Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Indirect costs associated with premature mortality among those with veno-occlusive disease/sinusoidal obstruction syndrome with multiorgan dysfunction post-hematopoietic stem-cell-transplant
Authors Zhou ZY, Tang W, Villa KF
Received 22 August 2018
Accepted for publication 8 November 2018
Published 17 December 2018 Volume 2019:11 Pages 13—22
DOI https://doi.org/10.2147/CEOR.S184883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Zheng-Yi Zhou,1 Wenxi Tang,1 Kathleen F Villa2
1Analysis Group, New York, NY, USA; 2Health Economics and Outcomes Research, Jazz Pharmaceuticals, Palo Alto, CA, USA
Purpose: The study objective was to develop an economic model to assess projected costs of lost productivity associated with premature deaths due to veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) with multiorgan dysfunction (MOD) among patients in the US who underwent hematopoietic stem-cell transplant (HSCT) in 2013.
Methods: Data sources included the US Census Bureau and Department of Health, epidemiologic research organizations, and medical research literature. The model considered only lost productivity associated with premature death, with lifetime salary assumed to reflect productivity. Average annual salary was assumed to be the same for HSCT survivors and the general population, with a working age range between 18 and 65 years. Key data inputs included number of HSCTs by graft type (allogeneic and autologous) performed in the US in 2013, HSCT-related mortality, mortality associated with VOD/SOS with MOD, and life-expectancy reduction for HSCT survivors vs the general population. Excess mortality equaled total deaths among patients with VOD/SOS and MOD minus deaths in these patients due to causes other than VOD/SOS with MOD.
Results: Among 18,284 patients who underwent HSCT in the US in 2013, the model estimated that 361 excess deaths due to VOD/SOS with MOD occurred (158 following allogeneic and 203 after autologous transplants). These deaths accounted for total lost work productivity of 5,990 years and $124,212,173 in lost wages, averaging 17 years and $343,791 per patient. A sensitivity analysis incorporating adjustment factors for epidemiologic and economic inputs calculated total financial loss of $84 million to $194 million.
Limitation: Estimates of post-HSCT VOD/SOS with MOD incidence and mortality were approximated, due to changing HSCT practices.
Conclusion: Premature death due to VOD/SOS with MOD imposes a substantial economic burden in this population in terms of lost productivity. Additional studies of this economic burden are warranted.
Keywords: veno-occlusive disease/sinusoidal obstruction syndrome, stem-cell transplantation, mortality, lost productivity/income, indirect costs, economic burden
Introduction
Hepatic veno-occlusive disease (VOD), also called sinusoidal obstruction syndrome (SOS), is a potentially fatal complication of hematopoietic stem-cell transplant (HSCT).1,2 VOD/SOS results from a pathophysiological cascade characterized by toxic injury to sinusoidal endothelial cells and hepatocytes in zone 3 of the hepatic acinus caused by the HSCT-conditioning regimen, and has also been observed to occur after nontransplant-associated chemotherapy.1,2 The estimated incidence of VOD/SOS among patients undergoing HSCT varies, but a pooled analysis found rates of approximately 8.7% and 12.9% among patients receiving autologous and allogeneic transplants, respectively.3 Clinical characteristics of VOD/SOS typically include hepatomegaly, weight gain, increased bilirubin (>2 mg/dL), and ascites that usually occur within 3 weeks post-HSCT.3,4 VOD/SOS has traditionally been diagnosed based on the Baltimore criteria (≤21 days post-HSCT, bilirubin ≥2 mg/dL, and two or more of hepatomegaly, ascites, and weight gain ≥5%)5 or the modified Seattle criteria (≤20 days post-HSCT, with two or more of bilirubin ≥2 mg/dL, hepatomegaly/right upper-quadrant pain, and >2% weight gain [sometimes ≥5%]).6,7 However, experts have recently called for revision of these diagnostic criteria to include a broader range of clinical presentations and updates to increase diagnostic sensitivity and specificity.2,8,9
The severity, course, and outcome of VOD/SOS have been difficult to predict.4,10,11 Severe VOD/SOS was traditionally defined retrospectively as death or nonresolution of symptoms by day +100.1 More recently, however, concomitant multiorgan dysfunction (MOD; eg, renal and/or pulmonary dysfunction) has been widely acknowledged to be a critical characteristic of severe VOD/SOS, irrespective of how the VOD/SOS pathophysiological cascade is triggered, allowing for practical, prospective assessment and treatment.2,4 Further, the European Society of Blood and Marrow Transplantation has proposed age-specific grading criteria for pediatric and adult patients, which may provide earlier identification of patients with severe disease prior to clinical MOD.8,9
VOD/SOS with MOD develops in ~20%–40% of patients with VOD/SOS who received HSCT, most frequently after allogeneic transplant,11–14 and may be associated with mortality rates >80%.3 In addition, VOD/SOS was estimated to increase first-year, per-patient direct HSCT costs by 42% or US $41,702 and 150% for patients with VOD/SOS and MOD.15 Therefore, VOD/SOS and MOD pose significant economic burdens in direct medical costs.
