Back to Journals » Clinical Optometry » Volume 9
Infantile nystagmus: an optometrist’s perspective
Authors Zahidi AA, Woodhouse JM, Erichsen JT, Dunn MJ
Received 26 May 2017
Accepted for publication 14 July 2017
Published 25 September 2017 Volume 2017:9 Pages 123—131
DOI https://doi.org/10.2147/OPTO.S126214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Mr Simon Berry
Asma AA Zahidi, J Margaret Woodhouse, Jonathan T Erichsen, Matt J Dunn
Research Unit for Nystagmus, School of Optometry and Vision Sciences, Cardiff University, Cardiff, UK
Abstract: Infantile nystagmus (IN), previously known as congenital nystagmus, is an involuntary to-and-fro movement of the eyes that persists throughout life. IN is one of three types of early-onset nystagmus that begin in infancy, alongside fusion maldevelopment nystagmus syndrome and spasmus nutans syndrome. Optometrists may also encounter patients with acquired nystagmus. The features of IN overlap largely with those of fusion maldevelopment nystagmus syndrome, spasmus nutans syndrome, and acquired nystagmus, yet the management for each subtype is different. Therefore, the optometrist’s role is to accurately discern IN from other forms of nystagmus and to manage accordingly. As IN is a lifelong condition, its presence not only affects the visual function of the individual but also their quality of life, both socially and psychologically. In this report, we focus on the approaches that involve optometrists in the investigation and management of patients with IN. Management includes the prescription of optical treatments, low-vision rehabilitation, and other interventions such as encouraging the use of the null zone and referral to support groups. Other treatments available via ophthalmologists are also briefly discussed in the article.
Keywords: eye movements, visual acuity, reading performance, low vision, null zone, optometric investigation
Introduction
Infantile nystagmus (IN), a constant involuntary to-and-fro movement of the eyes that persists throughout life, is one of three types of early-onset nystagmus that begin in infancy. Fusion maldevelopment nystagmus syndrome (FMNS), which was formerly known as latent nystagmus, and spasmus nutans syndrome (SNS) are the other two types. Previously, various terms have been used to refer to IN, the most common being congenital nystagmus. Despite this label, the condition is rarely present at birth. A study by Reinecke et al1 reported the development of nystagmus in the first 2 weeks of life in only three of 35 infants examined. Since IN typically develops within the first few months after birth, the term congenital has been replaced with infantile.2 The occurrence of IN in the general population is estimated to be 0.14%.3 This included nystagmus associated with visually impaired conditions and nystagmus with no known condition (idiopathic). The prevalence of any form of nystagmus (ie, not just IN) is estimated at 0.17% in people under the age of 18. In contrast, the prevalence is much higher in the adult population (0.27%).3 This increase is accounted for by cases of acquired nystagmus, which generally (but not always) occurs later in life. IN can occur spontaneously, or it may be inherited. Hereditary IN may be X-linked, recessive, or dominant.4 A number of genetic studies have discovered mutations in the FRMD7 gene in individuals with X-linked idiopathic IN.5 These mutations account for approximately 20%–57% of X-linked cases of idiopathic IN and 3.6%–7% of isolated cases.6,7
Clinical characteristics of IN
In IN, the eyes oscillate constantly and predominantly in the horizontal axis, although vertical and/or torsional movement may be present as a secondary component.8 The nystagmus occurs in both eyes and is usually conjugate. Thus, the amplitude of IN is similar in both eyes, ranging between 0.3° and 15.7°, with an average frequency of 2–3 Hz.8,9 A nystagmus cycle consists of two phases: an initiating slow phase in which the eyes slowly move away from the fixation point, and a corrective phase where the eye moves back toward the fixation point (Figure 1). This corrective phase can be either a slow or a fast eye movement. A pendular pattern is seen when both the initiating and corrective phases are slow movements, and a jerk pattern is seen when the corrective phase is a saccade. These patterns differ between individuals with IN, and also within the individuals themselves at different gaze angles or times.10
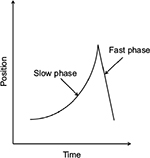  | Figure 1 Waveform showing the initiation (slow) phase and correction (fast) phase of a jerk nystagmus cycle. |
The IN waveform
The nystagmus waveform represents the position of the eye over time, and it is obtained by performing eye movement recordings. It is described in terms of its amplitude, frequency, and the overall pattern or shape of the oscillation. Nystagmus intensity is calculated by multiplying the nystagmus amplitude by the frequency, thus representing the average velocity of the eye movements.11 The intensity of an individual’s nystagmus is not typically constant and can be influenced by a number of factors. Among these factors is the direction of gaze. The intensity of IN is at a minimum at certain gaze angle(s) known as the null zone. The null zone is within 10° of the primary position (straight ahead) in 73% of individuals with IN.8 If the null zone is not in the primary position, patients may adopt an abnormal head posture (AHP) to place the eyes in this position. In many individuals (44%), nystagmus intensity also reduces during convergence.8,12,13 Furthermore, the state of attention and fatigue can affect nystagmus intensity.14 Stress, for example, has been found to increase intensity.15,16 These factors should be taken into consideration during an eye examination, as they may affect the outcome of clinical tests.
