Back to Journals » Journal of Inflammation Research » Volume 13
Inflammation, Bone Healing and Osteonecrosis: From Bedside to Bench
Authors Goodman SB , Maruyama M
Received 15 September 2020
Accepted for publication 22 October 2020
Published 13 November 2020 Volume 2020:13 Pages 913—923
DOI https://doi.org/10.2147/JIR.S281941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Stuart B Goodman,1,2 Masahiro Maruyama1
1Departments of Orthopaedic Surgery, Stanford University, Stanford, CA, USA; 2Departments of Bioengineering, Stanford University, Stanford, CA, USA
Correspondence: Stuart B Goodman
Departments of Orthopaedic Surgery, Stanford University, 450 Broadway Street, Redwood City, CA 94063, USA
Tel +1-650-721-7662
Fax +1-650-721-3470
Email [email protected]
Abstract: Osteonecrosis of the epiphyseal and metaphyseal regions of major weight-bearing bones of the extremities is a condition that is associated with local death of bone cells and marrow in the afflicted compartment. Chronic inflammation is a prominent feature of osteonecrosis. If the persistent inflammation is not resolved, this process will result in progressive collapse and subsequent degenerative arthritis. In the pre-collapse stage of osteonecrosis, attempt at joint preservation rather than joint replacement in this younger population with osteonecrosis is a major clinical objective. In this regard, core decompression, with/without local injection of bone marrow aspirate concentrate (BMAC), is an accepted and evidence-based method to help arrest the progression and improve the outcome of early-stage osteonecrosis. However, some patients do not respond favorably to this treatment. Thus, it is prudent to consider strategies to mitigate chronic inflammation concurrent with addressing the deficiencies in osteogenesis and vasculogenesis in order to save the affected joint. Interestingly, the processes of inflammation, osteonecrosis, and bone healing are highly inter-related. Therefore, modulating the biological processes and crosstalk among cells of the innate immune system, the mesenchymal stem cell-osteoblast lineage and others are important to providing the local microenvironment for resolution of inflammation and subsequent repair. This review summarizes the clinical and biologic principles associated with osteonecrosis and provides potential cutting-end strategies for modulating chronic inflammation and facilitating osteogenesis and vasculogenesis using local interventions. Although these studies are still in the preclinical stages, it is hoped that safe, efficacious, and cost-effective interventions will be developed to save the host’s natural joint.
Keywords: chronic inflammation, osteonecrosis, osteogenesis, vasculogenesis, bone healing, inflammation
Introduction
Inflammation: General Principles
Acute inflammation is the first step of the healing of all tissues and organs subjected to physical (mechanical), chemical, infectious, thermal, and other types of injurious stimuli. Such trauma leads to activation of the innate immune system, and subsequent release of cytokines, chemokines, reactive oxygen species, and other pro-inflammatory stimuli, and triggering of the complement and coagulation systems.1,2 These events are initiated by the recognition of specific chemical motifs called pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by pattern recognition receptors (PRRs) on/within the cells at the site of injury.3 PAMPs are derivatives of infectious organisms. DAMPs are molecular byproducts from dead or dying cells and are also referred to as endogenous danger signals. The most important PRRs include the Toll-like Receptors (TLRs), the C-type lectin receptors, the NOD-like receptors, the RIG-I-like receptors, and others.3 The acute inflammatory response of the innate immune system is a generalized broad response to eradicate or negate the offending stimulus, initiate the clearing of cellular debris, and begin the resolution and reconstruction of normal host tissue. Interestingly, the phase of repair and renewal is aided by the pro-inflammatory environment, that in the case of musculoskeletal and many other tissues, activates or licenses mesenchymal stem cells (MSCs) and endothelial progenitors to migrate to the injured area under the direction of chemokine gradients.4–8 Acute inflammation can lead to restoration of native host tissue, fibrosis, or chronic inflammation.
Chronic inflammation is a persistent injurious state in which acute inflammation and fibrosis continue, despite ongoing unsuccessful attempts at definitive resolution and repair.1,9,10 In simple terms, innate immune processes (and if applicable the more restricted antigen-specific adaptive immune system) cannot overcome the offending adverse stimulus to reconstitute normal anatomy and physiology.11,12 Thus, homeostasis is never achieved despite the continued mobilization of all biological resources. Chronic inflammation is also a state of heightened energy demands, in which organelles such as the mitochondria, endoplasmic reticulum, and other important components of the cell become exhausted, inefficient, dysfunctional, and dysregulated.13–15 If chronic inflammation persists, the resilience and survival of the organism are at risk.