Research to ascertain work-productivity loss associated with premature death due to VOD/SOS, however, is currently lacking. This parameter is particularly important to assess for post-HSCT patients, because ~70% of patients who receive HSCT are aged 60 years or younger,16 ie, within or before the prime years of life for work productivity. The economic model used for this study was developed to evaluate the cumulative indirect costs of lost productivity associated with premature deaths due to VOD/SOS with MOD among HSCT patients in the US.
Methods
Overall design
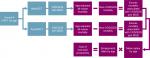
This was an Excel-based economic model of productivity loss due to premature death associated with VOD/SOS and MOD in patients who had undergone HSCT in the US (Figure 1). Controls included HSCT survivors and were modeled using US population data, such as US census and economic data, on employment rates and wages. Only lost work-related productivity of the patient associated with premature death was considered. Lost productivity related to disability, school, or daily activities was not considered. The age of the working population was considered to be 18–65 years.
Model inputs
Epidemiological model inputs for the total HSCT population included the number of transplants by type in the US16 and overall posttransplant mortality (Table 1).17
  | Table 1 Allogeneic and autologous HSCT and mortality by age-group (preretirement population) Abbreviations: HSCT, hematopoietic stem-cell transplant. |
Epidemiologic inputs for VOD/SOS populations included:
- incidence of VOD/SOS by HSCT-graft type (12.9% allogeneic, 8.7% autologous;3 hepatic VOD/SOS was first defined by the ICD 10 in October 2015)
- incidence of VOD/SOS for allogeneic HSCT patients identified by modified Seattle criteria and Baltimore criteria were estimated to be 13.8% and 8.8%, respectively12
- incidence of VOD/SOS with MOD among HSCT patients with VOD/SOS (27.6%), based on results from a study using a retrospective definition of severity11
- mortality rate due to VOD/SOS with MOD (84.3%) from a pooled analysis of 19 studies, which used varied definitions of severity3
- mortality was assumed to be directly related to VOD/SOS pathophysiology, irrespective of graft type19
Model inputs for control populations included:
- life expectancy by age-group in the general population18
- US general population averages for annual salary and employment rate by age-group (Table 2)
- reduction in life expectancy for HSCT survivors vs the general population by age-group (Table 2)
- reduced employment rate in HSCT survivors, defined as percentage of patients receiving HSCT who have not returned to their previous level of employment, presented as a weighted average of employment-rate reductions for autologous HSCT and allogeneic HSCT
- reductions in employment rate in HSCT survivors by years following HSCT, estimated to be 40% after 1 year and 31% after 2 years, based on a published analysis in 328 patients;24 the rate was assumed to be 0 after 3 years and onward
  | Table 2 General population average annual salary, employment rate, and reduction in life expectancy for HSCT patients by age-group Notes: aAverage salary was assumed to be the same for HSCT patients as for the general population.23 bReduced life expectancy was defined as percentage decrease in number of years that an HSCT patient is expected to live compared with the life expectancy of the general population. For example, a 25-year-old HSCT patient will have a life expectancy that is 28.8% lower than the 54.83-year life expectancy of a 25-year-old in the general population (54.83–[54.83×0.288]=39.04-year life expectancy). Life expectancy for the population aged 20–60 years was provided by published data,22 and percentage of reduction in life expectancy at age 60 years was used for the 61- to 64-year age-group. Abbreviations: HSCT, hematopoietic stem-cell transplant. |
Model data-input sources
Sources of the data inputs for this model included published literature; the Center for International Blood and Marrow Transplant Research (CIBMTR), US Department of Health and Human Services, Centers for Disease Control and Prevention, Health Resources and Services Administration, US Census Bureau, and Organization for Economic Co-operation and Development.
