Back to Journals » Orthopedic Research and Reviews » Volume 12
Infection Risk-Adjusted Antibiotic Prophylaxis Strategies in Arthroplasty: Short Review of Evidence and Experiences of a Tertiary Center in Spain
Authors Sanz-Ruiz P , Berberich C
Received 31 March 2020
Accepted for publication 21 June 2020
Published 6 August 2020 Volume 2020:12 Pages 89—96
DOI https://doi.org/10.2147/ORR.S256211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Pablo Sanz-Ruiz,1 Christof Berberich2
1Department of Orthopedics & Traumatology, University Hospital Gregorio Maranon, Madrid, Spain; 2Department of Medical Training, Heraeus Medical GmbH, Wehrheim, Germany
Correspondence: Christof Berberich
Department of Medical Training, Heraeus Medical GmbH, Philipp-Reis-Str. 8/13, Wehrheim 61273, Germany
Tel +49 6181 352996
Fax +49 6181 352916
Email [email protected]
Abstract: There is growing body of evidence that important patient-, procedure- and pathogen-related factors are linked to higher risks for prosthetic joint infections (PJI) following arthroplasty surgeries. The prior identification and optimization of such risk factors is considered paramount to minimize the incidence of these infections. Without any doubt, antibiotic prophylaxis remains one of the cornerstones among all preventive measures. However, the ideal antibiotic prophylaxis is still in debate and discussions have emerged, whether certain situations deserve adjustments or variations of the standard protocol taking into account antibiotic resistance surveillance data and patient risk factors for infections. This review aims to provide the reader with an overview of possible antibiotic prophylaxis strategies in response to these risks and discusses the clinical experiences so far obtained. We further present preliminary evidence that the use of a reinforced local antibiotic prophylaxis regimen with high-dose dual antibiotic-loaded bone cement may be an effective and easy-to-apply option in patients at high infection risks.
Keywords: arthroplasty, prosthetic joint infection, antibiotic prophylaxis, risk for infection, risk-adjustment, extension of prophylaxis, dual prophylaxis, antibiotic-loaded bone cement
Introduction
Periprosthetic joint infection (PJI) is a rare, but devastating complication following joint replacement. The implantation of extensive foreign material in arthroplasty and trauma surgeries increases the operational risk of infections due to the easy bacterial colonization and biofilm formation at the implant surfaces. The overall PJI incidence is in the range of 1–2% in primary arthroplasty, but 5% and higher in revision procedures.1 PJI treatment is complex and often requires multiple surgeries related with a high burden for the patient and high costs for the health-care system.2,3 The number of PJI cases is expected to further increase as a consequence of the worldwide growing numbers of primary and revision arthroplasty procedures. Despite higher awareness for this complication, infection rates have not changed much over the last two decades or tend to be even higher.4,5 This observation may be explained by the trend to operate more on older patients with higher ASA scores together with the spread of more resistant pathogens.6–8 In fact, there is increasing evidence that several patient-, pathogen- and procedure-related factors predispose patients to higher PJI risks than on average. Identifying these risk factors together with patients’ preoperative health optimization strategies, proper wound care and improvements in hygiene issues remain some of the core fundamental steps that can help minimizing the incidence of PJI. A more risk-adjusted antibiotic prophylaxis strategy has also been suggested as an additional easy-to-apply option within the framework of a more effective infection prevention. The current evidence of this rationale and our own experiences with a reinforced local antibiotic prophylaxis protocol for PJI risk patients in a large tertiary centre in Madrid, Spain, will be discussed in the following.