A total of 12 waveform patterns have been identified in IN.4 These 12 waveforms can be broadly categorized into pendular and jerk waveforms (Figure 2). The velocity of a pendular eye movement is equal in both directions. The eyes may change direction smoothly, producing a sinusoidal pendular waveform, or they may change direction abruptly, producing a triangular waveform.17 Jerk waveforms, however, consist of a slow phase in one direction followed by a corrective saccade, known as a fast phase. It is further described by the direction of the fast phase, whether it is left, right, up, or down. A specific characteristic of the jerk IN waveform that distinguishes it from other types of nystagmus is the accelerating slow phase that follows the foveation period; a period when the eye movement is relatively slower for a short period of time when the object of regard coincides with the fovea.17,18 An accelerating slow phase is unlikely to be visible to an examiner, so this can only be confirmed by using a high-speed eye tracking device.
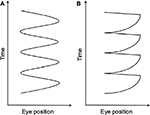  | Figure 2 (A) Pendular and (B) jerk waveform patterns in IN. Abbreviation: IN, infantile nystagmus. |
The evolution of the IN waveform during visual development has been investigated by a number of researchers. One of the earliest studies – in three infants – reported that IN started with large triangular waveforms, developing into pendular and, subsequently, jerk waveform nystagmus by 7 months to 1.5 years.1 However, a later study observed square-wave jerks (a form of saccadic intrusion) at 7 weeks of age, which then developed into a smaller amplitude (4°–20°) pendular nystagmus at 8 weeks.20 Over the next few weeks, the amplitude first increased (to approximately 30°) and then began to decrease to 4°–10° until 7.5 months. A more recent study was conducted on a group of 20 infants aged between 4 and 42 months in whose nystagmus a combination of two components was observed, ie, asymmetric pendular and pseudocycloid waveforms, which was previously undocumented.21 In addition, the researchers observed a decrease in the amplitude of both components of waveforms from birth until 1.5 years of age. Foveation duration also increased up to the age of 1.5–2 years, and thereafter remained relatively stable.
Although the exact type of IN waveform is not crucial for the diagnosis of IN itself, knowledge about the different waveforms is helpful in estimating the visual prognosis. For instance, pendular waveforms are more likely to be associated with an identifiable visual system pathology.22 Therefore, children with a pendular waveform that persists longer are suspected to have more poorly developed vision, as compared to children who transition to a jerk waveform earlier.23 Although access to eye movement recording equipment is limited in optometric practices, it is usually possible to determine whether a waveform is pendular or jerk without an eye tracker, just by direct observation. Jerk nystagmus, for instance, can be determined simply by observing the beat direction (ie, fast phase).
Visual acuity (VA)
VA varies widely in patients with IN, depending on the associated visual condition (if any). The mean VA of individuals with idiopathic IN (IN with no associated visual pathology) is 0.35 logMAR, which is better than that of individuals with associated visual system pathology (0.67 logMAR in patients with albinism, and 0.55 logMAR in the group with any other ocular pathology).8 The poor VA seen in patients with IN could be due to the underlying pathology, motion-blur-induced stimulus deprivation amblyopia, or a combination of both.24
Refractive error
Individuals with IN are more likely to have high refractive error than the general population. The range of refractive errors found in patients with IN is very broad. Some studies find that hyperopia is more common in IN,25 regardless of the VA,26 while others report that the trend is more myopic.27 The prevalence of corneal astigmatism (especially with-the-rule astigmatism) is also remarkably high in individuals with IN.28 The average amount of astigmatism in these individuals is 1.85DC; 57% of people with IN have astigmatism higher than 2.00DC.8 Astigmatism tends to increase with age29 and is suspected to be caused by the interaction between the cornea and the eyelids during the constant oscillations.28,30 Although full correction is given, patients with IN are better able to distinguish horizontal stimuli than vertical ones,24,31–33 which may be attributable to the presence of meridional amblyopia.33,34
Reading performance
Nystagmus intensity decreases when converging to view near objects in around 44% of individuals with IN.8,12 However, there is no significant improvement in near VA at this time, despite significant dampening of the nystagmus.11,9 Barot et al35 studied the reading performance of 71 individuals with IN and 20 controls, measuring reading acuity, near VA, and reading speed. Reading performance was 18.8% slower in participants with IN associated with albinism and 14.7% slower in idiopaths. However, near-normal reading speed can be accomplished by using a font size up to 0.6 logMAR larger than the near VA.35
Reversed optokinetic nystagmus (OKN) response
Another unique characteristic of IN is reversal of the OKN response. In a typical person without nystagmus, the fast phase of OKN beats in the opposite direction to the stimulus. However, some individuals with IN show a reversed response, ie, the fast phase of the OKN beats in the same direction as the stimulus.36,22
Oscillopsia
