Back to Journals » Cancer Management and Research » Volume 11
Infiltrating CD4 and CD8 lymphocytes in HPV infected uterine cervical milieu
Authors Maskey N , Thapa N, Maharjan M , Shrestha G , Maharjan N, Cai H, Liu S
Received 27 May 2019
Accepted for publication 23 July 2019
Published 14 August 2019 Volume 2019:11 Pages 7647—7655
DOI https://doi.org/10.2147/CMAR.S217264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Ninu Maskey,1 Niresh Thapa,2,3 Muna Maharjan,4 Girishma Shrestha,5 Narayani Maharjan,6 Hongbing Cai,2 Shangqin Liu1
1Department of Hematology, Zhongnan Hospital of Wuhan University, Wuchang, Wuhan, Hubei 430071, People’s Republic of China; 2Department of Gynecological Oncology, Zhongnan Hospital of Wuhan University, Hubei Cancer Clinical Study Center, Hubei Key Laboratory of Tumor Biological Behaviors, Wuhan 430071, People’s Republic of China; 3Karnali Academy of Health Sciences, Jumla, Nepal; 4Zhongnan Hospital of Wuhan University, Hope School of Nursing, Wuhan, Hubei, People’s Republic of China; 5Department of Pathology, Patan Hospital, Patan Academy of Health Sciences, Kathmandu, Nepal; 6Department of Clinical Laboratory Science, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China
Purpose: Tumor infiltrating lymphocytes (TILs) have been extensively described in anti-tumor immunity, but their functional alterations in the immunoediting processes during neoplastic progression in the uterine cervix are still not clear. Our aim was to gain insight into cervical tissue T cell populations, determine if there are any differences in the localization and quantity distribution of T lymphocytes, and to evaluate their role in disease regression or progression in the cervical neoplastic milieu.
Patients and methods: Serial section analysis of immunohistochemically stained CD4 and CD8 lymphocytes was performed on a total number of 72 samples, categorized into four cohorts: 23 HPV non-infected (HPV-) normal cervix, 20 HPV infected (HPV+) normal cervix, 17 HPV+ low grade cervical intraepithelial neoplasia (CIN), and 12 HPV+ high grade CIN.
Results: Low infiltrating lymphocytes (ILs) in normal cervix and high ILs in CIN were observed, while the trend of ILs increased with increasing grade of CIN, which was statistically significant (P<0.0001). Quantitative and localization analysis between the subsets of T cells showed that, in the epithelial layer, infiltrating CD8+ lymphocytes (CD8+ILs) were significantly higher than CD4+ILs in HPV+ normal cervix, while the trend decreased with increasing grade of CIN (P=0.011). Whereas, in the stromal layer, CD4+ILs were predominant in all study groups and no statistical difference was found between these groups. However, tumor infiltrating CD8+ lymphocytes (CD8+TILs) were noted to be significantly higher than CD4+TILs in severe dysplastic cases.
Conclusion: T cell infiltrates were predominant as the grade of the lesion progressed into more advanced lesions, which likely represent the lesions that have persisted over time. The variation in the infiltration rate and the location of CD4+ILs and CD8ILs may suggest the efficacious role of CD8 T cells in eliminating HPV infected cervical epithelial cells and also provides insight into the complex role of TILs in facilitating and mediating sustained anti-tumor responses, hence preventing tumor outgrowth.