Risk Factors for PJI
From a practical point of view, different factors that increase the operational risk of infections in arthroplasty procedures may be grouped in the three categories of patient-, pathogen- and procedures-related risk factors (see Figure 1). Because of mutual influence, the effects on one another are in clinical practice often combined.
 |
Figure 1 Categories of PJI risk factors (patient-, pathogen- and surgical procedure-related). |
Patient-Specific Risk Factors
Arthroplasty patients are not equally at risk for intra- or postoperative infections following joint replacement procedures. Numerous clinical reports, arthroplasty registry-based observations and our own experiences come to the same conclusion that 70–80% of all patients have at least one modifiable or unmodifiable infection risk factor.9–12 Among these are important comorbidities such as severe obesity (BMI ≥35), uncontrolled diabetes mellitus, severe cardiovascular disease, severe kidney dysfunction, chronic immunosuppression, malnutrition, anemia or antecedent bacteremia, all of them repeatedly described to be associated with higher PJI rates.13–17 Therefore, optimization of these comorbidities stays in the focus of the preoperative consultation, whenever this is possible, to make sure the patient is operated under the best possible conditions.
However, even though predictive patient risk judgement is somehow clinical practice, a validated and widely applicable risk classification tool is missing leaving the decision of who is a risk patient often to the surgeon intuition.
To overcome the variable and surgeon-specific interpretation of patient risk factors for infection in our institution, we have recently started to define our own risk classification algorithm based on our clinical experiences and taking into account the practicability and clinical relevance of some literature-proposed factors. Its calculation is based on the following general health- as well as orthopedics/trauma-specific risk factors (see Table 1).
 |
Table 1 Differentiation of General and Specific Risk Factors Which in Combination Justify in Our Experience the Classification of a Patient as High-Risk Patient for Infection |
In case of total knee arthroplasty (TKA), a patient at high risk for PJI was defined who presents at least 2 or more of these comorbidities or risk factors, in case of total hip arthroplasty (THA) one who presents at least 3 or more (see Figure 2).
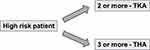 |
Figure 2 Classification of risk patients on basis of risk factors described in Table 1. |
In addition, isolated risk factors were identified which justify in our experience a direct classification as high-risk patient for infection. These were
- severe renal or cardiac insufficiency
- severe immunodeficiency (congenital, acquired or iatrogenic because of immunosuppressive medication)
- previous musculoskeletal infection
- urinary tract or rectal colonization (for hip arthroplasty)
- previous implant surgery in the same joint
- any previous revision arthroplasty
Pathogen-Specific Risk Factors
The majority of PJIs occurring within 1 year of surgery are initiated through the introduction of bacteria at the time of surgery, either via contamination of the prosthesis or the surrounding periprosthetic tissue. Once in contact with the surface of the implant, microorganisms colonize the surface of the implant and start to produce a biofilm. A significant factor in this process is the low inoculum of microorganisms needed to establish infection in the presence of the prosthetic material. Contiguous spread of infection from an adjacent site is the second mechanism by which infection can be initiated in the early postoperative time period, due to wound healing problems or persistent wound leakage.
Numerous studies have shown that gram-positive staphylococci are the most frequent bacteria causing PJI.18,19 Enterococci, Streptococci and gram-negative organisms are less common, but are also clinically relevant. Epidemiological studies from various hospitals have provided evidence that methicillin-resistant Staphylococcus aureus (MRSA) is still a major problem pathogen in PJI19 and that the prevalence of multi-drug resistant gram-negative bacteria is growing in some institutions.8
To which extent Staphylococcus aureus screening combined with preoperative decolonization adds to effective and cost-efficient PJI prophylaxis measures is still controversially discussed. The American Association of Orthopedic Surgeons (AAOS) concluded in their ortho guidelines 201520 that only limited strength evidence supports the use of universal preoperative chlorhexidine decolonization to reduce PJI after total hip arthroplasty (THA) and total knee arthroplasty (TKA). However, 85% of the participants of the PJI consensus meeting in Philadelphia agreed with the following statement in response to the question “What should be the process for MSSA and MRSA screening?”
