NR but had no significant effect on the incidence of in-hospital MACE. Multivariate analysis showed that stent length, stent diameter, and peak troponin I level were independent risk factors for SF-NR. Previous studies have reported that an incidence of SF-NR at the time of intervention was up to 50%. It is more common in patients with STEMI undergoing primary PCI.16–18 This study showed that the incidence of SF-NR after primary PCI was 29.7% in patients with STEMI. A significantly higher incidence was observed in group B than in group A (36.4% vs 23.2%, p=0.024).
The pathophysiological mechanisms of the SF-NR phenomenon, including ischemia-reperfusion injury, microvascular spasm, distal thromboembolism, endothelial dysfunction, and individual susceptibility, are complex.16,18–20 Inadequate epicardial coronary blood supply can result in endothelial cell dysfunction and interstitial edema of cardiomyocytes. This can compress the microvascular lumen and increase its stenosis, ultimately leading to the development of anatomical SF-NR. After revascularization, reperfusion injury leads to persistent coronary microvascular obstruction by activating endothelial cells to produce more oxygen radicals, mediating the release of inflammatory mediators and triggering intracellular calcium ion overload in cardiomyocytes, and other mechanisms. The function of the vascular endothelium is impaired in patients with STEMI, and thrombi are often present on the surface of the target vessel. During mechanical stent implantation, the longer the total length of the stent, the larger the area of contact with the vessel wall, thereby increasing the number of collateral vessels being affected accordingly. Meanwhile, guidewire pulling, stent release, and vascular recanalization during the intervention can cause cardiac sympathetic nervous system reflex, vasospasm induction, and microvascular constriction. In addition, stent deployment and balloon dilation tend to dislodge plaque fragments and cause mechanical obstruction, ultimately leading to microvascular ischemia and injury. The ruptured atherosclerotic plaque releases vasoconstrictive substances, such as thromboxane and 5-hydroxytryptamine, and inflammatory mediators, such as tumor necrosis factor-α, which are involved in leukocyte and platelet activation of the coagulation system, further aggravating microcirculatory ischemia.21 Advanced age, history of smoking, hypertension, diabetes, dyslipidemia, and renal failure are associated with SF-NR.22,23 In particular, longer duration of disease in patients with diabetes have indicated that the body is more likely to be in a state of prolonged hypercoagulation and inflammation and more likely to undergo vascular endothelial damage. This can aggravate oxidative stress in the body and eventually lead to MVD after PCI.24 Studies have shown that distal thromboembolism and ischemia-reperfusion injury are the main mechanisms of microvascular injury in patients with dyslipidemia and that high-dose statin therapy before PCI significantly reduces the risk of SF-NR.25,26
Furthermore, reports have suggested that longer the stents, higher are the chances of myocardial injury, which may lead to thromboembolism and hence, greater risk of complications. A long stent implantation is more prone to lead to stent under-expansion and malapposition, thus increasing the need for balloon post-dilation. Excessive post-dilation during primary PCI may increase type B natriuretic peptide, troponin I and hypersensitive C-reactive protein levels, resulting in MVD and consequently increasing the risk of SF-NR.20 However, a recent prospective observational study combining coronary physiology and intracoronary imaging showed that post-dilation in patients with STEMI is beneficial in increasing fractional flow reserve after PCI, with no significant effect on coronary microcirculation overall.27 In this study, balloon post-dilation did not increase the risk of SF-NR.
Longer stents were reported to be a risk factor for ST and in-stent restenosis, leading to long-term MACE.28–30 Patients with stent lengths >50 mm were significantly more likely to experience MACE and bleeding events in a current registry of 2399 patients treated with second-generation DES.19 All patients in our study experienced in-hospital MACE at a rate of 7.3%, with no significant difference between the two groups, possibly due to the low rate of in-hospital MACE in patients with STEMI.