Keywords: cervical screening test, human papilloma virus, cervical intraepithelial neoplasia, CD4, CD8, immunohistochemistry
Introduction
Cervical cancer is the second most commonly diagnosed cancer and third leading cause of cancer death among females in less developed countries. Over the past 40 years, cervical cancer rates have decreased by as much as 65% in several Western countries, where screening programs have long been established to detect precancerous cervical lesions that can be treated successfully.1 In developing countries, due to limited access to effective screening programs, cervical neoplasia is often identified at an advanced stage with symptoms, resulting in a higher rate of mortality from cervical cancer.2,3 In Nepal, it is consistently number one disease of incidence and mortality. Persistent infection with HPV is a known etiological factor in the development of cervical intraepithelial neoplasia (CIN) and cervical cancer. As reported by the Information Center on HPV and Cancer, Nepal reports a prevalence of HPV type 16 and or HPV type 18 among women with low grade squamous intraepithelial lesion (LSIL), high grade squamous intraepithelial lesion (HSIL), and cervical cancer of 30.2%, 63.4%, and 80.3% respectively.4–7 However, the latency period between primary infection and the appearance of cervical dysplasia is lengthy, which suggests that additional factors are involved in disease progression.8
Among the factors that influence the probability of a cervical HPV infection becoming persistent, cell-mediated immunity of an individual is considered to be an important mechanism in protection against the virus and elimination of virus-infected cells.9,10 Tumors possess a variety of cell membrane-bound antigens, recognized as non-self by the immune system which stimulate a cytotoxic immune response characterized by CD4, CD8, antigen-presenting cells, and other lymphoid elements' infiltration.11–15 These infiltrating cells are recognized as a response by immune surveillance mechanisms designed to inhibit tumor growth and spread.11,15,16 tumor infiltrating lymphocytes (TILs), mainly CD4 and CD8 T cells have been extensively described in anti-tumor immunity and knowledge of CD4 and CD8 T lymphocytes' interplay in mediating the control of tumor growth exists.17,18 However, little is known about the functional alterations of these T cell subsets in the immunoediting processes during neoplastic progression in the cervix. Also, to our knowledge, there is limited information regarding the distribution and localization of these molecules in CIN. Based on the speculation that the activation of a specific immune response may be reflected in the increase or decrease in the number of infiltrating subtypes of T cell in the site of the HPV infected precursor lesions, we focused on accurately determining the quantity (cells/mm2) of CD4+ T cells and CD8+ T cells in HPV- normal cervix, HPV+ normal cervix, and HPV+CIN of different grades.
Materials and methods
Study participants and procedures
After obtaining ethical approval from Nepal Health Research Council, Nepal and Department of Gynecological Oncology, Second Clinical College of Wuhan University, People’s Republic of China, the study participants were recruited from the different mobile cervical cancer screening clinics and Karnali Academy of Health Science conducted in Jumla, Nepal in 2016 and 2017. The screenings consisted of Papanicolaou smear and visual screening tests. The details of the screening tests were described in our previously published article.19
Briefly, samples were collected for cytology and punch biopsies from areas in the cervix assessed by visual inspection with acetic acid and/or visual inspection with Lugol’s iodine and regarded as abnormal. Biopsy specimens were fixed in 10% formalin and sent to the pathologist. Samples were not collected from women if they were menstruating on the day of enrollment. The women had negative cytology reports for intraepithelial lesion or malignancy, inflammatory, atypical squamous cells of undetermined significance, LSIL or HSIL. The histopathological results were defined as chronic cervicitis, normal cervix, low grade dysplasia (CIN I) or high grade dysplasia (CIN II– III) based on a consensus review by two experienced pathologists. Biopsy report is the gold standard for defining final disease status. HPV sampling was obtained from the women at the second visit for follow-up. Finally, 72 study participants were enrolled, classified into four groups: i) 23 HPV- normal cervix; ii) 20 HPV+ normal cervix; iii) 17 HPV+ low grade CIN; iv) 12 HPV+ high grade CIN. Participants with the following conditions were excluded from the study: pregnancy, women with any signs and symptoms of sexually transmitted infections, cytology- and/or biopsy-proven cervicitis tissues, any other neoplastic diseases, immunocompromised state and those under any anti-inflammatory or immunosuppressive treatment. The study was carried out according to the Declaration of Helsinki. All participants gave written informed consent.