Consensus statement: While this workgroup does NOT recommend universal screening and decolonisation of all patients undergoing joint arthroplasty, it accepts that pre-operative screening for Staphylococcus aureus (MSSA and MRSA) and decolonisation decreases the rate of SSI and the incidence of staphylococcal and non-staphylococcal infections.21
Procedure-Specific Risk Factors
Several studies are suggesting that increased surgical duration is associated with an increased risk for post-operative infections.22–24 The most likely explanation is the increased probability of contamination of the surgical site, either directly or from secondarily contaminated instruments. Based on the retrospective analysis of more than 92.000 patients undergoing TKA in the Ontario region of Canada between 2009 and 2016 Ravi et al recently found a statistically significant relation between procedure lengths and the number of deep infections.25 They observed that patients with a “long” surgical duration (≥100 min) suffered from a higher rate of PJI within a year of their surgery than patients with a “short” duration (1.1% versus 0.6%). Similar results were also obtained by Garbarino et al24 who observed higher PJI rates in cases >121 minutes duration of surgery (1.4%) compared to those <85 minutes (0.3%). The longer operation times, higher invasiveness of the procedure and the often more fragile health status of patients may also explain why infections after revision arthroplasty, even if performed for strictly aseptic reasons, are higher than in primary surgeries.
One of the most predisposing risk factors for infections following TKA or THA appears to be a previous septic etiology of an implant. For example prior PJI, although apparently cured, has been found to be related to a several-fold increased risk of deep infections following primary knee arthroplasty in the other joint. A PJI incidence of 6.1% in such a patient cohort was observed by Chalmers et al26 confirming previous results of a study in which the number of PJI cases after primary TKA or THA was 10 among 90 patients vs 0 in the matched control group without prior PJI treatment.27
Perioperative Antibiotic Prophylaxis (PAP)
Standard PAP
Perioperative antibiotic prophylaxis (PAP) is expected to prevent that contaminations turn into infections. It is clear that the probability of developing an infection is drastically increased if the initial contaminating flora in open wounds is not targeted by the prophylactic antibiotic drug. Effective PAP requires a correct timing, dosage and choice of the antibiotic. Because of their broad antimicrobial spectrum, good safety profile, low costs and ease of administration, cephalosporins of the first and second generations are recommended in many orthopedic guidelines for PAP.28,29 After analyzing a big patient pool (29.695 patients) in their institution, Wyles et al have recently reported that PJI rates were significantly higher when non-cefazolin antibiotics were used for perioperative TKA and THA prophylaxis.30 In particular, vancomycin, which is often used as an alternative option in cases of suspected patient allergy to penicillins and cephalosporins, does not appear to be as effective as cefazolin in preventing deep infections in arthroplasty.31
Most PAP guidelines encourage a “single-shot policy”, although a PAP prolongation up to 24 hours and beyond is still clinical practice in some countries. Both, registry data as well as clinical observational studies do not argue for a superiority of multiple doses in routine procedures except for long procedures and those requiring major blood transfusions.32
While a standard dose of 2 g intravenous cefazolin is usually applied for most arthroplasty patients, pharmacokinetic studies argue for an increase to a 3 g dose in obese patients weighing ≥120 kg, because of the poorer penetration and distribution of the drug in fat tissue.33 Possible PAP underdosing may also partially explain the recently observed higher rates of polymicrobial and gram-negative early PJI cases in THA procedures in an obese patient cohort compared to the non-obese group (rate of polymicrobial infections: 60.3% vs 33.3%, rate of infections with gram-negative: 12.9% vs 2.3%).34 These findings suggest that a “one size fits all” PAP strategy might not be always ideal and that possible adjustment of the standard antibiotic prophylaxis should be considered for specific patients.