The presence of SF-NR suggests poor clinical prognosis; therefore, necessitating its early identification and prevention. Diabetes, dyslipidemia, and hypertension are risk factors for SF-NR, and preoperative control of blood glucose levels and intensive statin therapy with primary PCI can significantly reduce coronary MVD.31,32 Reducing total ischemic time, avoiding long-stent implantation, high-pressure release stents, and multiple balloon dilation during primary PCI are can significantly reduce myocardial injury and decrease the risk of SF-NR. If SF-NR occurs during the intervention, vasodilator drugs, such as adenosine, nitroprusside, calcium channel blockers (nicardipine, verapamil, diltiazem), and antiplatelet agents (glycoprotein IIb/IIIa inhibitors), can be administered in the distal vessels via a microinfusion catheter. Coronary angiography showed a heavy thrombus load and vulnerable plaque, allowing the prophylactic use of thrombus aspiration or distal protection devices.33 A recent prospective study in patients with anterior STEMI showed that adjuvant treatment with pressure-controlled intermittent coronary sinus occlusion after primary PCI significantly reduces early infarct size.34 As one of the serious complications of PCI, finding effective approaches to reduce the incidence of SF-NR is essential to improve the efficacy of primary PCI and the clinical prognosis of patients with STEMI.
Study Limitations
This study has several limitations. First, this was a single-center retrospective study, the sample size was relatively small, and long-term follow-up data were lacking. Second, this study lacked data on bare metal stents, as DES is the main choice in the present era. And third, this study was performed to assess SF-NR using CTFC frames after coronary angiography, which lacked cardiovascular magnetic resonance data. Therefore, more extensive studies are necessary to elucidate the prognostic role of stent length in periprocedural outcomes of patients with STEMI.
Conclusion
Long stent length is an independent risk factor for SF-NR without influencing the risk of in-hospital MACE in patients with STEMI who had undergone primary PCI. Therefore, we recommend avoiding unnecessary use of long stent implantation to improve patient prognosis.
Data Sharing Statement
The data of this study are available from the Zhejiang Provincial People’s Hospital. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with the Declaration of Helsinki and approved by the Zhejiang Provincial People’s Hospital Ethics Committee. Written informed consents were obtained from the individuals enrolled in this study.
Consent for Publication
We have obtained the consent for publication from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed upon the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LGF18H020007.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Collet J, Thiele H, Barbato E., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
2. Lawton J, Tamis-Holland J, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. doi:10.1016/j.jacc.2021.09.006
3. Choo E, Kim P, Chang K, et al. The impact of no-reflow phenomena after primary percutaneous coronary intervention: a time-dependent analysis of mortality. Coron Artery Dis. 2014;25(5):392–398. doi:10.1097/mca.0000000000000108
4. Kim M, Cho J, Jeong H, et al. Long-Term Clinical Outcomes of Transient and Persistent No Reflow Phenomena following Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. Korean Circ J. 2016;46(4):490–498. doi:10.4070/kcj.2016.46.4.490
5. Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55(21):2383–2389. doi:10.1016/j.jacc.2009.12.054
6. Costa M, Angiolillo D, Tannenbaum M, et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol. 2008;101(12):1704–1711. doi:10.1016/j.amjcard.2008.02.053
7. Honda Y, Muramatsu T, Ito Y, et al. Impact of ultra-long second-generation drug-eluting stent implantation. Catheter Cardiovasc Interv. 2016;87(2):E44–53. doi:10.1002/ccd.26010
8. Chandrasekhar J, Baber U, Sartori S, et al. Effect of Increasing Stent Length on 3-Year Clinical Outcomes in Women Undergoing Percutaneous Coronary Intervention With New-Generation Drug-Eluting Stents: patient-Level Pooled Analysis of Randomized Trials From the WIN-DES Initiative. JACC Cardiovasc Interv. 2018;11(1):53–65. doi:10.1016/j.jcin.2017.11.020
9. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
10. Ganz W. The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med. 1985;313(16):1018. doi:10.1056/nejm198510173131611
