Back to Journals » Cancer Management and Research » Volume 9
Induction regimens for transplant-eligible patients with newly diagnosed multiple myeloma: a network meta-analysis of randomized controlled trials
Authors Zeng ZH, Chen JF, Li YX, Zhang R, Xiao L, Meng XY
Received 5 April 2017
Accepted for publication 22 June 2017
Published 10 July 2017 Volume 2017:9 Pages 287—298
DOI https://doi.org/10.2147/CMAR.S138932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zi-Hang Zeng,1,2 Jia-Feng Chen,1,2 Yi-Xuan Li,1,2 Ran Zhang,1,2 Ling-Fei Xiao,1,2 Xiang-Yu Meng1,2
1Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University, 2Department of Evidence-Based Medicine and Clinical Epidemiology, Second Clinical College of Wuhan University, Wuhan, People’s Republic of China
Objective: The aim of this study was to compare the early efficacy and survivals of induction regimens for transplant-eligible patients with untreated multiple myeloma.
Materials and methods: A comprehensive literature search in electronic databases was conducted for relevant randomized controlled trials (RCTs). Eligible studies were selected according to the predefined selection criteria, before they were evaluated for methodological quality. Basic characteristics and data for network meta-analysis (NMA) were extracted from included trials and pooled in our meta-analysis. The end points were the overall response rate (ORR), progression-free survival (PFS), and overall survival (OS).
Results: A total of 14 RCTs that included 4,763 patients were analyzed. The post-induction ORR was higher with bortezomib plus thalidomide plus dexamethasone (VTD) regimens, and VTD was better than the majority of other regimens. For OS, VTD plus cyclophosphamide (VTDC) regimens showed potential superiority over other regimens, but the difference was not statistically significant. The PFS was longer with thalidomide plus doxorubicin plus dexamethasone (TAD) regimens for transplant-eligible patients with newly diagnosed multiple myeloma (NDMM).
Conclusion: The NMA demonstrated that the VTD, VTDC, and TAD regimens are most beneficial in terms of ORR, OS, and PFS for transplant-eligible patients with NDMM, respectively.
Keywords: multiple myeloma, newly diagnosed, transplant-eligible, induction therapies, network meta-analysis
Introduction
MM is a common type of malignant plasma cell disorders. In Western countries, MM accounts for about 0.9% of all cancer deaths, and for about 10% of burdens of hematological malignancies.1,2 Over the past decade, the novel agents such as thalidomide,3 bortezomib,4,5 and lenalidomide6,7 have significantly improved the response rate and survival in patients with MM.8–10 Induction therapy followed by HDT (traditionally, high-dose melphalan) and ASCT is considered standard of care for NDMM patients aged <65 years.11
Until now, there are many Phase III RCTs reporting diverse induction regimens containing novel agents, such as the GIMEMA,12 MRC Myeloma IX trial,13 and HOVON-50.14 However, certain issues should be noted. First, some induction regimens have not yet been compared in a direct manner in RCTs. Second, a comprehensive evaluation and ranking for all available regimens have not yet been established with respect to the early efficacy and survival measures.
The NMA is a statistical analysis approach developed recently to address and resolve these problems ideally.15,16 NMA integrates information from direct and indirect comparison, by which a thorough assessment is realized. To compare the early efficacy (ie, ORR) and survivals (ie, PFS and OS) of different induction therapies for transplant candidates with newly diagnosed MM, the current NMA is performed.
Materials and methods
This NMA is reported according to the PRISMA-NMA.17
Search strategy
We searched databases including the PubMed, Embase, and Cochrane Library. The conference proceedings from the American Society of Hematology (2000–2016), European Hematology Association (2000–2016), and the American Society of Clinical Oncology (2000–2016), and the website of trial registries (eg, http://www.controlledtrials.com/ and https://www.ClinicalTrials.gov/ct), were also searched to find additional relevant studies. The search terms utilized were “multiple myeloma OR Myelomatosis OR plasmacytoma,” “autologous stem cell transplantation OR stem cell transplantation OR hematopoietic stem cell transplantation OR autologous transplantation OR transplant-eligible,” “initial therapy OR induction treatment OR induction therapy,” and “newly diagnosed OR untreated.” In addition, the references of RCTs were manually searched. The last search was performed on November 21, 2016.