Alternative and Combined Antibiotic Prophylaxis Strategies
Dual PAP
While many studies have been reinforcing the recommendation that a single shot of cephalosporins should be the choice and mode of application of PAP in arthroplasty, concerns regarding their limited coverage of resistant coagulase-negative Staphylococci and MRSA strains are emerging.35 Given the fact, that resistant bacteria in a region or hospital may vary with time and region, the European Center for Disease Control therefore advised in its technical report on PAP 2015 that a multi-disciplinary team in the hospital should regularly update the existing protocol and make periodic adjustments, if necessary, taking into consideration the local antibiotic susceptibility pattern and needs of patients at higher infection risks.36
A strategy to overcome the limitations of cephalosporins against multi-resistant Staphylococci could be the addition of a glycopeptide to the standard PAP in hospitals suffering from a high incidence of such multi-drug resistant bacteria. However, conflicting results have been reported after comparing the effectiveness of single versus dual antibiotics. While some studies reported no substantial decrease in overall postoperative infection rates with the use of a combination of vancomycin and cefazolin vs cefazolin alone, the incidence of MRSA infections appeared significantly reduced with this dual PAP regimen.37 Another study evaluated revision TKA patients who had combined cefazolin and vancomycin prophylaxis and showed a significant decline in their infection rates (3.13% vs 7.89%).38 Similarly, Tornero et al demonstrated that the addition of a high dose of teicoplanin to the standard prophylactic agent cefuroxime in response to the high MRSA burden in the hospital in Barcelona, Spain, led to a significant drop in the PJI rate from 3.51% to 1.26%.39 It was further shown that the lower number of overall infections after switching to this dual regimen was mostly due to noticeable reduction of MRSA cases. However, it should be noted the use of cefazolin and vancomycin is not without risks and may be associated with a significant increase in the cases of acute kidney failure as demonstrated by the study of Courtney et al.40
Prolongation of PAP
The theoretical benefit of a PAP extension beyond 24 hours to prevent the retrograde spread of skin flora bacteria due to slow wound healing must be balanced against the risk of more side effects which also includes the development of Clostridium difficile infections. There is little doubt that prolonged PAP should be prevented in routine arthroplasty procedures. However, in multi-morbid patients or revision arthroplasty procedures, a few studies are suggesting a substantial and clinically meaningful reduction of the PJI rate, if the duration of PAP had been extended to several days post-op. Claret et al described in a series of 341 aseptic revision TKA patients that the PJI rate was with 2.2% significantly lower in the long-prophylaxis group (prophylaxis until fifth day post-op) compared to the short-prophylaxis group in which a PJI rate of 6.9% was observed.41 Prolonged postoperative antibiotic application was the only variable associated independently with a lower rate of PJI. Likewise, Inabathula et al compared the impact of an extended antibiotic prophylaxis protocol on the number of PJI cases in two groups of high-risk patients. Those multi-morbid patients without extended antibiotic prophylaxis were 4.9 and 4.0 times more likely to develop PJI after TKA and THA, respectively, than high-risk patients with extended antibiotic prophylaxis until 7 days post-op.42 Although these results suggest possible benefits of extended antibiotic prophylaxis in high-risk patients undergoing arthroplasty, methodologic study limitations and potential drawbacks of a widespread adoption of such extended PAP protocols have soon started to be discussed after its publication.43
Combination of Systemic and Local Antibiotic Prophylaxis
Many surgeons in Europe use local antibiotics in cemented arthroplasty procedures in the form of antibiotic-loaded bone cement (ALBC) as a complementary strategy to PAP. The rationale behind this strategy is the formation of an additional antimicrobial “frontline” in the joint cavity itself. One of the major advantages of this approach is that high peak concentrations of bactericidal and concentration-dependant antibiotics, such as gentamicin, are achieved where contaminations may have occurred without exposing the patient to a major risk of systemic side effects. In fact, several clinical studies and arthroplasty registry results have provided evidence for lower revision rates if systemic and local antibiotic prophylaxis are combined.13,44,45 However, opponents of routine ALBC use in arthroplasty often point to the low strength of evidence and cite studies which did not find significant differences in the PJI rate between ALBC and plain cement.46 Interestingly, most of these negative studies have been performed in the US where ALBC use is officially only registered for the second stage of a septic revision protocol. These restrictions may have led to a clinical practice in which ALBC are more often used in PJI risk patients than in the “normal” patient. A final interpretation of these controversial results between many European countries and the US remains difficult and may also reflect the use of different bone cements per region loaded with different antibiotics and with varying antibiotic elution properties.