PCR for HPV DNA typing
Cervical cells were collected using cytobrush (Hybribio® Limited, People’s Republic of China) from the eligible women who were followed up for our previous screening test (cytology) report. DNA extraction and HPV genotyping were analyzed, as described in detail elsewhere.20 Briefly, DNA was extracted in Intrepid Cancer Diagnostics, Kathmandu from the cervical samples using the QIAamp DNA Mini kit (Qiagen NV, Venlo, the Netherlands) according to the manufacturer’s instructions. The presence of HPV DNA was primarily confirmed by HPV consensus PCR using PGMY09/PGMY11 designed primers to amplify a fragment of the HPV L1 gene visible at 450 bp and human leukocyte antigen (HLA) band at 230 bp as described in “Human papillomavirus laboratory manual” published by the World Health Organization.21 Detection of HLA confirmed that the samples contained a sufficient quantity of DNA. To limit to only HPV positive samples, gel electrophoresis analysis was performed and further HPV genotyping was analyzed by membrane hybridization (21 HPV GenoArray Diagnostic Kit, Hybrobio® Limited, People’s Republic of China) to detect the following 21 HPV types: HPV 6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, 81 (equivalent to CP8304) according to the manufacturer’s instruction. In this study, types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68 were included as high-risk HPV (HR-HPV) and remaining HPV types were considered as low-risk HPV.22
Immunohistochemistry
Immunohistochemistry was performed using our previously published protocol.23 Briefly, selected formalin-fixed paraffin-embedded sections were stained with antibodies against CD4 (rabbit monoclonal antibody, clone: EP204), CD8 (rabbit monoclonal antibody, clone: SP16), and p16INK4a (mouse monoclonal antibody, clone: 10). All the antibodies were bought from ZSGB-BIO, Beijing, People’s Republic of China and were in prediluted form. The staining was visualized using 3,3′-diaminobenzidine substrate kit and counterstained with hematoxylin.
Immune cell quantitation
Serial section analysis for CD4 and CD8 was performed. Images were captured focusing on regions of interest (ROIs) using an OLYMPUS BX51 microscope and a DP72 OLYMPUS digital camera, using DP2-BSW imaging software (Olympus Corporation, Tokyo, Japan). ROIs were defined as: in normal epithelium, stroma immediately beneath normal epithelium, CIN 1/2/3 epithelium, and stroma immediately beneath CIN 1/2/3. Cytoplasmic immunoreactivity for CD4 and CD8 were semi-quantitated by consensus of two pathologists who were blinded to the clinicopathological data. Stained lymphocytes were quantitated by normalization against the area of the ROI (mm2) and given a score of either 0 (≤5 lymphocytes), 1+ (6-50 lymphocytes), 2+ (51-99 lymphocytes) or 3+ (>100 lymphocytes). Cells that were scored as 1+ or 2+ were considered as low rate of infiltration and those scored 3+ were considered as high rate of infiltration.
Statistical analysis
Data analysis was performed using SPSS 20.0 (IBM Corporation, Armonk, NY, USA). Descriptive statistics were reported as frequency, mean, and percentage. OR and 95% CI were calculated using cross-tabulations to describe the association of potential risk factors with the rate of lymphocyte infiltration. χ2 test was used to compare the expression level of CD4 and CD8 between the groups. All P-values resulting from the tests of significance were considered significant at P<0.05.
Results
Type-specific HPV distribution of study group
The type-specific HPV distribution of the study group is summarized in Table 1. Seventy-two study participants were aged between 21 and 60 years (mean +SD, 37.57+8.08). Thirty-four women (69.39%) were infected with HR-HPV, including multiple infections. The most common HR-HPV type was 16 (16.33%, 8/49) followed by 18 (10.20%, 5/49), and 39 (8.16%, 4/49).
 |
Table 1 HPV type-specific distribution and CD4, CD8 immunoreactivity score of study group |
Immunoreactivity score of CD4 and CD8 in study group
The immunoreactivity score of CD4 and CD8 in our study group is shown in Table 1. Among 23 cases of HPV- normal cervix, CD4+ expression at score 1+ was noted in 19 cases (82.61%) and 2+ in four cases (17.39%). CD8 expression at score 1+ was detected in 21 cases (91.30%) and 2+ in two cases (8.7%). CD4 and CD8 expression at score 3+ was not observed in any of the cases.