Model outputs
Excess mortality was calculated as the estimated total number of deaths among patients with VOD/SOS and MOD post-HSCT, based on incidence and mortality rates reported in the literature,3,11 minus the estimated number of deaths among patients with VOD/SOS and MOD due to factors other than VOD/SOS with MOD (eg, HSCT, progressive disease, infection, graft-versus-host disease, and natural causes):
|
Specifically, numbers of patients receiving allogeneic and autologous HSCT were multiplied by rates of VOD/SOS from the literature (12.9% and 8.7%, respectively), then for MOD (27.6%), and finally for death due to MOD (84.3%). These numbers were then adjusted by subtracting all-cause mortality post-HSCT (3%–43% depending on graft type and age). Lost productivity years resulting from premature death were calculated as the number of years between initial age or age 18 years, whichever was higher, and retirement age (65 years) or life-expectancy estimates, whichever was lower. Total lost productivity years and indirect costs by age were obtained by multiplying each per-patient value by the number of excess deaths in each age-group. Work-productivity loss was expressed as the cumulative lost salary for all projected work years contributed by a patient, with a 3% discount for each additional year. Productivity loss due to VOD/SOS with MOD was calculated in 2014 US$ and compared with HSCT survivors.
Deterministic sensitivity analyses were performed to assess the effect of various epidemiological and economic parameters, such as incidence and mortality of VOD/SOS with MOD, retirement age, average salaries, and reductions in employment rate and life expectancy, on the observed results, with a range of low and high costs estimated for each variable based on the sources used or model-based calculations (Table 3).
  | Table 3 Deterministic sensitivity analysis modeling variables of work-productivity loss due to premature death from VOD/SOS with MOD vs HSCT survivors without VOD/SOS Notes: aBased on published epidemiological studies, the incidence of VOD/SOS for allogeneic HSCT patients under Seattle and Baltimore criteria was estimated to be 13.8% and 8.8%, respectively, based on a retrospective study of 845 patients12 and the ratio of incidence of VOD/SOS among autologous and allogeneic HSCT was estimated to be 0.674 (8.7%/12.9%) based on a meta-analysis of 135 studies.3 Therefore, the incidence of VOD/SOS for autologous HSCT patients was calculated to be 9.30% (13.8%×0.674) as per Seattle criteria or 5.93% (8.8%×0.674) as per Baltimore criteria. bModel assumption. Abbreviations: HSCT, hematopoietic stem-cell transplant; MOD, multiorgan dysfunction; SOS, sinusoidal obstruction syndrome; VOD, veno-occlusive disease. |
Results
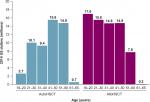
Based on this model for the year 2013, 361 excess deaths per year in the US are expected due to VOD/SOS with MOD in the HSCT population, including 203 excess deaths in patients undergoing autologous HSCT — the great majority in patients aged >50 years — and 158 excess deaths in patients receiving allogeneic HSCT (Figure 2). As shown in Table 1, 18,284 patients received HSCT in 2013, including 10,542 who received autologous and 7,742 who received allogeneic HSCT.17 Estimated incidence of VOD/SOS is incrementally higher in allogeneic vs autologous HSCT patients (12.9% vs 8.7%),3 which resulted in a slightly higher estimated number of VOD/SOS cases in allogeneic vs autologous HSCT patients in the study sample (999 vs 917, respectively). In addition, given that the estimated incidence of VOD/SOS with MOD in patients with VOD/SOS (27.6%)11 and its associated mortality rate (84.3%)3 were assumed to be the same in each group,19 deaths for patients with VOD/SOS with MOD were estimated to be slightly higher in allogeneic vs autologous HSCT (232 vs 213, respectively). However, such deaths could be due to any causes among patients with VOD/SOS with MOD, and may include such reasons as HSCT itself. Therefore, the “excess” death due to VOD/SOS with MOD was further calculated by taking into consideration all-cause mortality among patients who received HSCT, which is reported to be much higher in patients who received allogeneic vs autologous HSCT (28%–43% vs 3%–8% across different age-groups; Table 1).17 Therefore, a larger proportion of patients with allogeneic vs autologous HSCT were estimated to die from reasons associated with HSCT itself or various causes other than VOD/SOS with MOD. After subtracting these patients, the estimated “excess” mortality from VOD/SOS with MOD was slightly lower in allogeneic vs autologous HSCT patients (158 vs 203, respectively).