The cellular profile of the innate immune system comprises cells of the monocyte/macrophage lineage, especially local DAMP and PAMP sensing macrophages, polymorphonuclear leukocytes (neutrophils), dendritic cells, mast cells, specific lymphocyte subgroups including NK cells, and other cell types. Chronic inflammation involves the above cells as well as other T and B cell subgroups. Fibroblasts and vascular lineage cells appear in both acute and chronic inflammatory states. During the resolution of inflammation, pro-inflammatory M1 macrophages polarize to an anti-inflammatory, pro-reconstructive, pro-vascularization M2 phenotype, with reciprocal effects of local mesenchymal and vascular progenitors.1,16,17
Osteonecrosis: Definition and Etiology
Osteonecrosis encompasses a diverse set of conditions that lead to the death of the bone cells and marrow within a bone compartment.18–20 Osteonecrosis can be localized or widespread (multifocal). For simplicity, osteonecrosis of the femoral head (ONFH) will be used as the prototype condition. Many different predisposing factors are associated with osteonecrosis. In general, ONFH is due to traumatic events (eg, a displaced fracture of the femoral neck, hip dislocation, or vigorous attempts at closed reduction of a dislocated hip) or may be atraumatic, ie, not due to mechanical injury. Traumatic etiologies are thought to compromise the vascular supply to the local area directly. Atraumatic etiologies include the use of high dose corticosteroids, excessive alcohol intake, autoimmune diseases such as systemic lupus erythematosus (SLE), radiation, chemotherapy, hypercoagulable states, sickle cell disease, Gaucher’s disease, and other causes.21,22 Osteonecrosis usually afflicts the epiphyseal and metaphyseal areas of bone, and can lead to the collapse of bone and secondary degenerative arthritis. Osteonecrosis must be differentiated from insufficiency fractures due to overuse, pathological fractures in abnormal bone, and other conditions. Osteonecrosis often occurs in the large weight-bearing joints of the hip (femoral head), knee (femoral and tibial condyles), and humerus (head), but can occur in virtually any bone and location. The majority of cases are associated with corticosteroid use or alcohol abuse, usually in younger patients in their prime working years.23,24 Collapse of the involved joint advances to painful and debilitating end-stage arthritis. Therefore, it is important to diagnose osteonecrosis early so that potential inciting factors can be assessed and mitigated, limiting progression to the later stages. Furthermore, early diagnosis and treatment may possibly arrest or reverse the progression of disease, thereby retaining the patient’s own anatomical structures and avoiding joint replacement surgery. Unfortunately, a recent study reported from our tertiary care center with a special interest in osteonecrosis disclosed that 77% of cases were diagnosed in the late stages of ONFH, compromising joint saving procedures.25
Relationship Between Chronic Inflammation and Osteonecrosis
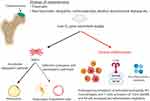
Despite the fact that numerous etiologies are associated with osteonecrosis, the final pathway involves inadequate oxygen and nutrient supply to the affected area (Figure 1). These events are associated with enhanced differentiation of MSCs along the adipogenic pathway, and deficient osteogenic and vasculogenic pathways.22,26 The lesions of multifocal osteonecrosis are often diagnosed at various stages of the disease. However, at some point, the affected anatomical areas demonstrate histological evidence of chronic inflammation, cell death, and compromised resolution and repair.27 Real-time image probe analysis shows that 6 weeks after induction of osteonecrosis by vascular cauterization in mice, activated macrophages and neutrophils persist locally.27 In other studies, steroid-associated osteonecrosis in rats resulted in upregulation of the PRR Toll-like Receptor 4 (TLR4), the downstream adapter protein for the majority of TLRs: Myeloid differentiation factor 88 (MyD88), the major transcription factor for inflammatory proteins: Nuclear Factor-Kappa B (NF-κB), and Monocyte Chemotactic Protein-1 (MCP-1).28
It is appreciated that many of the molecules related to acute and chronic inflammation, osteonecrosis, and bone healing are overlapping, and play major roles in activation of the innate immune system and tissue repair. NF-κB is the major pro-inflammatory transcription factor induced by injurious stimuli; pro-inflammatory factors activate or license MSCs.1 TLR4 is a PRR on the cell surface, and is activated by PAMPs, DAMPs, and other substances.29 TLR4 has two signaling pathways: the MyD88 dependent (TLR4/MyD88/NF-κB) pathway and the MyD88 independent (TLR4/TRIF/IRF3) pathway.30 The MyD88 dependent pathway activates NF-κB and promotes the expression of MCP-1, a chemokine.31 MCP-1 is a chemoattractant for cells of the monocyte-macrophage lineage and the MSC-osteoblast lineage.32 MCP-1 induces the proliferation of monocytes/macrophages and promotes the differentiation and activation of osteoclasts.33 In a porcine model, byproducts of necrotic bone have been shown to upregulate numerous pro-inflammatory cytokines in a mechanism that is dependent on TLR4 activation by macrophages.34 This observation has been confirmed in a rat model of steroid-associated ONFH, which demonstrated excessive activation of TLR4/NF-κB and suppression of the canonical Wnt/β-catenin pathway (the latter pathway regulates cell fate, cell migration, and organogenesis).35 In a study in which serum was collected from 20 patients with various stages of ONFH and compared with serum from normal controls, eight genes, including TLR4 were identified as potential serum biomarkers of the disease.36 The other biomarkers including BIRC3, CBL, CCR5, LYN, PAK1, PTEN, and RAF1 were related to inflammation, bone and cartilage metabolism, and vasculogenesis. This suggests that potential biological strategies to mitigate the adverse sequelae of osteonecrosis might entail curtailing chronic inflammation, and facilitating bone formation and vasculogenesis.