Study selection
The following selection criteria were used to evaluate the eligibility of references identified by the literature search: 1) RCT design; 2) the participants were transplant-eligible patients with newly diagnosed MM; 3) the studies compared different pre-ASCT induction therapies; and 4) the data of investigative outcome measures (ORR, PFS, or OS) were provided or could be calculated.
Outcome measures
The study end point was post-induction ORR (an objective response equal to or better than PR [≥PR]), OS (the time from randomization to the date of death), and PFS (the time from the start of the treatment to disease progression or death). The definition of PR and progression followed the International Myeloma Working Group Uniform Response Criteria.18
Data extraction
We utilized predesigned data extraction form to guide the data extraction process. We extracted patients’ baseline characteristics, outcomes, and number of events. For some trials in which the HR and its 95% CI for OS and PFS were not reported but the relevant data for imputation were provided, Tierney et al’s method19 was used for calculation. Besides, we reconstructed IPD using Guyot et al’s20 method and performed survival analysis to make a comparison between TAD and VTD in terms of OS, based on extracted arm data from two independent trials.21,22 All the abovementioned procedures were independently accomplished by two researchers (Z-HZ and J-FC). If any discrepancies were found, a third researcher (Y-XL) will be involved in discussing.
Statistical analysis
For ORR, we calculated the natural logarithmic OR and its SE as the effect size and precision measure input into the NMA model. For OS and PFS, the natural logarithmic of HR and its SE were utilized. The consistency of effect sizes among studies was assessed by I2 statistic indicating heterogeneity among individual studies.23 When the value of I2 ≤ 50%, we would apply fixed-effects model. Otherwise, the random-effects model would be used. Visualization of analysis results including the forest plot and rankogram was performed. All the statistical analyses were made using the R software version 3.2.5.
Results
Inclusion and basic characteristics of eligible studies
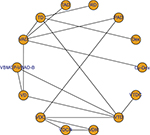
A total of 1,472 articles were obtained through our initial literature search, and 1,458 of these articles were excluded due to reasons indicated (Figure 1). Our NMA finally included 14 RCTs (full texts available for 13 studies12,14,21,22,24–32 and one conference abstract33). Details of induction regimens of included RCTs are provided in Table 1. A total of 4,763 patients were included in the NMA. In total, there were 14 induction regimens, by which 17 pairwise comparisons were established (Figure 2). Only two trials included multiple arm,26,32 and others included two arms.12,14,21–25,27–31,33 All 14 trials reported ORR outcome, but eight out of 14 trials reported OS and PFS outcome, respectively. The baseline characteristics of patients from each trial, including gender, median age, International Staging System stage, median β2-microglobulin, median albumin, and types of M protein, are provided in Table 2.
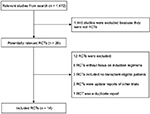  | Figure 1 Flow diagram showing literature search results. Abbreviation: RCTs, randomized controlled trials.
|
Risk of bias assessment
The Cochrane Collaboration’s tool for assessing the risk of bias was used to evaluate the quality of the included trials, except for one conference abstract.33 The results are shown in Figure 3. The included RCTs had low risks of selection bias about random sequence generation, attrition bias, reporting bias, and other biases, in which the rates of low risk were 100%, 92.3%, 92.3%, and 76.9%, respectively. Studies without clear information on allocation concealment, performance bias and detection bias accounted for 53.8%, 92.3%, and 92.3%, respectively.