  | Figure 2 Annual number of excess deaths in the total population across all ages (including patients aged >65 years). Notes: Calculated as number of deaths among HSCT patients with VOD/SOS and MOD, based on published incidence and mortality rates (Tables 1–3),3,11,12 minus the number of deaths among patients with VOD/SOS and MOD that were attributed to other (non-VOD/SOS and MOD) causes (eg, primary disease, other complications, and natural causes). Interim calculation by age and type of transplant (N=361). aAutoHSCT and alloHSCT combined may not add up to total due to rounding. Abbreviations: alloHSCT, allogeneic HSCT; autoHSCT, autologous HSCT; HSCT, hematopoietic SCT; MOD, multiorgan dysfunction; SOS, sinusoidal obstruction syndrome; VOD, veno-occlusive disease. |
For patients who died in 2013, death due to VOD/SOS with MOD among HSCT patients was associated with a total of 5,990 lost work-productivity years and an indirect cost of $124,212,173 in 2014 US$ (autologous, $53,580,337; allogeneic, $70,631,836) compared with HSCT survivors, averaging 17 lost work-productivity years at an indirect cost of $343,791 per patient (Table 4).
Patients with VOD/SOS and MOD who had undergone allogeneic HSCT were younger on average and had greater estimated work-productivity loss ($71 million over time for patients receiving HSCT in 2013) compared with patients who had received autologous HSCT ($54 million; Figure 3).
The deterministic sensitivity analysis using epidemiological and economic inputs (Table 3), including variations in the incidence and mortality of VOD/SOS with MOD, retirement age, average salaries, and reductions in employment rate and life expectancy, showed productivity loss of premature death due to VOD/SOS with MOD in all scenarios (Figure 4). These ranged from a low of ~$84 million when confined to patients diagnosed with VOD/SOS via Baltimore criteria5 to a high of $194 million based on the high end of the annual discount rate. Other factors to which the model was most sensitive were incidence of VOD/SOS with MOD and average annual salary for HSCT survivors.
Discussion
Existing studies have reported high direct medical costs associated with HSCT, with VOD/SOS, and with VOD/SOS with MOD.25,26 Specifically, direct medical costs associated with hospitalization for those with VOD/SOS and MOD have been evaluated and reported to be $140,000–$250,000 per patient,15,27 However, data have not been available on the indirect costs expected to be a consequence of potentially fatal complications, such as VOD/SOS with MOD. Although VOD/SOS with MOD is a comparatively rare complication of HSCT, this model found that VOD/SOS with MOD represents a substantial indirect cost to society. The model estimates that in each year, patients who develop VOD/SOS with MOD will incur total lifetime work-productivity loss of over $124 million due to reduced life expectancy. Results of a sensitivity analysis demonstrated that total productivity loss could be as high as $194 million per year. The estimated average total productivity loss per patient was approximately $344,000. This model likely underestimates the total indirect costs of premature death due to VOD/SOS with MOD, as it does not include productivity loss among children and caregivers, patients aged >65 years who might otherwise continue to work (30%),20,21 or unpaid workers (eg, homemakers and stay-at-home parents). Moreover, VOD/SOS with MOD may occur outside the HSCT setting, such as high-dose chemotherapy alone.2,28
These data are all the more important to consider, given the high and growing prevalence of patients undergoing HSCT.4,29,30 Of the 11.7 million people who had cancer in the US in 2007, 8% (936,000) were estimated to have hematologic cancers.29 In addition, CIBMTR data indicate that use of HSCT has increased steadily over the past 30 years, with a current annual rate of more than 21,000 patients receiving this procedure in the US, and is projected to continue increasing.31–33 Along with this general increase, the proportion of patients aged ≥60 years among all patients receiving autologous and allogeneic HSCT rose markedly, from less than 20% and 5%, respectively, in the period 1993–1999 to 50% and 30%, respectively, in 2015.33 Nonetheless, the majority of HSCT patients receiving either type of transplant remain of working age or have their entire working lives before them: as of 2009, CIBMTR data showed that 14% of all HSCT recipients were aged <18 years.29 Therefore, the productivity loss due to premature death in the total HSCT population is likely to be of considerable magnitude, as demonstrated by the present data.