Strategies to Mitigate Chronic Inflammation and Enhance Osteogenesis and Vasculogenesis in ONFH
Healing of chronic critical-size bone defects due to trauma (delayed union, nonunion), previous infection, periprosthetic osteolysis, and other causes is similar, in many ways, to defect that are encountered in osteonecrosis. To a lesser or greater degree, all of these etiologies have a component of chronic inflammation with localized bone necrosis, fibrosis, deficient osteogenesis and vasculogenesis, and fatty infiltration of the tissues. Consequently, research from in vitro and in vivo models of healing of critical-size bone defects is relevant to treating osteonecrotic lesions. Strategies and methodologies that have been used to solve these difficult clinical scenarios from our laboratory and others will be reviewed in this light.
Inhibition of Chronic Inflammation
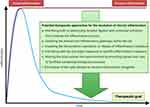
Given the fact that osteonecrosis is associated with chronic inflammation, it seems prudent to consider interfering with these processes. Potential approaches must be delivered in a temporal and spatially sensitive manner, as soft and hard tissue healing after acute injury is dependent on a short period (usually several days) of acute inflammation for subsequent resolution and initiation of repair by licensing MSCs and other cells.4,5,37–42
Given these facts, below are possible methods to mitigate chronic inflammation (Figure 2):
(a). interfering with or obstructing receptor ligation and continued activation that prolongs the inflammatory process,
(b). inhibiting the relevant pro-inflammatory pathways within the cell,
(c). impeding the transcription, translation, or release of inflammatory mediators,
(d). interfering with the end-organ response to specific inflammatory mediators,
(e). altering the local cellular microenvironment by providing signals and cues to facilitate competing biological processes,
(f). elimination of the cells relevant to chronic inflammation altogether.
Many of these strategies have been used in the treatment of systemic chronic inflammatory diseases such as rheumatoid arthritis (RA). Pharmacologic agents for RA include antimetabolites and other chemotherapeutic agents, disease-modifying drugs and biologics that directly or indirectly interference with specific cytokines, chemokines, and other pro-inflammatory molecules such as Tumor Necrosis Factor alpha (TNFα), Interleukins (IL) such as IL-1β and IL-6, etc. Although these drugs are highly efficacious in treating RA, systemic delivery of these medications with potential serious adverse effects is not pragmatic for chronic inflammation due to osteonecrosis and critical-size bone defects.43 Thus, local delivery is probably the preferred route. With respect to mitigating chronic inflammation in clinical scenarios relevant to osteonecrosis and critical-size bone defects, the following local approaches have promise: inhibition of specific TLRs, most prominently TLR4; interference with the following: the adapter protein MyD88, the transcription factor NF-κB, or the chemokines MCP-1 and Macrophage Inhibitory Factor (MIF); altering the macrophage polarization state from an M1 pro-inflammatory to an M2 anti-inflammatory phenotype via local delivery of IL-4 or IL-13. Our laboratory and others have utilized some of these strategies in models simulating chronic inflammation associated with wear particle disease.41,44–52 Infusion of IL-4, an anti-inflammatory cytokine is one important putative strategy to curtail chronic inflammation in a wide variety of clinical conditions.41,53–56 IL-4 protein can be delivered directly, via scaffolds or other devices, or as genetically modified MSCs that over-express IL-4 constitutively, or in response to upregulation of NF-κB.41,48,57–62 This approach is one of the great interests of our laboratory’s treatment of chronic bone defects and osteonecrosis.1,63 Other potential immunotherapeutic approaches include delivery of IL-13, IL-10, IL-1Ra, TNFsR, etc.64
Local Delivery of Biomolecules and/or Cells for Osteogenesis and Vasculogenesis (Figure 3)
Biomolecules and Drugs
Local delivery of growth factors and other molecules that enhance osteogenesis and vasculogenesis, or inhibit osteoclasts for the treatment of bone defects and osteonecrosis is not a new concept.65 These factors include members of the Transforming Growth Factor (TGF) superfamily including TGFβ and Bone Morphogenic Proteins (BMPs), Fibroblast Growth Factor (FGF), Vascular Endothelial Growth Factor (VEGF), Platelet-Derived Growth Factor (PDGF), Insulin-like Growth Factor (IGF), Hepatocyte Growth Factor (HGF), Parathyroid Hormone (PTH), and others.66,67 These agents have been delivered locally as proteins within various polymers, scaffolds, types of cements, etc. The biomolecules are absorbed, entrapped, immobilized, or coated as drug delivery systems and then released by diffusion, matrix or crosslinker degradation.67 Other drugs for local delivery include corticosteroids and other sterols, statins, and bisphosphonates.67,68 These biomolecules are often multifunctional, modulate numerous pathways including the inflammatory cascade, osteogenesis, and vasculogenesis, and have other biological targets. Although some of these interventions have been explored in extensive preclinical and limited clinical studies for the healing of critical sized defects, few have been used clinically for the treatment of osteonecrosis.69–72 In fact, the clinical trials have not led to widespread acceptance and implementation. Systemic treatment with bisphosphonates or statins has not proven to be effective for ONFH in a recent systematic review, however local treatment may prove to be efficacious.73 The challenges of the necrotic, avascular harsh biological environment in osteonecrotic lesions may be too demanding for pharmacologic therapy alone to be successful.