  | Figure 3 Graph of assessments of risk of bias. Notes: Green, yellow, and red represent low, unclear, and high risk of bias, respectively. |
ORR
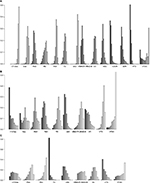
A total of 13 included trials were able to be used to evaluate ORR with NMA.12,14,22,24–33 We selected the fixed-effects model due to nonsignificant heterogeneity (I2 = 24%). The forest plot with Cy-Dex as the reference is shown in Figure 4. For the pairwise comparison of regimens, VTD had significantly higher ORR than other regimes, except for VDCR and VDR to which the superiority was nonsignificant (Table S1). VDR, VDCR, VDC, VD, VBMCP-VBAD-B, TD, RD, and PAD had significantly higher ORR than VAD, Dex, and Cy-Dex. TAD showed significantly higher ORR than Cy-Dex and VAD, but had significantly poorer ORR than PAD, VBMCP-VBAD-B, VDC, VDCR, VDR, and VTD. Meanwhile, VDCR had significantly higher ORR than VDR. VBMCP-VBAD-B resulted in significantly better ORR than VD and TD. No statistically significant difference was found for other comparisons. VTD was ranked the best regimen in terms of ORR according to rankogram which showed the rank probability (Figure 5).
OS
Eight studies involving 10 regimens were included in NMA for OS.12,14,21,22,24,26,29,31 The data of VTD vs TAD were based on reconstructed IPD obtained by Guyot et al’s19 approach. According to the value of I2 (1%), the fixed-effects model was used in NMA. Results showed that VTD was significantly better than TAD and VAD, and PAD was also significantly superior to VAD. Meanwhile, Cy-Dex had a shorter OS than the other nine regimens (Figure 4). On the other hand, there was no statistically significant difference among VTDC, Cy-Dex, Dex, VD, VBMCP-VBAD-B, PAD, VTD, and TD (Table S2). VTDC was ranked the best regimen for OS with relatively higher probability (Figure 5).
PFS
Eight out of 14 trials reported data on PFS.12,14,21,24–26,29,31 The fixed-effects model was used for NMA, because of the value of I2 (18%). The forest plot of TAD vs other eight regimens is shown in Figure 4. PAD, VD, VTD, VBMCP-VBAD-B, TAD, and VTDC had significant superiority when compared with TD (Table S3). PAD, VD, VTD, VBMCP-VBAD-B, TAD, and VTDC had significantly better PFS than TD. Furthermore, TAD and PAD resulted in significantly better PFS than VAD. VBMCP-VBAD-B had significantly better PFS than VTD. No statistically significant difference was found for other comparisons. TAD was ranked the best regimen for PFS with relatively higher probability (Figure 5).
Discussion
Since the introduction of the proteasome inhibitor bortezomib and IMiDs such as thalidomide and lenalidomide, the novel agents have shown increased benefits compared with traditional regimens such as VAD regimen, the standard induction in the 1990s.28,29,34 Two meta-analyses found that bortezomib-based induction regimens result in improvements in both PFS and OS, compared with nonbortezomib-based induction regimens, despite higher risk of toxicity.35,36 The better complete response, ORR, and PFS of velcade plus thalidomide-based regimens vs velcade-based or thalidomide-based regimens were also demonstrated in another meta-analysis.37 In addition, three-drug induction regimens combining bortezomib and Dex became the standard of care for transplant-eligible NDMM patients in 2014, because of the better efficacy.24,26,34,38 To date, no comprehensive overall comparison involving multiple induction options has been realized yet. NMA, first proposed by Lumley,15 can integrate direct and indirect evidence and make a global comparison of various regimens.