Among long-term HSCT survivors, 10- to 20-year survival has generally been estimated in studies to be in the range of 70%–90%.22,34–40 For patients surviving 10 years, life expectancy may approach that of the general population, although this cohort continues to experience increased morbidity and mortality risks.22,23,34,40–42 Therefore, premature death occurring shortly after HSCT imposes a considerable indirect cost in lost decades of productive life for a large number of patients in the US, as indicated by this analysis.
The current analysis outlines the expected indirect costs associated with VOD/MOD-related mortality. However, the difficulties among the overall population of HSCT survivors are important to consider in the present context, because they illustrate that premature death represents only a portion of the total indirect cost related to VOD/SOS with MOD. Some studies in the literature have demonstrated the adverse impact on work productivity in the years following HSCT. For example, one study found that ~40%–60% of adult survivors of allogeneic transplant who were employed pretransplant were employed at 1 year post-HSCT.24,43 Further, among those with full-time employment, survivors reported that they could accomplish 80%–87% of what they had been able to achieve before the transplant.43 In another study, a third of allogeneic transplant recipients who had been employed prior to HSCT were no longer employed in the following year; in the overall survey population, 26% reported that household income had decreased >50%.44 However, a separate study looking at longer-term outcomes found that 72% of HSCT patients were working at 10 years posttransplant, which was similar to the 74% rate in matched controls.23 A Swiss study in 203 patients at 12-years post-HSCT (median age 50 years) found that while 77% were working full- or part-time, 37% were receiving a work-disability pension compared to an expected pension incidence of 3.2% of the Swiss working population.45 Employment was also examined in a study of adult survivors of pediatric allogeneic transplantation, which found that secondary-school graduation rates for girls were similar to those of the general population, but the rate for boys was approximately half of population norms; even so, job distribution was similar to that of the general population.46 Besides employment difficulties, HSCT survivorship may be associated with costs and work productivity loss due to hospitalizations and follow-up care resulting from infectious and noninfectious complications frequently encountered with HSCT.24,34
The present analysis helps quantify an important aspect of the burden of illness of VOD/SOS with MOD, advance this area of research, and provide a basis for future studies. The estimated work-productivity losses per patient of approximately $344,000 associated with death due to VOD/SOS with MOD found in this study, combined with direct costs over time of $100,000 to more than $400,000 for HSCT alone15,25,26 and approximately $150,000 more for additional hospital costs due to VOD/SOS and MOD,15,27 provide a more complete and accurate assessment of the total potential costs of HSCT. The present sensitivity analysis also demonstrates the qualitative robustness of the model. In addition, these data serve to remind physicians of an important clinical association — the potential for VOD/SOS to lead to MOD — which may be overlooked in general assessments of the economic burden of HSCT.