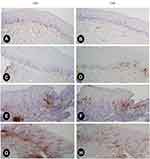
According to high and low lymphocyte infiltration category, all 23 cases fell under low CD4 and CD8 infiltration (Figure 1A and B).
In a cohort of 20 HPV+ normal cervix, CD4+ and CD8+ expression at score 1+ was noted in 13 cases (65%), 2+ in four cases (20%), and 3+ in three cases (15%). Among 17 cases of HPV+ low grade CIN, CD4+ and CD8+ expression at score 2+ was noted in nine cases (52.94%) and ten cases (58.82%) respectively, 3+ in cases eight cases (47.06%) and seven cases (41.18%) respectively whereas, in HPV+ high grade CIN, all the cases showed a score of 3+ (100%).
Although the expression of CD4+ infiltrating lymphocytes (CD4+ILs) and CD8+ILs fell under the same scoring range in most of the HPV+ cases, a significant difference was noticed in the quantity and the distribution location of CD4ILs and CD8ILs between epithelial and stromal region, which is reported in detail in the following section.
Association of infiltration rate of lymphocytes between HPV+ normal cervix and CIN of different grades
The association of infiltration rate of lymphocytes between HPV+ normal cervix and CIN of different grades is displayed in Table 2. Low rate of ILs in normal cervix and high rate of ILs in CIN were observed, while the trend of ILs increased with increasing grade of CIN, which was statistically significant (15% vs 47.06% vs 100% respectively, P<0.0001).
 |
Table 2 Association of infiltration rate of lymphocytes between HPV+ normal cervix and CIN of different grades |
Association of cervical pathology and potential risk factors with the rate of ILs
Table 3 shows the univariate analysis of cervical pathology and potential risk factors with the rate of ILs. CIN is associated with high ILs and the difference in the rate of ILs between CIN and normal cervix was statistically significant (P<0.0001). Potential risk factors such as parity (>4) and contraceptive use were found to be associated with high ILs (P=0.005, 0.047 respectively) which is related to CIN.
 |
Table 3 Univariate analysis of different variables with the rate of infiltrating lymphocytes |
Localization and quantity distribution of CD4 and CD8 in HPV+ normal and CIN group
Table 4 shows the comparison of localization and quantity distribution between CD4ILs and CD8ILs in HPV+ normal and HPV+ CIN group. In a cohort of HPV+ normal cervix, CD8 lymphocytes were noticed predominantly in the epithelial region whereas, CD4 was mostly in the stromal region (Figure 1C and D). When the infiltrating CD4+ and CD8+ lymphocytes were counted separately in epithelial and stromal layer, we found that the number of CD8+ILs was higher than CD4+ILs cells in the epithelial layer (CD8h, 70%) while the number of CD4+ILs was higher than CD8+ILs (CD4h, 80%) in stromal layer. In low grade CIN group, ILs were significantly higher than in normal cervix (Figure 1E and F). When the subsets of TILs were counted separately in both regions, we found CD4h in both epithelial and stromal layer (58.82% and 82.35% respectively). In high grade CIN, CD4TILs and CD8TILs were significantly higher than in low grade CIN (Figure 1G and H). Individual counting of the subsets of TILs in epithelial and stromal region showed CD4h (66.67% and 75% respectively).
 |
Table 4 Comparison of localization and quantity distribution between CD4ILs and CD8ILs in HPV+ normal and HPV+ CIN group |
Based on these results, we conclude that there was variation in the number of CD4ILs and CD8ILs in epithelial layer between normal cervix and CIN of different grades and this difference was statistically significant (P=0.011) whereas, no difference was noted in stromal layer as CD4+ILs were predominantly noted in all three groups (P=0.779), though CD8h could be noticed in severe dysplastic cases (Figure 2A and B).