In our NMA, we found the superiority of VTD regimen for ORR. In GIMEMA and PETHEMA/GEM trial, Cavo et al12 and Rosiñol et al26 comparing VTD with TD showed significantly higher ORR for VTD. Meanwhile, reduced doses of VTD had higher ORR than VD, without statistical significance.25 In IFM 2013-04 trial, VTD was significantly superior to VDC.27 Although VTD resulted in an improvement in ORR when compared with VTDC, VTDC shows better performance in both PFS and OS in the NMA. For OS, VTDC is superior to others in our NMA. In a Phase II trial by Ludwig et al,21 VTDC showed nonsignificant superiority compared to VTD (HR = 0.87, 95% CI: 0.44–1.72, P = 0.69). Interestingly, in our mixed comparisons, the same result was observed (HR = 0.87, 95% CI: 0.44–1.7). For the PFS outcome, TAD had a better PFS than the other eight regimens, including VTD (HR = 0.66, 95% CI: 0.39–1.1) and VTDC (HR = 0.91, 95% CI: 0.44–1.9). However, TAD showed the significant inferiority when compared with VTD in terms of ORR (OR = 0.21, 95% CI: 0.12–0.38) and OS (HR = 1.8, 95% CI: 1.0–3.1), and similar trend was found for the comparison of TAS vs VTDC in terms of OS (HR = 2.0, 95% CI: 0.85–4.9). According to our NMA, VTDC was considered as more beneficial regimen in survival, which was ranked as the best and second regimen for OS and PFS, respectively. VTD and TAD were also promising regimens for transplant-eligible NDMM patients, especially for VTD, which was ranked as the best and second regimen for ORR and OS. On the other hand, in addition to efficacy measures, toxicity profiles, which are not meta-analytically analyzed in this article, may also be of great importance for the choice of treatment. A meta-analysis found that bortezomib-based induction regimens increased the risk of adverse events with a grade ≥3, compared with nonbortezomib-based induction regimens (63% vs 59%).36 Another meta-analysis showed that bortezomib–thalidomide-based regimens were linked to higher incidence of peripheral neuropathy than bortezomib-based regimens or thalidomide-based regimens (OR = 1.64, 95% CI: 0.77–3.46, P = 0.2).37 Furthermore, apparent toxicity was observed for quadruple regimens in some trials.21,32 In further studies, a comprehensive analysis concerning toxicity will provide valuable information for decision-making.
There are some limitations of this NMA. First, many comparisons in the NMA were based on single study; thus, the results might be altered if a single trial result has modifications. Second, some Phase II trials were included, but these trials had limited large sample size. Third, the survival of NDMM could be influenced by transplantation schemes, consolidation therapy, and maintenance therapy. Therefore, the results of this NMA should be interpreted with cautions, and more high-quality studies with adequate sample size are needed to confirm our findings.
Conclusion
The NMA demonstrated that the VTD, VTDC, and TAD regimens are most beneficial in terms of ORR, OS, and PFS for transplant-eligible patients with NDMM, respectively.
Abbreviations
ASCT, autologous stem cell transplantation; BCNU, bischloroethylnitrosourea; Cy-Dex, cyclophosphamide plus dexamethasone; Dex, dexamethasone; HDT, high-dose therapy; HR, hazard ratio; IMiDs, immunomodulatory drugs; IPD, individual patient data; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; NMA, network meta-analysis; OR, odds ratio; ORR, overall response rate; OS, overall survival; PAD, bortezomib plus doxorubicin plus dexamethasone; PFS, progression-free survival; PR, partial response; PRISMA-NMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Network Meta-analysis; RCTs, randomized controlled trials; RD, lenalidomide plus dexamethasone; SE, standard error; TAD, thalidomide plus doxorubicin plus dexamethasone; TD, thalidomide plus dexamethasone; VAD, vincristine plus doxorubicin plus dexamethasone; VBMCP-VBAD-B, BCNU plus vincristine plus melphalan plus prednisone plus dexamethasone plus bortezomib; VD, bortezomib plus dexamethasone; VDC, bortezomib plus dexamethasone plus cyclophosphamide; VDCR, bortezomib plus dexamethasone plus cyclophosphamide plus lenalidomide; VDR, bortezomib plus dexamethasone plus lenalidomide; VTD, bortezomib plus thalidomide plus dexamethasone; VTDC, bortezomib plus thalidomide plus dexamethasone plus cyclophosphamide.