However, because the economic impact of VOD/SOS with MOD is not well characterized in the literature, there are limitations on the available input data. For example, reporting to CIBMTR is voluntary, and it is estimated that the database captures 60%–90% of related donor allogeneic transplants and 65%–75% of autologous transplants.33 Further, the diagnostic code for VOD/SOS was introduced in the ICD10, complicating identification of VOD/SOS (and VOD/SOS with MOD and VOD/SOS-associated mortality) before 2015. Estimates for incidence of VOD/SOS, associated MOD, and mortality due to VOD/SOS with MOD have been variable over time as clinical practice in HSCT has changed: some researchers have found higher incidence of VOD/SOS over time and across studies,3 while others have reported decreasing incidence in single-center settings.12 Although increasing the use of reduced intensity conditioning may affect incidence of VOD/SOS,2 one study in postallogeneic patients reported an overall VOD/SOS rate of 9% in those given reduced-intensity conditioning.47 Mortality due to causes other than VOD/SOS and MOD was estimated, and was assumed to be the same as HSCT patients who had died from causes other than VOD/SOS and MOD. Therefore, additional research is needed to assess more completely the total costs of this important, high-risk complication of HSCT. In addition, as this model does not include productivity loss among children and caregivers, patients aged >65 years who might otherwise continue to work (30%),20,21 and unpaid workers (eg, homemakers and stay-at-home parents), it likely underestimates total indirect costs. Of note, VOD/SOS in the absence of MOD12,48 is also associated with early mortality; however, this lost productivity is not included in the model. The reductions in employment rate in HSCT survivors by years following HSCT, estimated to be 40% after 1 year and 31% after 2 years, were also based on limited data: a study in 328 patients.24
Conclusion
VOD/SOS with MOD imposes a substantial economic burden in terms of excess deaths and lost productivity. This model, focusing on work-productivity loss due to premature death, found that VOD/SOS with MOD could represent a cumulative indirect cost of $124 million and as high as $194 million for US patients treated in 2013 in 2014 US$, with an estimated average cost of approximately $344,000 per patient. This cost is in addition to an estimated mean direct hospitalization cost of $250,000 per patient associated with VOD/SOS and MOD post-HSCT. Future research is warranted to assess the additional indirect costs associated with VOD/SOS with and without MOD.
Author contributions
All authors were responsible for the study conception and design, involved in the collection and assembly of data, participated in study-data analysis, interpretation, and manuscript writing, and provided their final approval of this manuscript.
Acknowledgments
This research was funded by Jazz Pharmaceuticals Inc, the manufacturer of defibrotide. The authors thank Larry Deblinger and The Curry Rockefeller Group, LLC of Tarrytown, NY for providing medical writing support and editorial assistance in formatting, proofreading, and copy editing, and fact-checking, which was funded by Jazz Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Jazz Pharmaceuticals also reviewed and edited the publication for scientific accuracy.
Disclosure
WT and ZYZ are consultants for Jazz Pharmaceuticals. KFV is an employee of Jazz Pharmaceuticals and holds stock and/or stock options in Jazz Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85(11):3005–3020. | ||
Mohty M, Malard F, Abecassis M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50(6):781–789. | ||
Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16(2):157–168. | ||
Dignan FL, Wynn RF, Hadzic N. Haemato-oncology task force of the British Committee for Standards in Haematology and the British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163(4):444–457. | ||
Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–783. | ||
McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. | ||
Corbacioglu S, Cesaro S, Faraci M, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379(9823):1301–1309. | ||
Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51(7):906–912. | ||
Corbacioglu S, Carreras E, Ansari M, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53(2):138–145. | ||
Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11(9):1729–1736. | ||
Carreras E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92(10):3599–3604. | ||
Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17(11):1713–1720. | ||
Strouse C, Richardson P, Prentice G, et al. Defibrotide for treatment of severe veno-occlusive disease in pediatrics and adults: an exploratory analysis using data from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2016;22(7):1306–1312. | ||
Cesaro S, Pillon M, Talenti E, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90(10):1396–1404. | ||
Cao Z, Villa KF, Lipkin CB, Robinson SB, Nejadnik B, Dvorak CC. Burden of illness associated with sinusoidal obstruction syndrome/veno-occlusive disease in patients with hematopoietic stem cell transplantation. J Med Econ. 2017;20(8):871–883. | ||