Regression/progression of disease
Out of 17 low grade CIN cases, seven underwent cryotherapy and ten opted for follow-up in 6 months. Six months’ follow-up data showed that of those patients who underwent cryotherapy, six patients were disease-free and one patient did not show up for follow-up, whereas of those patients who opted for follow-up, only one patient developed high grade CIN, seven patients were disease-free, and two did not show up for follow-up. Among 12 high grade CIN cases, loop electrosurgical excision procedure (LEEP) was done in eight cases; simple hysterectomy was done in four cases where the women already had a complete family. None of the patients showed recurrence on follow-up in 6 months.
According to our second 6 months’ follow-up data, seven low grade CIN participants who underwent cryotherapy were disease-free, one patient who opted for follow-up developed high grade CIN, and the remaining six patients were disease-free. None of the high grade CIN patients who underwent LEEP or hysterectomy showed recurrence.
Discussion
Only a small proportion of women infected with HPV develop cervical cancer and the risk of progression to malignancy is very high with persistent HPV infection. Host immune response seems to play a role in eliminating the viral infection and preventing cancer progression.10 In this study, we characterized the subpopulations of T lymphocytes that infiltrate various grades of cervical neoplasia in immunocompetent women and focused on the density and the distribution location of infiltrating T cells to gain insight into cervical tissue T cell populations that could play a role in the regression or progression of dysplastic lesions.
In a cohort of HPV- and HPV+ normal cervical tissue, low CD4+ILs and CD8+ILs were noted. Though, a noteworthy difference that can be spotted between these two cohorts is, CD8+ cells were observed predominantly infiltrating the epithelial layer in HPV+ normal group, while this observation was not significant in HPV- normal group. Whereas, CD4+ cells were noticed predominantly in the stromal layer in both groups. When the cells were counted separately in epithelial and stromal regions, the number of CD8+ILs were found to be higher than CD4+ILs in epithelial layer in HPV+ normal group, suggesting that HPV infected non-neoplastic epithelial cells are highly susceptible to killing by CD8 T cells. However, the presence of CD4+ cells predominantly in the stromal layer cannot deny the efficacious role of CD4 T helper cells in priming cytotoxic CD8 lymphocyte.
In the CIN group, higher lymphocytic infiltration than in the normal cervix group was observed. Nonetheless, a progressive increase in the T cell infiltration was detected as the grade of the lesion progressed, and CD4 T cells were often aggregated into stromal clusters beneath the squamous intraepithelial lesions. However, no significant difference could be seen in the distribution location of CD8+TILs between epithelial and stromal layer. Higher density of CD4+TILs than CD8+TILs in the stromal layer demonstrates the role of CD4 T cells in facilitating and mediating sustained anti-tumor responses which is far beyond the mere task of providing helper signals to CD8 T cells. But, the CD4TILs predominance scenario in the stromal layer was reversed in severe dysplastic cases ie, a higher number of CD8TILs than CD4TILs was noticed, which is another substantial finding of our study.
Tumor outgrowth is mainly controlled by CD4 and CD8 T cells.24 During tumor development, cancer immunoediting ensues with its three phases namely elimination, equilibrium, and escape.25,26 In HPV infected cervical milieu, the persistent occurrence of HPV infection initiates cellular transformation into nascent tumor lesions, stimulating CD4 and CD8 T cells and mediating an immune response specifically to eliminate these lesions, thus protecting the host from cancer. When the immune response is incapable of completely clearing the tumor cells during the elimination phase, it promotes the generation of tumor cell variants with decreased immunogenicity. Still, the ability of the immune system to maintain occult cancer in an equilibrium state exists.27,28 Though our follow-up cases are few in number, 60% of our low grade CIN cases who opted for follow-up every 6 months showed non-progression of the disease by 12 months, which reveals that the immune system is able to maintain the emerging cancer in an equilibrium state and prevent tumor outgrowth.