Disclosure
The authors report no conflicts of interest in this work.
References
Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115–1123. | ||
Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183(1):25–35. | ||
Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. | ||
Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. | ||
Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. | ||
Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–4053. | ||
Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–3464. | ||
Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–2526. | ||
Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. | ||
Engelhardt M, Terpos E, Kleber M, et al; European Myeloma Network. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99(2):232–242. | ||
Cavo M, Rajkumar SV, Palumbo A, et al; International Myeloma Working Group. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117(23):6063–6073. | ||
Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. | ||
Morgan GJ, Davies FE, Gregory WM, et al; National Cancer Research Institute Haematological Oncology Clinical Studies Group. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2011;97(3):442–450. | ||
Lokhorst HM, van der Holt B, Zweegman S, et al; Dutch-Belgian Hemato-Oncology Group (HOVON). A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115(6):1113–1120. | ||
Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313–2324. | ||
van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–299. | ||
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. | ||
Durie BG, Harousseau JL, Miguel JS, et al; International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. | ||
Tierney JF, Stewart LA, Ghersi D. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. | ||
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. | ||
Ludwig H, Greil R, Masszi T, et al. Bortezomib, thalidomide and dexamethasone, with or without cyclophosphamide, for patients with newly diagnosed multiple myeloma: 5-year follow-up. Br J Haematol. 2015;171(3):344–354. | ||
Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR, Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–436. | ||
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. | ||
Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–2955. | ||
Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752–5758. | ||
Rosiñol L, Oriol A, Teruel AI, et al; Programa para el Estudio y la Terapéutica de las Hemopatías Malignas/Grupo Español de Mieloma (PETHEMA/GEM) group. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. | ||
Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127(21):2569–2574. | ||
Cavo M, Zamagni E, Tosi P, et al; Bologna 2002 Study. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106(1):35–39. | ||
Harousseau JL, Attal M, Avetloiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–4629. | ||
Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide, dexamethasone (VCD) versus bortezomib, doxorubicin, dexamethasone (PAd) in newly-diagnosed myeloma. Leukemia. 2015;29(8):1721–1729. | ||
Mellqvist UH, Lenhoff S, Johnsen HE, et al. Cyclophosphamide plus dexamethasone is an efficient initial treatment before high-dose melphalan and autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of a randomized comparison with vincristine, doxorubicin, and dexamethasone. Cancer. 2008;112(1):129–135. | ||
Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in newly diagnosed multiple myeloma. Blood. 2012;119(19):4375–4382. | ||
Kumar L, Mookerjee A, Sharma A, et al. Low dose dexamethasone plus lenalidomide (Len-dexa) versus thalidomide (Thal-dexa) as induction therapy for newly diagnosed multiple myeloma: a phase III, randomized study. Clin Lymphoma Myeloma Leuk. 2015;15:e146–e146. | ||
Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076–3084. | ||
Nooka AK, Kaufman JL, Behera M, et al. Bortezomib-containing induction regimens in transplant-eligible myeloma patients: a meta-analysis of phase 3 randomized clinical trials. Cancer. 2013;119(23):4119–4128. | ||
Sonneveld P, Goldschmidt H, Rosiñol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with newly diagnosed multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31(26):3279–3287. | ||
Huang H, Zhou L, Peng L, Fu W, Zhang C, Hou J. Bortezomib-thalidomide-based regimens improved clinical outcomes without increasing toxicity as induction treatment for untreated multiple myeloma: a meta-analysis of phase III randomized controlled trials. Leuk Res. 2014;38(9):1048–1054. | ||
Rosiñol L, Kumar S, Moreau P, Cavo M. Initial treatment of transplant-eligible patients in multiple myeloma. Expert Rev Hematol. 2014;7(1):43–53. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.