Our Own Experiences with a Low-Dose Gentamicin-Loaded Bone Cement
We have recently demonstrated that the implementation of routine use of the low-dose ALBC PALACOS R+G (Heraeus Medical GmbH, Wehrheim, Germany) for all cemented primary hip and knee procedures in our institution led to a reduction of PJI cases by 60–70% compared to non-ALBC.47 It is important to note that the infection rate of uncemented prostheses in the same observation periods remained unchanged, thus suggesting that the drop in infection cases might be in fact related to the switch in bone cement.
Prophylaxis with a High-Dose Dual ALBC in Risk Patients
Several in-vitro studies have demonstrated that antibiotic combinations in bone cements, such as the combination of gentamicin and clindamycin, lead to a more potent and more sustained antimicrobial growth inhibition.48 As proof of concept, this hypothesis has been recently tested in the clinical setting of a quasi-randomized controlled clinical study in 848 neck of femur fracture patients in the UK.49 It was shown that the initially high deep and superficial infection rates in this frail patient cohort were significantly lower, if hemiprostheses were cemented with the high-dose dual antibiotic-loaded cement COPAL G+C (loaded with 1 g gentamicin and 1 g clindamycin, Heraeus Medical GmbH, Germany) in the intervention group instead with the low-dose cement PALACOS R+G (loaded with 0.5 g gentamicin, Heraeus Medical GmbH, Germany) in the control group. A deep and total SSI rate of 3.5% and 5.3%, respectively, was observed in the low-dose single ALBC group compared to 1.1% and 1.7%, respectively, in the high-dose dual ALBC group. The rate of other complications in both groups was comparable. Three years later, a similar outcome in the neck of femur fracture patients was independently reproduced by the group of Savage et al,50 thus confirming that prophylaxis with high-dose dual ALBC in cemented hemiprostheses instead with conventional low-dose single ALBC might indeed lead to less surgical infections in this vulnerable patient cohort.
Our Own Experiences with the Dual High-Dose Cement COPAL G+C
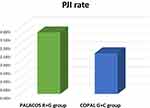
With the promising results from the trauma trials in mind, we wanted to test the hypothesis, whether the use of high-dose dual ALBC would also reduce the infection rate in patients at high-infection risks in elective surgeries. To our knowledge, this is the first report in which the effect of adjusting the local antibiotic prophylaxis to the infection risk has been studied in elective procedures and compared to the conventional standard of care. We analyzed the PJI rate in all cemented primary arthroplasty operations performed in the time period from 2015 to 2018 on a total of 2551 patients (primary arthroplasties included TKA, THA & hemiarthroplasty procedures) as a matter of the ALBC used. A total of 2368 patients (=92.8%) showing a mixed low- and high-risk profile received the low-dose single ALBC PALACOS R+G. A total of 183 patients (=7.2%) with an exclusive high-risk profile according to our above described recent risk stratification algorithm received the high-dose dual ALBC COPAL G+C Figure 3. With a minimum follow-up period of 1 year, a PJI rate of 3.7% was found in the PALACOS and a PJI rate of 2.45% in the COPAL cohort. These differences, although not statistically significant because of the mixed patient profile in the PALACOS group, would correspond to a reduction of the number of PJI cases of 34% in the COPAL group against the expectation of a higher infection rate in this throughout high-risk patient cohort. However, we cannot entirely exclude that individual surgeon technique-related factors have led to a minor bias in these results although only experienced consultants were operating randomly on patients within both groups.