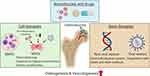 |
Figure 3 Potential approaches for local delivery of biomolecules, cell therapies, and gene therapies to enhance osteogenesis and vasculogenesis in osteonecrosis. |
Cell Therapies
Cell therapy for ONFH and other bones afflicted with this disease is no longer experimental. The use of concentrated autologous iliac crest bone graft in conjunction with core decompression (CD) in the early stages of osteonecrosis is evidenced based.18,74–78 Perhaps the most compelling study is the one reported by Hernigou et al in 2018, in which the authors performed simultaneous bilateral CD in 125 sequential patients; in one of the two hips undergoing CD, they added bone marrow aspirate concentrate (BMAC).76 The number of MSCs (or colony-forming unit-fibroblasts [CFU-Fs]) injected into the CD site ranged from 45,000 to 180,000 cells with a mean of 90,000 ± 25,000 cells. After 20–30 years of follow-up, the addition of BMAC was found to reduce the rate of collapse of the femoral head from 72% to 28%; the percentage of patients undergoing hip replacement was reduced from 76% to 24%. Using quantitative MRI, the volume in the femoral head occupied by the osteonecrotic lesion in the group receiving BMAC decreased from 44.8% to 12%. These are compelling data. The concept of addition BMAC to CD for treatment of ONFH would benefit from a large prospective randomized multicenter study.
Extensive preclinical and laboratory analysis has been performed on BMAC and is reviewed in recent publications.74,79–82 Numerous factors are relevant to the number and vitality of the harvested cells, including the age and gender of the patient, the presence of medical co-morbidities and medications such as corticosteroids and others, smoking, obesity, etc. It is important to note that BMAC is not MSCs, but a conglomerate of different mononuclear cell types including macrophages, lymphocytes, mast cells, and other cells. In fact, only about 1 CFU-F was present in 30,000 nucleated cells harvested from the anterior iliac crest, which equates to about 600 CFU-Fs per cc of bone marrow aspirate.82
Despite the apparent success of BMAC for the treatment of osteonecrosis, several questions remain. Is BMAC the preferred cells to inject for osteonecrosis? How many cells of different lineages are necessary? Are MSCs alone sufficient? Can MSCs be altered to mitigate inflammation and facilitate healing of the osteonecrotic lesion? Are allograft-derived MSCs equally efficacious? Although no definitive answers are currently available for these questions, some pertinent observations need mentioning. There is substantial evidence that the addition of macrophages will augment the osteogenic capabilities of MSCs, probably by licensing the latter cells and engaging in continuous MSC-macrophage crosstalk to enhance tissue repair.17,38,41,53,62,83–85 These findings substantiate injecting an agglomerate of MSCs and hematopoietic lineage cells. Nevertheless, autologous and allogenic MSCs alone (and/or their byproducts such as exosomes) are being isolated, expanded and delivered to heal bone defects and for treatment of osteonecrosis.86–90 In the USA, the FDA has rather stringent regulatory guidelines for the use of cells and their byproducts, which must undergo “minimal manipulation” prior to use in humans.91 A recent publication from the present authors summarizes significant modifications to the phenotype of MSCs (more than minimal manipulation) to facilitate bone healing.9 Some of these techniques include preconditioning with biologics, exposure of MSCs to low oxygen environments, and gene therapy/genetic manipulation of cells. Some other approaches include optimizing techniques for isolation, expansion, and storage of MSCs, and improving the physical, chemical, topographic, electrical, and other properties of the carrier or scaffold used, and the defect in the host into which the cells are implanted.
Gene Therapies
Gene therapy and the genetic manipulation of cells for the purpose of musculoskeletal tissue healing are an exciting concept and have been reviewed elsewhere.9,92 Gene therapy may be accomplished using chemical and physical methodologies without viruses to transport DNA or microparticles into cells; by the use of gene activated matrices (GAMs) or other platforms that support the release of genetic material to the surrounding cells according to predetermined temporal and spatial parameters; via the use of viral vectors to engineer autologous or allogeneic cells ex vivo with subsequent injection of these cells in vivo; or by direct transfer of genes into cells in vivo.9 Gene therapy has also been used as a treatment for osteonecrosis, mostly in preclinical studies.93–100 We have used BMAC, MSCs, preconditioned MSCs, and genetically modified MSCs that overexpress IL-4 injected into the CD tract with/without a novel 3D printed, customized functionally graded scaffold as a treatment for ONFH in rabbits.1,101 In preclinical studies, the addition of IL-4 over-expressing MSCs in the acute phase of osteonecrosis may hamper regenerative efforts by suppressing the acute inflammatory reaction that is necessary for bone healing. Other strategies are currently being assessed.