Despite the constant oscillation of the eyes that produce retinal image motion, people with nystagmus generally perceive the world as stable.37 Therefore, they rarely complain of oscillopsia38 (perception of the world swinging back and forth).39 In contrast, oscillopsia is commonly experienced by patients with acquired nystagmus.22 However, reports of oscillopsia are not necessarily indicative of acquired nystagmus, but should be an indication for further investigation. Individuals with IN can sometimes experience oscillopsia intermittently in situations such as when they are tired, excited, stressed, or concentrating.8
Head nodding
Another occasional feature of IN is head nodding.8 This head oscillation is independent of the oscillation occurring in the eyes, often being in a different direction or with a different phase. Head nodding clearly does not compensate for the nystagmus eye movements, and its etiology is unclear. It can be suppressed by the patient voluntarily, but may recur if the patient’s attention is distracted from doing so.40 This feature of IN is also present in SNS, but in SNS, the head oscillation is in synchrony with the eyes. Key distinguishing features of SNS and IN can be seen in eye movement recordings, as the waveform of SNS is asymmetrical between the two eyes, pendular, high in frequency, and low in amplitude.2,22
Associated conditions vs idiopathic IN
IN can present with anterior visual pathway abnormalities, or it can present without any detectable abnormalities at all (ie, it can be idiopathic). Anterior visual pathway abnormalities occur in 38%–91% of cases of IN.3,8,41,42 This includes, but is not limited to, congenital cataracts, retinal dystrophies, and degenerations such as Leber’s congenital amaurosis, optic nerve disorders such as optic nerve hypoplasia and optic atrophy, foveal hypoplasia, aniridia, albinism, achromatopsia, and achiasma. Only 9% of cases of IN are reported as idiopathic,41,42 although Lorenz and Gampi43 and Abadi and Bjerre8 reported a higher prevalence. However, since the idiopathic diagnosis can only be reached after all possible associated visual conditions have been excluded, the prevalence of IN depends to some extent upon the persistence of the investigator and the exhaustiveness of clinical testing. Children with IN associated with sensory abnormalities tend to have poorer vision than those who are idiopathic. Therefore, it is important to distinguish the two so that appropriate management can be given.
Optometric investigation of IN
Although unlikely, an optometrist may come across a patient with nystagmus (whether a child or an adult) who has not been seen by the hospital eye services. Referral of these cases is essential so that an underlying cause can be identified, if possible. The optometrist must ascertain the appropriate speed of referral by deciding, following a detailed discussion of history and symptoms, whether the nystagmus is likely to be infantile or acquired – the latter requiring emergency referral. Often, the history can be very informative in making this determination. The difficulty in making such a differential diagnosis by signs is due to the fact that the features of IN overlap largely with those of acquired nystagmus, FMNS, and SNS. However, some key features are distinguishable. For example, features of acquired nystagmus include a pronounced vertical component, complaints of oscillopsia, and saccadic oscillations without apparent slow phases (technically, saccadic oscillations are not a form of nystagmus, but these conditions are often misdiagnosed as such and require onward referral).22 Note that asymmetrical movements between the two eyes raises the likelihood of the nystagmus being acquired, although this feature may not be seen with the naked eye. IN is not a progressive condition, and changes in nystagmus are not expected in adulthood. Therefore, any suspected changes in nystagmus in adults should always be referred to the hospital eye service as they could represent (however unlikely) the development of comorbid acquired nystagmus.
On the other hand, FMNS is often associated with infantile esotropia and amblyopia (which disrupts binocularity).44 In FMNS, nystagmus might not be observable under binocular viewing (without eye movement recording) but becomes apparent when one eye is occluded. The nystagmus fast phase will be seen beating toward the uncovered eye, while remaining conjugate. FMNS respects Alexander’s Law, ie, that the nystagmus gets more intense in abduction. This means that if the left eye is viewing, the nystagmus is more intense in leftgaze, and vice versa. Moreover, in IN, an increase in the intensity can sometimes be seen when one eye is covered, indicating the presence of a latent component. Therefore, the cover test should be performed to determine the presence of a latent component or FMNS.
Key features of SNS include a low amplitude, high frequency (often >10 Hz), and asymmetrical pendular nystagmus that may be conjugate or disconjugate. In addition, children with SNS usually present with AHP and head nodding.2,22 Also, oscillations of the eyes often reduce to subclinical levels after a few years,45,46 giving the impression that the nystagmus has disappeared.
Performing tests on patients with nystagmus can be a clinical challenge requiring good skills. For example, tests that require the eyes to be still – such as noncontact tonometry – may be difficult to undertake. However, the alternative of using a contact tonometer has a higher risk of causing corneal abrasion. The clinician will need to use their individual judgment in each situation depending on the patient and test being performed. Consider using the patient’s null zone to dampen nystagmus while carrying out tests such as perimetry. As mentioned earlier, stress can increase nystagmus intensity, so the practitioner should do everything possible to minimize the patient’s anxiety and discomfort.