11. Gibson C, Cannon C, Daley W, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. doi:10.1161/01.cir.93.5.879
12. Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: state of the art. Arch Cardiovasc Dis. 2015;108(12):661–674. doi:10.1016/j.acvd.2015.09.006
13. Wijaya IP, Rumende CM, Nasution SA. The association between 24-h blood pressure variability and major adverse cardiac events (MACE) in hospitalized patients with acute myocardial infarction: a retrospective cohort study. Egypt Heart J. 2021;73(1):88. doi:10.1186/s43044-021-00213-1
14. Kelshiker MA, Seligman H, Howard JP, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022;43(16):1582–1593. doi:10.1093/eurheartj/ehab775
15. Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2015;66(4):403–469. doi:10.1016/j.jacc.2014.12.018
16. Durante A, Camici P. Novel insights into an “old” phenomenon: the no reflow. Int J Cardiol. 2015;187:273–280. doi:10.1016/j.ijcard.2015.03.359
17. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54(4):281–292. doi:10.1016/j.jacc.2009.03.054
18. Salinas P, Jimenez-Valero S, Moreno R, et al. Update in pharmacological management of coronary no-reflow phenomenon. Cardiovasc Hematol Agents Med Chem. 2012;10(3):256–264. doi:10.2174/187152512802651024
19. Ejiri K, Sawano M, Numasawa Y, et al. Association of Second-Generation Drug-Eluting Stent Length With 2-Year Adverse Clinical Outcomes Among Japanese Patients With Ischemic Heart Disease. JAMA Network Open. 2020;3(8):e2012546. doi:10.1001/jamanetworkopen.2020.12546
20. Wu G, Zong G, Chen J, et al. Changes of plasma B-type natriuretic peptide levels after high-pressure post-dilation following coronary stent deployment. PLoS One. 2013;8(12):e82357. doi:10.1371/journal.pone.0082357
21. Kleinbongard P, Böse D, Baars T, et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108(3):344–352. doi:10.1161/circresaha.110.235713
22. Kurtul A, Murat SN, Yarlioglues M, Duran M, Celik IE, Kilic A. Mild to Moderate Renal Impairment Is Associated With No-Reflow Phenomenon After Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction. Angiology. 2015;66(7):644–651. doi:10.1177/0003319714546738
23. Namazi M, Mahmoudi E, Safi M, et al. The No-reflow Phenomenon: is it Predictable by Demographic factors and Routine Laboratory Data? Acta Biomed. 2021;92(5):e2021297. doi:10.23750/abm.v92i5.10053
24. Zhao SR, Huang R, Liu F, Li Y, Gong Y, Xing J. Study on Correlation between Type 2 Diabetes and No-Reflow after PCI. Dis Markers. 2022;2022:7319277. doi:10.1155/2022/7319277
25. Reindl M, Reinstadler SJ, Feistritzer HJ, et al. Relation of Low-Density Lipoprotein Cholesterol With Microvascular Injury and Clinical Outcome in Revascularized ST-Elevation Myocardial Infarction. J Am Heart Assoc. 2017;6(10):48. doi:10.1161/jaha.117.006957
26. Zhao JL, Yang YJ, Pei WD, Sun YH, Chen JL. The effect of statins on the no-reflow phenomenon: an observational study in patients with hyperglycemia before primary angioplasty. Am J Cardiovasc Drugs. 2009;9(2):81–89. doi:10.1007/bf03256579
27. Karamasis GV, Kalogeropoulos AS, Gamma RA, et al. Effects of stent postdilatation during primary PCI for STEMI: insights from coronary physiology and optical coherence tomography. Catheter Cardiovasc Interv. 2021;97(7):1309–1317. doi:10.1002/ccd.28932
28. Naidu S, Krucoff M, Rutledge D, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8061-patient XIENCE V United States study. JACC Cardiovasc Interv. 2012;5(6):626–635. doi:10.1016/j.jcin.2012.02.014
29. Shirai S, Kimura T, Nobuyoshi M, et al. Impact of multiple and long sirolimus-eluting stent implantation on 3-year clinical outcomes in the j-Cypher Registry. JACC Cardiovasc Interv. 2010;3(2):180–188. doi:10.1016/j.jcin.2009.11.009
30. Suh J, Park D, Lee J, et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug-eluting stent implantation. JACC Cardiovasc Interv. 2010;3(4):383–389. doi:10.1016/j.jcin.2009.10.033
31. Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41(8):1387–1393. doi:10.1016/s0735-1097(03
32. Li XD, Yang YJ, Hao YC, et al. Effect of pre-procedural statin therapy on myocardial no-reflow following percutaneous coronary intervention: a meta analysis. Chin Med J. 2013;126(9):1755–1760.
33. Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc Interv. 2017;10(3):215–223. doi:10.1016/j.jcin.2016.11.059
34. Egred M, Bagnall A, Spyridopoulos I, et al. Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: piCSO in ACS study. Int J Cardiol Heart Vasc. 2020;28:100526. doi:10.1016/j.ijcha.2020.100526
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