Summary
The use of biomolecules, drugs, cells, and gene therapy for the treatment of osteonecrosis is very enticing. However, these treatments are generally in the preclinical stage except for BMAC therapy, and must be weighed against numerous potential risks including unintended adverse effects on neighboring cells, and the development of immunogenicity, mutagenicity, and carcinogenesis. Furthermore, the timing, dose and optimal platform for delivery, and issues related to cost-effectiveness must be addressed.
Discussion
Chronic inflammation is detrimental to all tissues and organs. This process generally leads to the replacement of normal host tissue by an undesirable fibrovascular stromal scar laden with acute and chronic inflammatory cells. This substitute tissue does not have the anatomical, physiological, metabolic, and functional integrity of the host tissue. In clinical cases in which substantial portions of an organ are afflicted by chronic inflammation, the operational performance of vital processes may be jeopardized, eg, in chronic hepatitis, nephritis, diabetes, cardiopulmonary disease, RA, aging, and other disorders.102,103 The associated morbidity and mortality are substantial.104
In this regard, bones and joints are no different. Chronic inflammation is often seen in inflammatory arthritis, chronic osteomyelitis, nonunion of fractures, and osteonecrosis. This is manifested as persistent unresolved overactivity of the innate, and in some cases, the adaptive immune systems. In osteonecrosis, despite various associated predisposing factors, chronic inflammation in response to DAMPs impedes neovascularization and osteogenesis. This situation will progress to joint collapse and end-stage arthritis if it is not arrested. The situation is even more dire in cases of multifocal osteonecrosis.105,106
The optimal treatment for early-stage osteonecrosis involves strategies for mitigation of chronic inflammation, and fostering of osteogenesis and vasculogenesis prior to joint collapse. In these cases, joint preservation is a much better option than joint replacement, due to the patient’s young age. However, the exact treatment for these complex cases, often in the presence of persisting predisposing factors (eg, continued high dose corticosteroids for the treatment of SLE) sometimes restricts the medical practitioner’s options.
Systemic pharmacological approaches appear to have little utility in the treatment and prevention of osteonecrosis in the adult.73 Early diagnosis is important so that treatment options can be reviewed and implemented. This suggests that high-risk patients need to be identified and screened, at least with a comprehensive history and possibly with selective non-invasive imaging such as MRI.25
Local treatment with CD, possibly with the addition of biological adjuncts such as BMAC seems reasonable in the pre-collapse stages. Research should address what component(s) of the BMAC and what doses optimize reconstitution of the osteonecrotic defect. Specific biological approaches might focus on the issues of attendant chronic inflammation, and their adverse effects on osteogenesis and vasculogenesis. Custom design of cells and biologics derived from the patient’s own tissues, though currently not approved by the FDA as they would involve more than minimal manipulation, might further improve the aims and goals of regenerative medicine for osteonecrosis. Custom-designed mechanically based implants could potentially delay physical collapse of bone and cartilage, and provide important signaling cues for tissue regeneration. Such implants that are 3D printed and biodegradable are currently being tested in preclinical studies in our laboratory.101 It is hoped that some of these technologies will prove to be safe, efficacious, and cost-effective. In this way the pain, disability, and morbidity of the millions of patients with osteonecrosis worldwide might be assuaged.
Acknowledgments
The authors acknowledge the generous support of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health (Grant No. R01AR063717, R01AR073145 and RO1AR72613), and the Ellenburg Chair in Surgery, Stanford University.
Disclosure
Prof. Dr. Stuart B Goodman reports a patent for a customized load-bearing and bioactive functionally graded implant for the treatment of osteonecrosis issued to Stanford University. The authors report no other conflicts of interest in this work.
References
1. Maruyama M, Rhee C, Utsunomiya T, et al. Modulation of the inflammatory response and bone healing. Front Endocrinol (Lausanne). 2020;11:386. doi:10.3389/fendo.2020.00386
2. Goodman SB, Pajarinen J, Yao Z, Lin T. Inflammation and bone repair: from particle disease to tissue regeneration. Front Bioeng Biotechnol. 2019;7:230. doi:10.3389/fbioe.2019.00230
3. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650.
4. Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89.
5. Lin T, Pajarinen J, Nabeshima A, et al. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res Ther. 2017;8(1):277.
6. Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32(2):395–409.
7. Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133–143.
8. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686.
9. Goodman SB, Lin T. Modifying MSC phenotype to facilitate bone healing: biological approaches. Front Bioeng Biotechnol. 2020;8:641.
10. Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17(6):393–402. doi:10.1089/ten.teb.2011.0182
11. Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi:10.1016/j.bone.2016.02.020
12. Pahwa R, Goyal A, Bansal P, Jialal I. Chronic Inflammation. In: StatPearls Publishing Copyright © 2020. Treasure Island (FL): StatPearls Publishing LLC; 2020.