Pasquini MC, Wang Z. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR summary slides [webpage on the Internet]; 2013, [cited 2016 Jul 26]. Available from: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Documents/2013%20Summary%20Slides-%20Final%20Web%20Version%20%20V2%204.14.2014.pptx. Accessed November 22, 2017. | ||
Health Resources and Services Administration. US patient survival report [webpage on the Internet]. Rockville: US Department of Health and Human Services [cited 2016 20 Apr]. Available from: http://bloodcell.transplant.hrsa.gov/RESEARCH/Transplant_Data/US_Tx_Data/Survival_Data/survival.aspx. Accessed November 22, 2017. | ||
Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1–119. | ||
Carreras E, Rosiñol L, Terol MJ, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13(12):1448–1454. | ||
United States Census Bureau. Historical income tables: people. Table P-10. Age—people (both sexes combined) by median and mean. 2013 [webpage on the Internet]. Washington, DC: US Department of Commerce [updated Aug 10, 2017]. Available from: https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-income-people.html. Accessed November 22, 2017. | ||
Organisation for Economic Co-operation and Development [webpage on the Internet]. Labour force statistics by sex and age. Available from: http://stats.oecd.org/Index.aspx?DatasetCode=LFS_D#. Accessed 2015 Feb 23. | ||
Martin PJ, Counts GW, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011–1016. | ||
Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23(27):6596–6606. | ||
Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19(1):242–252. | ||
Majhail NS, Mau LW, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone Marrow Transplant. 2013;48(2):294–300. | ||
Broder MS, Quock TP, Chang E, et al. The cost of hematopoietic stem-cell transplantation in the United States. Am Health Drug Benefits. 2017;10(7):366–374. | ||
Dvorak CC, Nejadnik B, Cao Z, Robinson SB, Lipkin C, Villa KF. Hospital cost associated with veno-occlusive disease (VOD) in patients with hematopoietic stem cell transplant (HSCT). Poster presented at: 57th American Society of Hematology Annual Meeting and Exposition; December 7; 2015; Orlando, FL. | ||
Kantarjian HM, Deangelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4(8):e387–e398. | ||
Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–1501. | ||
Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. | ||
D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23(9):1417–1421. | ||
Majhail NS, Farnia SH, Carpenter PA, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21(11):1863–1869. | ||
Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic cell transplantation (HCT): 2016 summary slides [webpage on the Internet]; 2016. Available from: https://www.cibmtr.org/referencecenter/slidesreports/summaryslides/Pages/index.aspx#DownloadSummarySlides. Accessed October 31, 2017. | ||
Majhail NS, Rizzo JD. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48(9):1145–1151. | ||
Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147(1):129–139. | ||
Majhail NS, Bajorunaite R, Lazarus HM, et al. High probability of long-term survival in 2-year survivors of autologous hematopoietic cell transplantation for AML in first or second CR. Bone Marrow Transplant. 2011;46(3):385–392. | ||
Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. | ||
Goldman JM, Majhail NS, Klein JP, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28(11):1888–1895. | ||
Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. | ||
Nivison-Smith I, Simpson JM, Dodds AJ, Ma DD, Szer J, Bradstock KF. Relative survival of long-term hematopoietic cell transplant recipients approaches general population rates. Biol Blood Marrow Transplant. 2009;15(10):1323–1330. | ||
Vanderwalde AM, Sun CL, Laddaran L, et al. Conditional survival and cause-specific mortality after autologous hematopoietic cell transplantation for hematological malignancies. Leukemia. 2013;27(5):1139–1145. | ||
Baker KS, Armenian S, Bhatia S. Long-term consequences of hematopoietic stem cell transplantation: current state of the science. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S90–S96. | ||
Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. J Cancer Surviv. 2010;4(1):33–44. | ||
Khera N, Chang YH, Hashmi S, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1375–1381. | ||
Tichelli A, Gerull S, Holbro A, et al. Inability to work and need for disability pension among long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(10):1436–1442. | ||
Freycon F, Trombert-Paviot B, Casagranda L, et al. Academic difficulties and occupational outcomes of adult survivors of childhood leukemia who have undergone allogeneic hematopoietic stem cell transplantation and fractionated total body irradiation conditioning. Pediatr Hematol Oncol. 2014;31(3):225–236. | ||
Tsirigotis PD, Resnick IB, Avni B, et al. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplant. 2014;49(11):1389–1392. | ||
Yakushijin K, Atsuta Y, Doki N, et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant. 2016;51(3):403–409. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.