CD4 and CD8 lymphocytes in cervical cancer patients have been studied by others suggesting an altered CD4+ and CD8+ ratio in cervical cancer tissues, and peripheral blood might be of prognostic significance.29 The number of CD8+ lymphocytes infiltrating cervical tumor mass was found greater than that of CD4+ lymphocytes in most of the cases in a study by Ghosh et al,30 which corresponds to our finding where CD8+TILs were higher than CD4+TILs in severe dysplastic cases. Prayitno et al31 showed expression of CD8+ and MHC-I in cervical cancer with HPV infection are in mild category indicating the importance of further research in cervical cancer immunology. A significant increment of CD3 lymphocytes in the epithelium and stroma of CIN III was discovered by Carrero et al,32 suggesting a casual role of CD3 lymphocytes in disease progression. Bedoya et al33 has reported that CD8+ T cells are more abundant than CD4+ and Foxp3+ cells in both the stroma and epithelium, regardless of CIN grade, though the difference was not found to be statistically significant. Based on the aforementioned contradictory studies, there is a need for further research on the expression of T cells and other molecules involved in the regulation of T cell responses and their implications in disease progression. Overall, in the current study, we showed that T cell infiltrates are predominant as the grade of the lesion progressed to more advanced lesions, which likely represent the lesions that have persisted over time. Cytotoxic CD8 T cell infiltrates appear to be principal effectors in eliminating HPV infected pre-neoplastic cervical epithelial cells and severe dysplastic cells, and are orchestrated by CD4 responses. Immune cells display a complex role facilitating and mediating sustained anti-tumor responses maintaining equilibrium phase, thus, preventing tumor outgrowth.
Conclusion
Although our present study provides IittIe insight into the true roles of T cell subsets in tumor progression, it offers an attractive research area to identify the cells and molecules involved in the regulation of T cell responses, which may serve as novel diagnostic and/or prognostic markers.
Acknowledgments
This work was supported by the Research Fund from the National Natural Science Foundation of China (No 81470007 and 81272627). The authors acknowledge all the participants in the study, Karnali Academy of Health Sciences and the staff, Swati Shah, Mina Rana, Satachhi Devkota, Jwala Upadhya, Nira Rai, and Savina Thapa for assistance during data collection and screening.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21294
2. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 [Internet]. GLOBOCAN 2012 v1.0; 2013. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
3. Miller AB, ed. Epidemiologic Studies in Cancer Prevention and Screening. New York: Springer Science & Business Media; 2012.
4. Insinga RP, Perez G, Wheeler CM, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev. 2011;20(2):287–296. doi:10.1158/1055-9965.EPI-10-0791
5. Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO/IARC information centre on HPV and cancer (HPV information Centre). Human Papollomavirus and related diseases in Nepal. Summary Report 17 June 2019. Available from: https://hpvcentre.net/statistics/reports/NPL.pdf. Accessed August 04, 2019.
6. Pun CB, Pradhananga KK, Siwakoti B, Subedi K, Moore MA. Malignant neoplasm burden in Nepal - data from the seven major cancer service hospitals for 2012. Asian Pac J Cancer Prev. 2016;16:8659–8663. doi:10.7314/APJCP.2015.16.18.8659
7. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
8. Sheu BC, Chang WC, Lin HH, Chow SN, Huang SC. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. J Obstet Gynaecol Res. 2007;33:103–113. doi:10.1111/j.1447-0756.2007.00492.x
9. Guzm an-Olea E, Bermu dez-Morales VH, Peralta-Zaragoza O, Torres-Poveda K, Madrid-Marina V. Molecular mechanism and potential targets for blocking HPV- induced lesion development. J Oncol. 2012;20:278312.