13. Pucino V, Certo M, Varricchi G, et al. Metabolic checkpoints in rheumatoid arthritis. Front Physiol. 2020;11:347.
14. Bhatia D, Capili A, Choi ME. Mitochondrial dysfunction in kidney injury, inflammation, and disease: potential therapeutic approaches. Kidney Res Clin Pract. 2020;39(3):244–258. doi:10.23876/j.krcp.20.082
15. van Niekerk G, Engelbrecht AM. Inflammation-induced metabolic derangements or adaptation: an immunometabolic perspective. Cytokine Growth Factor Rev. 2018;43:47–53. doi:10.1016/j.cytogfr.2018.06.003
16. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi:10.1002/path.4133
17. Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445–1453. doi:10.1016/j.exphem.2009.09.004
18. Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J. 2017;99–B(10):1267–1279.
19. Koo K-H, Mont MA. Osteonecrosis. Berlin, Germany: Springer-Verlag; 2014.
20. Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6(8):590–601. doi:10.5312/wjo.v6.i8.590
21. Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015;97(19):1604–1627. doi:10.2106/JBJS.O.00071
22. Seamon J, Keller T, Saleh J, Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. doi:10.1155/2012/601763
23. Yoon BH, Jones LC, Chen CH, et al. Etiologic classification criteria of ARCO on femoral head osteonecrosis part 1: glucocorticoid-associated osteonecrosis. J Arthroplasty. 2019;34(1):163–168 e161. doi:10.1016/j.arth.2018.09.005
24. Yoon BH, Kim TY, Shin IS, Lee HY, Lee YJ, Koo KH. Alcohol intake and the risk of osteonecrosis of the femoral head in Japanese populations: a dose-response meta-analysis of case-control studies. Clin Rheumatol. 2017;36(11):2517–2524. doi:10.1007/s10067-017-3740-4
25. Boontanapibul K, Steere JT, Amanatullah DF, Huddleston JI, Maloney WJ, Goodman SB. Diagnosis of osteonecrosis of the femoral head: too little, too late, and independent of etiology. J Arthroplasty. 2020;35(9):2342–2349. doi:10.1016/j.arth.2020.04.092
26. Motomura G, Yamamoto T, Miyanishi K, Yamashita A, Sueishi K, Iwamoto Y. Bone marrow fat-cell enlargement in early steroid-induced osteonecrosis–a histomorphometric study of autopsy cases. Pathol Res Pract. 2005;200(11–12):807–811. doi:10.1016/j.prp.2004.10.003
27. Phipps MC, Huang Y, Yamaguchi R, et al. In vivo monitoring of activated macrophages and neutrophils in response to ischemic osteonecrosis in a mouse model. J Orthop Res. 2016;34(2):307–313. doi:10.1002/jor.22952
28. Tian L, Wen Q, Dang X, You W, Fan L, Wang K. Immune response associated with Toll-like receptor 4 signaling pathway leads to steroid-induced femoral head osteonecrosis. BMC Musculoskelet Disord. 2014;15:18.
29. Takagi M, Takakubo Y, Pajarinen J, et al. Danger of frustrated sensors: role of Toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements. J Orthop Transl. 2017;10:68–85.
30. Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel). 2017;5(4).
31. Deng YY, Lu J, Ling EA, Kaur C. Monocyte chemoattractant protein-1 (MCP-1) produced via NF-kappaB signaling pathway mediates migration of amoeboid microglia in the periventricular white matter in hypoxic neonatal rats. Glia. 2009;57(6):604–621.
32. Siddiqui JA, Partridge NC. CCL2/monocyte chemoattractant protein 1 and parathyroid hormone action on bone. Front Endocrinol (Lausanne). 2017;8:49.
33. Mulholland BS, Forwood MR, Morrison NA. Monocyte chemoattractant protein-1 (MCP-1/CCL2) drives activation of bone remodelling and skeletal metastasis. Curr Osteoporos Rep. 2019;17(6):538–547.
34. Adapala NS, Yamaguchi R, Phipps M, Aruwajoye O, Kim HKW. Necrotic bone stimulates proinflammatory responses in macrophages through the activation of Toll-like receptor 4. Am J Pathol. 2016;186(11):2987–2999.
35. Pei J, Fan L, Nan K, et al. Excessive activation of TLR4/NF-kappaB interactively suppresses the canonical Wnt/beta-catenin pathway and induces SANFH in SD rats. Sci Rep. 2017;7(1):11928.
36. Li T, Zhang Y, Wang R, et al. Discovery and validation an eight-biomarker serum gene signature for the diagnosis of steroid-induced osteonecrosis of the femoral head. Bone. 2019;122:199–208.
37. Carvalho JL, Braga VB, Melo MB, et al. Priming mesenchymal stem cells boosts stem cell therapy to treat myocardial infarction. J Cell Mol Med. 2013;17(5):617–625.
38. Croes M, Oner FC, Kruyt MC, et al. Proinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitment. PLoS One. 2015;10(7):e0132781.
39. Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18(9):1584–1592.
40. Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–884.