Similar to this trend we also observed a statistically significant reduction of PJI cases in aseptic knee revision arthroplasty (risk reduction of 57%), if the high-dose dual cement COPAL G+C was used for the cementation of the revision prosthesis instead of the low-dose cement PALACOS R+G.51
Conclusions
Prior infection risk stratification of patients followed by adjustments of the antibiotic prophylaxis regimen in special risk situations may be one interesting option among other preoperative optimization protocols to decrease the burden of PJI. Such adjustments may relate to the systemic and/or to the local antibiotic prophylaxis option. The latter has proven effective and safe in the neck of femur fracture patients and in our elective arthroplasty procedures in patients at higher risks. Further studies are needed to test this strategy in a wider context and to weigh possible benefits against potential adverse effects and costs.
Disclosure
Christof Berberich is an employee of Heraeus Medical GmbH. The authors report no other conflicts of interest in this work.
References
1. Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4(7):482–494. doi:10.1302/2058-5241.4.180092
2. Lamagni T. Epidemiology and burden of prosthetic joint infections. J Antimicrob Chemother. 2014;69(Suppl 1):i5–10. doi:10.1093/jac/dku247
3. Kunutsor SK, Beswick AD, Peters TJ, et al. Health care needs and support for patients undergoing treatment for prosthetic joint infection following hip or knee arthroplasty: a systematic review. PLoS One. 2017;12(1):e0169068. doi:10.1371/journal.pone.0169068
4. Wang FD, Wang YP, Chen CF, Chen HP. The incidence rate, trend and microbiological aetiology of prosthetic joint infection after total knee arthroplasty: a 13 years’ experience from a tertiary medical center in Taiwan. J Microbiol Immunol Infect. 2018;51(6):717–722. doi:10.1016/j.jmii.2018.08.011
5. Lenguerrand E, Whitehouse MR, Beswick AD, Toms AD, Porter ML, Blom AW. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open. 2017;7(7):e014056. doi:10.1136/bmjopen-2016-014056
6. Pilz V, Hanstein T, Skripitz R. Projections of primary hip arthroplasty in Germany until 2040. Acta Orthop. 2018;89(3):308–313. doi:10.1080/17453674.2018.1446463
7. Robertsson O, Ranstam J, Sundberg M, W-Dahl A, Lidgren L. The Swedish knee arthroplasty register: a review. Bone Joint Res. 2014;3(7):217–222. doi:10.1302/2046-3758.37.2000289
8. Benito N, Franco M, Ribera A, et al. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect. 2016;22(8):
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726–9.e1. doi:10.1016/j.arth.2011.09.013
10. Dale H, Fenstad AM, Hallan G, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012;83(5):449–458. doi:10.3109/17453674.2012.733918
11. Badawy M, Espehaug B, Fenstad AM, et al. Patient and surgical factors affecting procedure duration and revision risk due to deep infection in primary total knee arthroplasty. BMC Musculoskelet Disord. 2017;18(1):544. doi:10.1186/s12891-017-1915-4
12. Lenguerrand E, Whitehouse MR, Beswick AD, et al. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis. 2019;19(6):589–600. doi:10.1016/S1473-3099(18)30755-2
13. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38–47. doi:10.2106/JBJS.G.01686
14. Machado S, Marta M, Rodrigues P, et al. Risk factors for infection after total knee arthroplasty and prevention strategies. Orthop Proc. 2018;97:122.
15. Alamanda VK, Springer BD. The prevention of infection: 12 modifiable risk factors. Bone Joint J. 2019;101-B:3–9. doi:10.1302/0301-620X.101B1
16. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710–1715. doi:10.1007/s11999-008-0209-4
17. Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150866. doi:10.1371/journal.pone.0150866
18. Osmon DR. Microbiology and antimicrobial challenges of prosthetic joint infection. J Am Acad Orthop Surg. 2017;25(Suppl 1):S17–S19. doi:10.5435/JAAOS-D-16-00639
19. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386–2391. doi:10.1128/AAC.06246-11