Nystagmus intensity increases with occlusion in patients with a latent component, which may produce an artefactual reduction in VA. To avoid this increase in intensity, fogging should be used to remove fixation of the untested eye, using convex lenses between +4.00DS and +10.00DS.47 Studies have shown that patients with IN take longer to direct their gaze toward a fixated target and report slowness to see.48,49 Therefore, giving patients more time to respond when reading the acuity chart would be beneficial to both the patient and practitioner.
Although performing refraction on a patient with nystagmus may seem challenging, an accurate refraction can still be obtained. The use of trial frames and wide-aperture trial lenses (as opposed to a phoropter) are advantageous, as they allow the patient to move their head freely toward – if not into – their null zone.
Slit-lamp examination and funduscopy are essential to rule out conditions commonly associated with IN. The practitioner should look for structural abnormalities such as iris transillumination (albinism) and congenital cataracts. During funduscopy, attention should be paid to abnormalities in the optic disc (optic nerve hypoplasia), fovea (foveal hypoplasia in albinism or aniridia), and fundus pigmentation. Optical coherence tomography should be used to assess for foveal hypoplasia. Given that idiopathic IN is a diagnosis by exclusion, the hospital eye service can be expected to perform electroretinography and visual evoked potentials to assess for disorders such as congenital stationary night-blindness and cone dysfunction, which can be difficult to detect with funduscopy alone.
Management by the optometrist
In this paper, we focus on the approaches that involve optometrists in the management of individuals with IN. This includes the prescription of optical treatments, low-vision rehabilitation, and other interventions such as use of the null zone and support groups.
Optical prescription
As might be expected, correction of refractive error has been shown to improve the VA of individuals with IN.50,51 Correction of even the smallest significant amount of refractive error can result in subjective improvements in vision. Therefore, the management should begin with correction of refractive errors, usually with spectacles. However, the use of spectacles may not be ideal in patients with an AHP caused by an eccentric null zone, as it may require them to use an eccentric point of the lens. This will induce prismatic and peripheral lens effect aberrations, leading to blurred vision and perhaps asthenopia. In such cases, contact lenses may be prescribed.
The use of contact lenses to correct refractive error has been reported to improve VA by at least one line when compared to the use of spectacles.52,53 The presence of contact lenses on the eyes appears to dampen nystagmus intensity, an effect that does not occur when the eye is anesthetized.54 This suggests that correction of refractive error may not be the primary mechanism involved, and that the sensory feedback from the contact lenses on the cornea or lids might have a moderating impact on the nystagmus. The presence of high with-the-rule astigmatism in patients with IN leads to a higher sensitivity to rotational instability of the contact lens, resulting in reduced VA.55 Therefore, special care must be taken when fitting contact lenses to these patients. The use of rigid gas-permeable lenses may provide a more stable fit compared to soft lenses in these cases.55
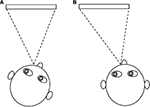
Prisms may be used to reduce AHP by shifting the eyes into the null position while the head is in the primary position. As an example, base right prisms can be used to shift the eyes into left gaze for patients with a leftward null zone, allowing the head to remain straight (Figure 3). Be aware that eccentric null positions will require high powered prisms, which may be heavy. An alternative is to use Fresnel stick-on prisms, although neither option may be cosmetically acceptable to the patient. Patients with IN who have a null zone in the convergent position may benefit from wearing base out spectacle prisms that will induce convergence and thus dampen nystagmus intensity when viewing distant targets.56 This can be achieved by prescribing 7Δ base out in front of both eyes, with –1.00DS to compensate for the accommodation that accompanies the convergence.57,40 In rare cases, some nystagmats may present with a divergence null. In such cases, base-in prisms would be beneficial.58
Low-vision rehabilitation
As discussed above, the VA of individuals with IN is usually reduced. Although some may have 6/6 or better vision, most are visually impaired, affecting their ability to perform daily tasks. For this group of individuals, low-vision rehabilitation is beneficial. This includes increasing the font size of texts together with the use of optical or electronic magnification devices. The use of devices such as tablets is ideal, as these can provide a wide range of magnifications. Additionally, other adaptive strategies during viewing (eg, use of the null zone) can also be trained.
Use of the null zone
Many of the interventions available for IN aim to utilize the null zone, which has been shown to improve VA.59 Nystagmats sometimes adopt an AHP to achieve the required gaze position, but long-term use of such head postures may lead to a restriction of neck movement.60 Besides using prisms, adjustment to the surroundings, such as changing the seating position during viewing, can be made to place the eyes into the null zone while keeping the head straight. Such adjustments may improve the patient’s comfort for long periods of viewing. Figure 4 illustrates an example of environmental changes that can be made in a classroom for a child with a null zone in left gaze.
Support groups
IN is a lifelong condition that affects not only visual function but also the quality of life of patients and those who are close to them.61,62 A recent study by McLean et al61 investigated the impact of nystagmus on the daily life of patients older than 16 years. The authors found that the physical appearance of nystagmus causes individuals to suffer from low self-esteem. The constant oscillation of the eyes and AHP made participants feel that they stand out and/or do not fit in with their peers. This can lead to social withdrawal. An earlier study by Pilling et al62 on the social and visual function in nystagmus discovered that children with nystagmus have poorer social function compared to adults.