41. Loi F, Córdova LA, Zhang R, et al. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7(1):15.
42. Noronha NC, Mizukami A, Caliari-Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):131.
43. Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39(8):1559–1582.
44. Gibon E, Ma T, Ren PG, et al. Selective inhibition of the MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. J Orthop Res. 2012;30(4):547–553.
45. Lin TH, Sato T, Barcay KR, et al. NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng Part A. 2015;21(5–6):875–883.
46. Pajarinen J, Lin T, Nabeshima A, et al. IL-4 reverses wear particle induced osteolysis by modulating macrophage polarization and bone turnover.
47. Pearl JI, Ma T, Irani AR, et al. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32(24):5535–5542.
48. Sato T, Pajarinen J, Behn A, et al. The effect of local IL-4 delivery or CCL2 blockade on implant fixation and bone structural properties in a mouse model of wear particle induced osteolysis. J Biomed Mater Res A. 2016;104(9):2255–2262.
49. Sato T, Pajarinen J, Lin TH, et al. NF-kappaB decoy oligodeoxynucleotide inhibits wear particle-induced inflammation in a murine calvarial model. J Biomed Mater Res A. 2015;103(12):3872–3878.
50. Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. J Orthop Res. 2004;22(1):13–20.
51. Cheng T, Zhang X. NFkappaB gene silencing inhibits wear particles-induced inflammatory osteolysis. Med Hypotheses. 2008;71(5):727–729.
52. Nabeshima A, Pajarinen J, Lin TH, et al. Mutant CCL2 protein coating mitigates wear particle-induced bone loss in a murine continuous polyethylene infusion model. Biomaterials. 2017;117:1–9.
53. Spiller KL, Nassiri S, Witherel CE, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207.
54. Spiller KL, Vunjak-Novakovic G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv Transl Res. 2015;5(2):101–115.
55. Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488.
56. Spiller KL, Freytes DO, Vunjak-Novakovic G. Macrophages modulate engineered human tissues for enhanced vascularization and healing. Ann Biomed Eng. 2015;43(3):616–627.
57. Lin T, Kohno Y, Huang JF, et al. Preconditioned or IL4-secreting mesenchymal stem cells enhanced osteogenesis at different stages. Tissue Eng Part A. 2019;25(15–16):1096–1103.
58. Lin T, Kohno Y, Huang JF, et al. NFkappaB sensing IL-4 secreting mesenchymal stem cells mitigate the proinflammatory response of macrophages exposed to polyethylene wear particles. J Biomed Mater Res A. 2018;106(10):2744–2752.
59. Lin T, Pajarinen J, Kohno Y, et al. Transplanted interleukin-4–secreting mesenchymal stromal cells show extended survival and increased bone mineral density in the murine femur. Cytotherapy. 2018;20(8):1028–1036.
60. Lin T, Pajarinen J, Nabeshima A, et al. Establishment of NF-kappaB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy. 2017;19(9):1025–1034.
61. Pajarinen J, Tamaki Y, Antonios JK, et al. Modulation of mouse macrophage polarization in vitro using IL-4 delivery by osmotic pumps. J Biomed Mater Res A. 2015;103(4):1339–1345.
62. Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–3802.
63. Ueno M, Lo CW, Barati D, et al. Interleukin-4 overexpressing mesenchymal stem cells within gelatin-based microribbon hydrogels enhance bone healing in a murine long bone critical-size defect model. J Biomed Mater Res A. 2020;108(11):2240–2250.
64. Gelse K, von der Mark K, Schneider H. Cartilage regeneration by gene therapy. Curr Gene Ther. 2003;3(4):305–317.
65. Cui Q, Botchwey EA. Emerging ideas: treatment of precollapse osteonecrosis using stem cells and growth factors. Clin Orthop Relat Res. 2011;469(9):2665–2669.
66. Bai Y, Yin G, Huang Z, et al. Localized delivery of growth factors for angiogenesis and bone formation in tissue engineering. Int Immunopharmacol. 2013;16(2):214–223.
67. Balmayor ER. Targeted delivery as key for the success of small osteoinductive molecules. Adv Drug Deliv Rev. 2015;94:13–27.
68. Chen CH, Chang JK, Lai KA, Hou SM, Chang CH, Wang GJ. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64(5):1572–1578.
69. Kuroda Y, Kawai T, Goto K, Matsuda S. Clinical application of injectable growth factor for bone regeneration: a systematic review. Inflamm Regen. 2019;39:20.
70. Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355:S314–335.
71. Mont MA, Jones LC, Seyler TM, Marulanda GA, Saleh KJ, Delanois RE. New treatment approaches for osteonecrosis of the femoral head: an overview. Instr Course Lect. 2007;56:197–212.
72. Kuroda Y, Matsuda S, Akiyama H. Joint-preserving regenerative therapy for patients with early-stage osteonecrosis of the femoral head. Inflamm Regen. 2016;36:4.
73. Lee YJ, Cui Q, Koo KH. Is there a role of pharmacological treatments in the prevention or treatment of osteonecrosis of the femoral head?: a systematic review. J Bone Metab. 2019;26(1):13–18.