10. Nguyen HH, Broker TR, Chow LT, et al. Immune responses to human papillomavirus in genital tract of women with cervical cancer. Gynecol Oncol. 2005;96(2):452–461. doi:10.1016/j.ygyno.2004.10.019
11. Reck M, Paz-Ares L. Immunologic checkpoint blockade in lung cancer. Semin Oncol. 2015;42:402–417. doi:10.1053/j.seminoncol.2015.02.013
12. Helissey C, Champiat S, Soria JC. Immune checkpoint inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2015;27:108–117. doi:10.1097/CCO.0000000000000167
13. He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 Pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5:13110. doi:10.1038/srep13110
14. Domingues D, Turner A, Silva MD, et al. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy. 2014;6:1221–1235. doi:10.2217/imt.14.82
15. Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J Cancer. 2014;33:434–4. doi:10.5732/cjc.014.10122
16. Wagner SN, Schultewolter T, Wagner C, et al. Immune response against human primary malignant melanoma: a distinct cytokine mRNA profile associated with spontaneous regression. Lab Invest. 1998;78:541–550.
17. Davoodzadeh Gholami M, Kardar GA, Saeedi Y, Heydari S, Garssen J, Falak R. Exhaustion of T lymphocytes in the tumor microenvironment: significance and effective mechanisms. Cell Immunol. 2017;322:1–14. doi:10.1016/j.cellimm.2017.10.002
18. Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N. Cd4 and cd8 t lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75:689. doi:10.1007/s00018-017-2686-7
19. Niresh T, Girishma S, Muna M, et al. Burden of cervical neoplasia in mid-western rural nepal: a population-based study. J Gynecol Oncol. 2018;29(5):e64. doi:10.3802/jgo.2018.29.e64
20. Niresh T, Muna M, Girishma S, et al. Prevalence and type-specific distribution of human papillomavirus infection among women in mid-western rural, nepal- a population-based study. BMC Infect Dis. 2018;18(1):338. doi:10.1186/s12879-018-3109-6
21. World_Health_Organization. Human Papillomavirus Laboratory Manual First. Geneva: World Health Organization; 2010.
22. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens-part B: biological agents. Lancet Oncol. 2009;10:321–322. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1470204509700968. Accessed July 29, 2019.
23. Hu M, Li K, Maskey N, et al. Overexpression of the chemokine receptor CXCR3 and its correlation with favorable prognosis in gastric cancer. Hum Pathol. 2015;46(12):1872–1880. doi:10.1016/j.humpath.2015.08.004
24. Shankaran V, Ikeda H, Bruce AT, et al. IFN gamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi:10.1038/35074122
25. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi:10.1016/j.coi.2014.01.004
26. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi:10.1126/science.1203486
27. Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi:10.1038/nature06309
28. Verdegaal EM, de Miranda NF, Visser M, et al. Neoantigen landscape dynamics during human melanoma–T cell interactions. Nature. 2016;536:91–95. doi:10.1038/nature18945
29. Das D, Sarkar B, Mukhopadhyay S, Banerjee C, Biswas SM. An altered ratio of cd4+ and cd8+ t lymphocytes in cervical cancer tissues and peripheral blood – a prognostic clue? Asian Pac J Cancer Prev. 2018;19(2):471.
30. Ghosh AK, Moore M. Tumour-infiltrating lymphocytes in cervical carcinoma. Eur J Cancer. 1992;28(11):1910–1916. doi:10.1016/0959-8049(92)90034-Y
31. Prayitno A, Asnar E, Astirin OP, Putra ST. The expression of CD8+ and MHC-I in cervical cancer with HPV infection. J Cancer Ther. 2013;4:15–18. doi:10.4236/jct.2013.46A2003
32. Carrero Y, Callejas D, Alaña F, Silva C, Mindiola R, Mosquera J. Increased vascular endothelial growth factor expression, CD3‐positive cell infiltration, and oxidative stress in premalignant lesions of the cervix. Cancer. 2009;115:3680–3688. doi:10.1002/cncr.v115:16
33. Bedoya AM, Jaramillo R, Baena A, et al. Location and density of immune cells in precursor lesions and cervical cancer. Cancer Microenviron. 2013;6(1):69–77. doi:10.1007/s12307-012-0097-8
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.