Besides confidence issues, many individuals with nystagmus feel frustrated with the lack of understanding from the general public regarding their condition. Many also report feeling abandoned by the medical community. This can lead to feelings of hopelessness and fear of failing.61 The findings from these studies demonstrate the importance of raising awareness of nystagmus and the need for support groups for patients. Therefore, it is important for patients to get the correct information and support regarding their condition. In the UK, patients with any form of nystagmus can be referred to Nystagmus Network (http://nystagmusnetwork.org), a charity and support group that provides information and support for family members and children who are growing up with nystagmus.63 Child patients should always be referred to the Local Educational Authority’s VI support service.
Treatments for IN
A number of treatments by ophthalmologists are available for nystagmus depending on the type and presenting symptoms. These treatments do not completely cure the nystagmus, but rather modify the waveform and/or reduce AHP. A review by Thurtell and Leigh64 discusses the various therapies available for different types of nystagmus, including both surgical and pharmaceutical options. As with optical treatments, the aims are to improve the VA, correct any AHP, and treat any strabismus.47 Some treatments also aim to reduce nystagmus intensity.47,64
Surgical treatment
Several surgical procedures have been reported to improve nystagmus in patients with IN. Surgery is indicated when there is a null point at eccentric gaze causing a significant AHP, presence of strabismus, or to improve VA through the use of convergence.65,66 One of the earliest surgical procedures to be developed involved recession and resection of the extraocular muscles to move the null zone into the primary position of gaze (Kestenbaum surgery).67 Artificial divergence surgery is another method involving recession of both medial rectus muscles in patients with nystagmus that dampens on convergence.68 A combination of this surgery with the Kestenbaum technique has been shown to produce a better visual outcome compared to performing each procedure on its own.69,70 Following the success of the Kestenbaum surgery in improving VA in patients with IN, it was hypothesized that similar outcomes would be produced through a combined procedure of tenotomy (detachment of the muscles) and reattachment at the same site of the muscle origin.71 Subjective improvement in VA has been reported. However, there are only limited reports of improvement in clinically measured VA.71–74
Pharmacological treatment
Various drugs have been reported to reduce nystagmus intensity in IN.75 The only ones to have gone through a randomized controlled trial are memantine and gabapentin. The improvement in vision seen with the prescription of memantine was 0.15 logMAR compared to placebo (0.04 logMAR). Slightly less improvement in VA was seen with gabapentin (0.09 logMAR).75–77
Cannabis and baclofen have also shown potential for improving VA in IN, but these medications have not undergone rigorous testing. The effect of smoking cannabis was shown through eye movement recordings and improved VA in one case study of a 19-year-old patient with nystagmus.78 Baclofen is known to reduce nystagmus amplitude and AHP and increase VA, and is often used in patients with periodic alternating nystagmus, a form of IN that reverses direction.79 The reader is directed to recent review articles for a full discussion of other pharmacological treatments of IN.80–82
Conclusion
The principal aim of an optometrist’s investigation of a patient with nystagmus should be to distinguish the characteristics that differentiate IN from the various other forms of the condition. This can be difficult, as many of the features overlap in the various subtypes. Correct diagnosis is crucial however, as proper management may at the least improve quality of life, and a referral might even save a life. Management of IN does not involve a cure, but rather minimization of the intensity, thereby improving the physical appearance, and potentially improving visual function. Management of IN depends on understanding and treating the associated visual system pathology (if any) and maximizing use of the null zone. Small changes in clinical measurements may impact the patient’s visual function significantly. Therefore, it is advantageous for optometrists to have adequate knowledge of the therapies available.
The potential impact of this lifelong condition on the visual function and psychological well-being of those who have it warrants a thorough clinical examination and management. It is well worth providing the patient with a good understanding of their own condition through additional care and attention.
Disclosure
The authors report no conflicts of interest in this work.