74. Goodman SB. The biological basis for concentrated iliac crest aspirate to enhance core decompression in the treatment of osteonecrosis. Int Orthop. 2018;42(7):1705–1709.
75. Goodman SB, Hwang KL. Treatment of secondary osteonecrosis of the knee with local debridement and osteoprogenitor cell grafting. J Arthroplasty. 2015;30(11):1892–1896.
76. Hernigou P, Dubory A, Homma Y, et al. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018;42(7):1639–1649.
77. Hernigou P, Flouzat-Lachaniette CH, Delambre J, et al. Osteonecrosis repair with bone marrow cell therapies: state of the clinical art. Bone. 2015;70:102–109.
78. Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–1009.
79. Hernigou P, Trousselier M, Roubineau F, et al. Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin Orthop Surg. 2016;8(1):1–8.
80. Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002;(395):66–80.
81. Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19(1):117–125.
82. Hernigou P, Poignard A, Zilber Z, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40–45.
83. Lu LY, Loi F, Nathan K, et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. 2017;35(11):2378–2385.
84. Batoon L, Millard SM, Raggatt LJ, Pettit AR. Osteomacs and bone regeneration. Curr Osteoporos Rep. 2017;15(4):385–395.
85. Vi L, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30(6):1090–1102.
86. Tan SHS, Wong JRY, Sim SJY, et al. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio. 2020;7:100067.
87. Houdek MT, Wyles CC, Martin JR, Sierra RJ. Stem cell treatment for avascular necrosis of the femoral head: current perspectives. Stem Cells Cloning. 2014;7:65–70.
88. Huang S, Xu L, Zhang Y, Sun Y, Li G. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 2015;24(12):2643–2655.
89. Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration–bench to bedside. J Biomed Mater Res B Appl Biomater. 2009;89(1):252–263.
90. Li Z, Liao W, Zhao Q, et al. Angiogenesis and bone regeneration by allogeneic mesenchymal stem cell intravenous transplantation in rabbit model of avascular necrotic femoral head. J Surg Res. 2013;183(1):193–203.
91. Administration FaD. Regulatory considerations for human cells, tissues, and cellular and tissue- based products: minimal manipulation and homologous use; 2017. Available from: https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
92. Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11(4):234–242.
93. Shu P, Sun DL, Shu ZX, et al. Therapeutic applications of genes and gene-engineered mesenchymal stem cells for femoral head necrosis. Hum Gene Ther. 2020;31(5–6):286–296.
94. Yang F, Xue F, Guan J, Zhang Z, Yin J, Kang Q. Stromal-Cell-Derived Factor (SDF) 1-alpha overexpression promotes bone regeneration by osteogenesis and angiogenesis in osteonecrosis of the femoral head. Cell Physiol Biochem. 2018;46(6):2561–2575.
95. Pan ZM, Zhang Y, Cheng XG, Gao GC, Wang XR, Cao K. Treatment of femoral head necrosis with bone marrow mesenchymal stem cells expressing inducible hepatocyte growth factor. Am J Ther. 2016;23(6):e1602–e1611.
96. Zhang C, Ma J, Li M, Li XH, Dang XQ, Wang KZ. Repair effect of coexpression of the hVEGF and hBMP genes via an adeno-associated virus vector in a rabbit model of early steroid-induced avascular necrosis of the femoral head. Transl Res. 2015;166(3):269–280.
97. Ding H, Gao YS, Hu C, et al. HIF-1α transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits. PLoS One. 2013;8(5):e63628.
98. Hang D, Wang Q, Guo C, Chen Z, Yan Z. Treatment of osteonecrosis of the femoral head with VEGF165 transgenic bone marrow mesenchymal stem cells in mongrel dogs. Cells Tissues Organs. 2012;195(6):495–506.
99. Wen Q, Ma L, Chen YP, Yang L, Luo W, Wang XN. Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells. Gene Ther. 2008;15(23):1523–1535.
100. Tang TT, Lu B, Yue B, et al. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89(1):127–129. doi:10.1302/0301-620X.89B1.18350
101. Maruyama M, Nabeshima A, Pan CC, et al. The effects of a functionally-graded scaffold and bone marrow-derived mononuclear cells on steroid-induced femoral head osteonecrosis. Biomaterials. 2018;187:39–46. doi:10.1016/j.biomaterials.2018.09.030
102. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol a Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi:10.1093/gerona/glu057
103. Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;5(Suppl 5):S422–s428. doi:10.1513/AnnalsATS.201602-095AW
104. Kahn JM, Le T, Angus DC, et al. The epidemiology of chronic critical illness in the United States*. Crit Care Med. 2015;43(2):282–287. doi:10.1097/CCM.0000000000000710
105. Sun W, Shi Z, Gao F, Wang B, Li Z. The pathogenesis of multifocal osteonecrosis. Sci Rep. 2016;6:29576. doi:10.1038/srep29576
106. Symptomatic multifocal osteonecrosis. A multicenter study. Collaborative Osteonecrosis Group. Clin Orthop Relat Res. 1999;(369):312–326.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.