20. AAOS ortho guidelines 2015. Available from: http://www.orthoguidelines.org/guideline-detail?id=1514.
21. Lazarinis S, Lidgren L, Stefánsdóttir A, Dahl AW. Consensus document on prosthetic joint infections. Acta Orthop. 2013;84(6):507–508. doi:10.3109/17453674.2013.867399
22. Peersman G, Laskin R, Davis J, Peterson M, Richart T. Prolonged operative time correlates with increased infection rate after total knee arthroplasty. HSS J. 2006;2(1):70–72. doi:10.1007/s11420-005-0130-2
23. Cheng H, Chen B, Soleas I, et al. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect. 2017;18(6):722–735. doi:10.1089/sur.2017.089
24. Garbarino L, Gold P, Sodhi N, et al. The effect of operative time on in-hospital length of stay in revision total knee arthroplasty. Ann Transl Med. 2019;7(4):66. doi:10.21037/atm.2019.01.54
25. Ravi B, Jenkinson R, O’Heireamhoin S, et al. Surgical duration is associated with an increased risk of periprosthetic infection following total knee arthroplasty: a population-based retrospective cohort study. EClinicalMedicine. 2019;16:74–80. doi:10.1016/j.eclinm.2019.09.015
26. Chalmers BP, Weston JT, Osmon DR, Hanssen AD, Berry DJ, Abdel MP. Prior hip or knee prosthetic joint infection in another joint increases risk three-fold of prosthetic joint infection after primary total knee arthroplasty: a matched control study. Bone Joint J. 2019;101-B(7_Supple_C):91–97. doi:10.1302/0301-620X.101B7.BJJ-2018-1189.R1
27. Bedair H, Goyal N, Dietz MJ, et al. A history of treated periprosthetic joint infection increases the risk of subsequent different site infection. Clin Orthop Relat Res. 2015;473(7):2300–2304. doi:10.1007/s11999-015-4174-4
28. Prokuski L, Clyburn TA, Evans RP, Moucha CS. Prophylactic antibiotics in orthopaedic surgery. Instr Course Lect. 2011;60:545–555.
29. Siddiqi A, Forte SA, Docter S, Bryant D, Sheth NP, Chen AF. Perioperative antibiotic prophylaxis in total joint arthroplasty: a systematic review and meta-analysis. J Bone Joint Surg Am. 2019;101(9):828–842. doi:10.2106/JBJS.18.00990
30. Wyles CC, Hevesi M, Osmon DR, et al. 2019 John Charnley Award: increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: the value of allergy testing for antibiotic prophylaxis. Bone Joint J. 2019;101-B(6_Supple_B):9–15. doi:10.1302/0301-620X.101B6.BJJ-2018-1407.R1
31. Kheir MM, Tan TL, Azboy I, Tan DD, Parvizi J. Vancomycin prophylaxis for total joint arthroplasty: incorrectly dosed and has a higher rate of periprosthetic infection than cefazolin. Clin Orthop Relat Res. 2017;475(7):1767–1774. doi:10.1007/s11999-017-5302-0
32. Tan TL, Shohat N, Rondon AJ, et al. Perioperative antibiotic prophylaxis in total joint arthroplasty: a single dose is as effective as multiple doses. J Bone Joint Surg Am. 2019;101(5):429–437. doi:10.2106/JBJS.18.00336
33. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14:73–156. doi:10.1089/sur.2013.9999
34. Löwik C, Zijlstra WP, Knobben BAS, et al. Obese patients have higher rates of polymicrobial and gram-negative early periprosthetic joint infections of the hip than non-obese patients. PLoS One. 2019;14(4):e0215035. doi:10.1371/journal.pone.0215035
35. Nodzo SR, Boyle KK, Frisch NB. Nationwide organism susceptibility patterns to common preoperative prophylactic antibiotics: what are we covering? J Arthroplasty. 2019;34(7S):302–306. doi:10.1016/j.arth.2019.01.017
36. Available from: https://www.ecdc.europa.eu/en/publications-data/systematic-review-and-evidence-based-guidance-perioperative-antibiotic-prophylaxis.