References
Reinecke RD, Guo S, Goldstein HP. Waveform evolution in infantile nystagmus: An electro-oculo-graphic study of 35 cases. Binocul Vis. 1988;3(4):191–202. | ||
Avallone JM, Bedell HE, Birch EE, et al. A classification of eye movement abnormalities and strabismus. 2001;2010 (November 18, 2010):Report of a National Eye Institute Sponsored Works. Available from http://www.nei.nih.gov/news/statements/cemas.pdf. | ||
Sarvananthan N, Surendran M, Roberts EO, et al. The prevalence of nystagmus: The Leicestershire Nystagmus Survey. Investig Ophthalmol Vis Sci. 2009;50(11):5201–5206. | ||
Hertle RW, Dell’Osso LF. Nystagmus in Infancy and Childhood. New York, NY: Oxford University Press; 2013. | ||
Self J, Lotery A. A review of the molecular genetics of congenital idiopathic nystagmus (CIN). Ophthalmic Genet. 2007;28(4):187–191. | ||
Self JE, Shawkat F, Crispin MT, et al. Allelic variation of the FRMD7 gene in congenital idiopathic nystagmus. Arch Ophthalmol. 2007; 125(9):1255–1263. | ||
Thomas S, Proudlock FA, Sarvananthan N, et al. Phenotypical characteristics of idiopathic infantile nystagmus with and without mutations in FRMD7. Brain. 2008;131(Pt 5):1259–1267. | ||
Abadi RV, Bjerre A. Motor and sensory characteristics of infantile nystagmus. Br J Ophthalmol. 2002;86(10):1152–1160. | ||
Hertle RW, Maldanado VK, Maybodi M, Yang D. Clinical and ocular motor analysis of the infantile nystagmus syndrome in the first 6 months of life. Br J Ophthalmol. 2002;86(6):670–675. | ||
Dell’Osso LF, Daroff R. Congenital nystagmus waveforms and foveation strategy. Doc Ophthalmol. 1975;39(1):155–182. | ||
Hanson KS, Bedell HE, White JM, Ukwade MT. Distance and near visual acuity in infantile nystagmus. Optom Vis Sci. 2006;83(11):823–829. | ||
Dickinson CM. The elucidation and use of the effect of near fixation in congenital nystagmus. Ophthalmic Physiol Opt. 1986;6(3):303–311. | ||
Gradstein L, Goldstein HP, Wizov SS, Hayashi T, Reinecke RD. Relationships among visual acuity demands, convergence, and nystagmus in patients with manifest/latent nystagmus. J AAPOS. 1998;2(4):218–229. | ||
Abadi RV, Dickinson CM. Waveform characteristics in congenital nystagmus. Doc Ophthalmol. 1986;64(2):153–167. | ||
Cham KM, Anderson AJ, Abel LA. Task-induced stress and motivation decrease foveation-period durations in infantile nystagmus syndrome. Investig Ophthalmol Vis Sci. 2008;49(7):2977–2984. | ||
Jones PH, Harris CM, Woodhouse JM, Margrain TH, Ennis FA, Erichsen JT. Stress and visual function in infantile nystagmus syndrome. Investig Ophthalmol Vis Sci. 2013;54(13):7943–7951. | ||
Scheiman M, Wick B. Binocular Vision. 3rd ed. Philadelphia, PA: Lippincot William & Wilkins; 2008. | ||
Felius J, Fu VLN, Birch EE, Hertle RW, Jost RM, Subramanian V. Quantifying nystagmus in infants and young children: relation between foveation and visual acuity deficit. Investig Ophthalmol Vis Sci. 2011;52(12):8724–8731. | ||
Dunn MJ. Clinical assessment of nystagmus. Optom Today. 2016;56(7):80–85. | ||
Gottlob I. Infantile nystagmus: development documented by eye movement recordings. Investig Ophthalmol Vis Sci. 1997;38(3):767–773. | ||
Theodorou M, Clement R, Taylor D, Moore A. The development of infantile nystagmus. Br J Ophthalmol. 2015;99(5):691–695. | ||
Leigh RJ, Zee DS. The Neurology of Eye Movements. 5th ed. New York, NY: Oxford University Press; 2015. | ||
Felius J, Muhanna ZA. Visual deprivation and foveation characteristics both underlie visual acuity deficits in idiopathic infantile nystagmus. Investig Ophthalmol Vis Sci. 2013;54(5):3520–3525. | ||
Dunn MJ, Margrain TH, Woodhouse JM, Ennis F, Harris CM, Erichsen JT. Grating visual acuity in infantile nystagmus in the absence of image motion. Invest Ophthalmol Vis Sci. 2014;55(4):2682–2686. | ||
Healey N, McLoone E, Mahon G, Jackson AJ, Saunders KJ, McClelland JF. Investigating the relationship between foveal morphology and refractive error in a population with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2013;54(4):2934–2939. | ||
Healey N, McClelland JF, Saunders KJ, Jackson AJ. Longitudinal study of spherical refractive error in infantile nystagmus syndrome. Ophthalmic {&} Physiol Opt J Br Coll Ophthalmic Opt. 2014;34(3):369–375. | ||
Sampath V, Bedell HE. Distribution of refractive errors in albinos and persons with idiopathic congenital nystagmus. Optom Vis Sci. 2002;79(5):292–299. | ||
Wang J, Wyatt LM, Felius J, et al. Onset and progression of with-the-rule astigmatism in children with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2010;51(1):594–601. | ||
Fresina M, Benedetti C, Marinelli F, Versura P, Campos EC. Astigmatism in patients with idiopathic congenital nystagmus. Graefes Arch Clin Exp Ophthalmol. 2013;251(6):1635–1639. | ||
Dickinson CM, Abadi RV. Corneal topography of humans with congenital nystagmus. Ophthalmic Physiol Opt. 1984;4(1):3–13. | ||
Abadi RV, Pascal E. Visual resolution limits in human albinism. Vis Res. 1991;31(7–8):1445–1447. | ||
Loshin DS, Browning RA. Contrast sensitivity in albinotic patients. Am J Optom Physiol Opt. 1983;60(3):158–166. Available from http://www.ncbi.nlm.nih.gov/pubmed/6846493. | ||
Bedell HE, Loshin DS. Interrelations between measures of visual acuity and parameters of eye movement in congenital nystagmus. Invest Ophthalmol Vis Sci. 1991;32(2):416–421. | ||
Harvey EM. Development and treatment of astigmatism-related amblyopia. Optom Vis Sci. 2009;86(6):634–639. | ||
Barot N, McLean RJ, Gottlob I, Proudlock FA. Reading performance in infantile nystagmus. Ophthalmology. 2013;120(6):1232–1238. | ||
Wong AMF. Eye Movement Disorders. New York, NY: Oxford University Press; 2008. | ||
Bedell HE. Perception of a clear and stable visual world with congenital nystagmus. Optom Vis Sci. 2000;77(11):573–581. | ||
Lee AG, Brazis PW. Localizing forms of nystagmus: symptoms, diagnosis, and treatment. Curr Neurol Neurosci Rep. 2006;6(5):414–420. | ||
Brickner RM. Oscillopsia - A new symptom commonly occurring in multiple sclerosis. Arch Neurol Psych. 1936;36(3):586–589. | ||
Khanna S, Dell’Osso LF. The diagnosis and treatment of infantile nystagmus syndrome (INS). ScientificWorldJournal. 2006;6:1385–1397. | ||
Gelbart SS, Hoyt CS. Congenital nystagmus: A clinical perspective in infancy. Graefe’s Arch Clin Exp Ophthalmol. 1988;226(2):178–180. | ||
Weiss AH, Biersdorf WR. Visual sensory disorders in congenital nystagmus. Ophthalmology. 1989;96(4):517–523. | ||
Lorenz B, Gampe E. Analysis of 180 patients with sensory defect nystagmus (SDN) and congenital idiopathic nystagmus (CIN). Klin Monatsbl Augenheilkd. 2001;218:3–12. | ||
Dell’Osso LF, Schmidt D, Daroff RB. Latent, manifest latent, and congenital nystagmus. Arch Ophthalmol. 1979;97(10):1877–1885. Available from http://www.ncbi.nlm.nih.gov/pubmed/485910. | ||
Weissman BM, Dell’Osso LF, Abel LA, Leigh RJ. Spasmus nutans. A quantitative prospective study. Arch Ophthalmol. 1987;105(4):525–528. | ||
Gottlob I, Wizov SS, Reinecke RD. Spasmus nutans. A long-term follow-up. Invest Ophthalmol Vis Sci. 1995;36(13):2768–2771. | ||
Ansons AM, Davis H, Mein J. Diagnosis and management of ocular motility disorders. 3rd ed. Oxford: Blackwell Science Ltd; 2001. | ||
Wang ZI, Dell’Osso LF. Being “slow to see” is a dynamic visual function consequence of infantile nystagmus syndrome: model predictions and patient data identify stimulus timing as its cause. Vis Res. 2007;47(11):1550–1560. | ||
Dunn MJ, Margrain TH, Woodhouse JM, Erichsen JT. Visual processing in infantile nystagmus is not slow. Invest Ophthalmol Vis Sci. 2015;56(9):5094–5101. | ||
Hertle RW. Examination and refractive management of patients with nystagmus. Surv Ophthalmol. 2000;45(3):215–222. | ||
Anderson J, Lavoie J, Merrill K, King RA, Summers CG. Efficacy of spectacles in persons with albinism. J AAPOS. 2004;8(6):515–520. | ||
Allen ED, Davies PD. Role of contact lenses in the management of congenital nystagmus. Br J Ophthalmol. 1983;67(12):834–836. | ||
Biousse V, Tusa RJ, Russell B, et al. The use of contact lenses to treat visually symptomatic congenital nystagmus. J Neurol Neurosurg Psychiatry. 2004;75(2):314–316. | ||
Dell’Osso LF, Traccis S. Contact-lenses and congenital nystagmus. Clin Vis Sci. 1988;3(3):229–232. | ||
Jayaramachandran P, Proudlock FA, Odedra N, Gottlob I, McLean RJ. A randomized controlled trial comparing soft contact lens and rigid gas-permeable lens wearing in infantile nystagmus. Ophthalmology. 2014; 121(9):1827–1836. | ||
Serra A, Dell’Osso LF, Jacobs JB, Burnstine RA. Combined gaze-angle and vergence variation in infantile nystagmus: two therapies that improve the high-visual-acuity field and methods to measure it. Investig Ophthalmol Vis Sci. 2006;47(6):2451–2460. | ||