37. Sewick A, Makani A, Wu C, O’Donnell J, Baldwin KD, Lee GC. Does dual antibiotic prophylaxis better prevent surgical site infections in total joint arthroplasty? Clin Orthop Relat Res. 2012;470(10):2702–2707. doi:10.1007/s11999-012-2255-1
38. Liu C, Kakis A, Nichols A, Ries MD, Vail TP, Bozic KJ. Targeted use of vancomycin as perioperative prophylaxis reduces periprosthetic joint infection in revision TKA. Clin Orthop Relat Res. 2014;472(1):227–231. doi:10.1007/s11999-013-3029-0
39. Tornero E, García-Ramiro S, Martínez-Pastor JC, et al. Prophylaxis with teicoplanin and cefuroxime reduces the rate of prosthetic joint infection after primary arthroplasty. Antimicrob Agents Chemother. 2015;59(2):831–837. doi:10.1128/AAC.03949-14
40. Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis Is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res. 2015;473(7):2197–2203. doi:10.1007/s11999-014-4062-3
41. Claret G, Tornero E, Martínez-Pastor JC, et al. A prolonged post-operative antibiotic regimen reduced the rate of prosthetic joint infection after aseptic revision knee arthroplasty. Surg Infect. 2015;16(6):775–780. doi:10.1089/sur.2015.044
42. Inabathula A, Dilley JE, Ziemba-Davis M, et al. Extended oral antibiotic prophylaxis in high-risk patients substantially reduces primary total hip and knee arthroplasty 90-day infection rate. J Bone Joint Surg Am. 2018;100(24):2103–2109. doi:10.2106/JBJS.17.01485
43. DeFrancesco CJ, Fu MC, Kahlenberg CA, Miller AO, Bostrom MP. Extended antibiotic prophylaxis may be linked to lower peri-prosthetic joint infection rates in high-risk patients: an evidence-based review. HSS J. 2019;15(3):297–301. doi:10.1007/s11420-019-09698-8
44. Espehaug B, Furnes O, Havelin LI, Engesaeter LB, Vollset SE. The type of cement and failure of total hip replacements. J Bone Joint Surg Br. 2002;84(6):832–838. doi:10.1302/0301-620X.84B6.0840832
45. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335–341. doi:10.1080/17453670710015229
46. King JD, Hamilton DH, Jacobs CA, Duncan ST. The hidden cost of commercial antibiotic-loaded bone cement: a systematic review of clinical results and cost implications following total knee arthroplasty. J Arthroplasty. 2018;33:3789. doi:10.1016/j.arth.2018.08.009
47. Sanz-Ruiz P, Matas-Diez JA, Sanchez-Somolinos M, Villanueva-Martinez M, Vaquero-Martin J. Is the commercial antibiotic-loaded bone cement useful in prophylaxis and cost saving after knee and hip joint arthroplasty? The transatlantic paradox. J Arthroplasty. 2017;32(4):1095–1099. doi:10.1016/j.arth.2016.11.012
48. Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466(6):1492–1498. doi:10.1007/s11999-008-0203-x
49. Sprowson AP, Jensen C, Chambers S, et al. The use of high-dose dual-impregnated antibiotic-laden cement with hemiarthroplasty for the treatment of a fracture of the hip: the fractured hip infection trial. Bone Joint J. 2016;98-B(11):1534–1541. doi:10.1302/0301-620X.98B11.34693
50. Savage P, McCormick M, Al-Dadah O. Arthroplasty infection rates in fractured neck of femur: single vs dual antibiotic cement. Ann R Coll Surg Engl. 2019;101:514–518. doi:10.1308/rcsann.2019.0054
51. Sanz-Ruiz P, Matas-Diez JA, Sanchez-Somolinos M, Vaquero-Martín J. Is dual antibiotic loaded bone cement more effective and cost-efficient than a single antibiotic loaded bone cement to reduce prosthetic joint infections in patients at higher infection risks? - A retrospective study in aseptic revision knee arthroplasty. J Arthroplasty. 2020. doi:10.1016/j.arth.2020.06.045
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.