Dell’Osso LF. Improving visual acuity in congenital nystagmus. Neuro-ophthalmology Symp Univ Miami Bascom Palmer Eye Inst. 1973;(v. 7):98–106. Available from http://books.google.co.uk/books?id=xM4EAQAAIAAJ. | ||
Stahl JS, Plant GT, Leigh RJ. Medical treatment of nystagmus and its visual consequences. J R Soc Med. 2002;95(5):235–237. | ||
Da Costa ACRV, Lopes MCB, Nakanami CR. Influence of head posture on the visual acuity of children with nystagmus. Arq Bras Oftalmol. 2014;77(1):8–11. | ||
Morris B, Smith V, Elphick J, Laws DE. Compensatory head posture and neck problems: is there an association? A cohort study of nystagmus patients. Eye (Lond). 2009;23(2):279–283. | ||
McLean RJ, Windridge KC, Gottlob I. Living with nystagmus: a qualitative study. Br J Ophthalmol. 2012;96(7):981–986. | ||
Pilling RF, Thompson JR, Gottlob I. Social and visual function in nystagmus. Br J Ophthalmol. 2005;89(10):1278–1281. | ||
Sanders J. The UK Nystagmus Network (NN). Semin Ophthalmol. 2006;21:61. | ||
Thurtell MJ, Leigh RJ. Therapy for nystagmus. J Neuroophthalmol. 2010;30(4):361–371. | ||
Anderson JR. Causes and treatment of congenital eccentric nystagmus. Br J Ophthalmol. 1953;37(5):267–281. | ||
Hertle RW, Anninger W, Yang D, Shatnawi R, Hill VM. Effects of extraocular muscle surgery on 15 patients with oculo-cutaneous albinism (OCA) and infantile nystagmus syndrome (INS). Am J Ophthalmol. 2004;138(6):978–987. | ||
Nelson LB, Ervin-Mulvey LD, Calhoun JH, Harley RD, Keisler MS. Surgical management for abnormal head position in nystagmus: the augmented modified Kestenbaum procedure. Br J Ophthalmol. 1984;68(11):796–800. | ||
Spielmann A. Clinical rationale for manifest congenital nystagmus surgery. J AAPOS. 2000;4(2):67–74. | ||
Zubcov AA, Stark N, Weber A, Wizov SS, Reinecke RD. Improvement of visual acuity after surgery for nystagmus. Ophthalmology. 1993;100(10):1488–1497. | ||
Gräf M, Droutsas K, Kaufmann H. Surgery for nystagmus related head turn: Kestenbaum procedure and artificial divergence. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch für Klin und Exp Ophthalmol. 2001;239(5):334-341. | ||
Dell’Osso LF, Hertle RW, Williams RW, Jacobs JB. A new surgery for congenital nystagmus: effects of tenotomy on an achiasmatic canine and the role of extraocular proprioception. J AAPOS. 1999;3:166–182. | ||
Dell’Osso LF. Extraocular muscle tenotomy, dissection, and suture: a hypothetical therapy for congenital nystagmus. J Pediatr Ophthalmol Strabismus. 1998;35(4):232–233. | ||
Hertle RW, Dell’Osso LF, FitzGibbon EJ, Thompson D, Yang D, Mellow SD. Horizontal rectus tenotomy in patients with congenital nystagmus: results in 10 adults. Ophthalmology. 2003;110(11):2097–2105. | ||
Wang ZI, Dell’Osso LF, Prakash S, Chen X. Smooth-pursuit changes after the tenotomy and reattachment procedure for infantile nystagmus syndrome: model predictions and patient data. J Pediatr Ophthalmol Strabismus. 2012;49(5):295–302. | ||
Sarvananthan N, Proudlock FA, Choudhuri I, Dua H, Gottlob I. Pharmacologic treatment of congenital nystagmus. Arch Ophthalmol. 2006;124(6):916–918. | ||
Shery T, Proudlock FA, Sarvananthan N, McLean RJ, Gottlob I. The effects of gabapentin and memantine in acquired and congenital nystagmus: a retrospective study. Br J Ophthalmol. 2006;90(7):839–843. | ||
McLean RJ, Sheth V, Abbas A, Pradeep A, Proudlock FA, Gottlob I. A randomized controlled crossover trial of gabapentin and memantine in infantile nystagmus. In: Abstracts of the European Neuro-Ophthalmology Society (EUNOS) 12th Meeting; 2015;39:S43. | ||
Pradeep A, Thomas S, Roberts EO, Proudlock FA, Gottlob I. Reduction of congenital nystagmus in a patient after smoking cannabis. Strabismus. 2008;16(1):29–32. | ||
Comer RM, Dawson ELM, Lee JP. Baclofen for patients with congenital periodic alternating nystagmus. Strabismus. 2006;14(4):205–209. | ||
McLean RJ, Gottlob I. The pharmacological treatment of nystagmus: a review. Expert Opin Pharmacother. 2009;10(11):1805–1816. | ||
Strupp M, Thurtell MJ, Shaikh AG, Brandt T, Zee DS, Leigh RJ. Pharmacotherapy of vestibular and ocular motor disorders, including nystagmus. J Neurol. 2011;258(7):1207–1222. | ||
Thurtell MJ, Leigh RJ. Treatment of nystagmus. Semin Neurol. 2015;35(5):522–526. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.