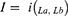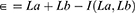Back to Journals » Clinical Interventions in Aging » Volume 17
Inclusion of Potentially Inappropriate Medicines for the Older Adults in the Brazilian Consensus in Accordance with International Criteria
Authors Pecce Bento A , Costa Pereira L , Ramos Garcia K , Ramos Ferreira LF, da Silva EV, Karnikowski M
Received 4 May 2021
Accepted for publication 15 July 2021
Published 16 February 2022 Volume 2022:17 Pages 151—161
DOI https://doi.org/10.2147/CIA.S318578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Andréa Pecce Bento,1 Leonardo Costa Pereira,2 Kerolyn Ramos Garcia,1 Luiz Fernando Ramos Ferreira,3 Emília Vitória da Silva,1 Margô Karnikowski1
1Sciences and Health Technologies Program, University of Brasilia, Brasilia, DF, Brazil; 2Physical Education, University UNIEURO, Brasilia, DF, Brazil; 3Academic League of Project Management, Florence College, São Luis, Maranhão, Brazil
Correspondence: Margô Karnikowski, University Campus, s/n, Metropolitan Center, Brasília, DF, 72220-275, Brazil, Tel/Fax +5 613107 8418, Email [email protected]
Aim: The use of potentially inappropriate medications (PIM) can impair the safety and effectiveness of pharmacotherapy in the older adults. Thus, several countries have lists and criteria to indicate these drugs, in order to promote the safety of prescription and the rational use of drugs in geriatric practice.
Objective: This study sought to contribute to the inclusion of PIM for the older adults in the Brazilian criterion (BCPIM/2016) – current list used in Brazil and reference in Latin American countries – through expert approval, comparing convergences with international AGS lists BEERS/2019, STOPP/START/2015, PRISCUS/2010 and EU (7)-PIM List/2015.
Methods: This is a critical analysis of potentially inappropriate medications for use in the older adults present in the list of Brazilian criteria, together with their absence of some drugs that are on international lists (BEERS/2019; Priscus/2010; Stopp/Start/2015; EU7-PIM list/2015). This study was subdivided in 6 stages: selection of national criteria, classification of drugs according to Anatomic Therapeutic Chemical, comparison between BCPIM/2016 with international lists, selection of drugs not included in the Brazilian list, selection of experts for evaluation and suggestions about drugs not included in the Brazilian list and the synthesis of the analysis carried out by the specialists.
Results: We cataloged 66 drugs marketed in Brazil that are on international lists, but not in the Brazilian consensus, of which 24 were validated by experts as necessary for inclusion in this consensus, considering the risks and benefits in health care for the older adults. However, the lists have divergences and similarities between them. We observed that eight drugs were common to all criteria studied, mainly related to the nervous system.
Conclusion: The results suggest the need for periodic validation of PIM against research clinics, new drugs and the inclusion of this agenda by the Ministry of Health in the revision of the National List of Essential Drugs and other Clinical Protocols and Therapeutic Prescription Guidelines for the older adults.
Keywords: list of potentially inappropriate drugs, seniors, aging, side effects, drug-related adverse reactions
Introduction
Functional changes and homeostasis usually appear naturally due to the senescence physiology and impact on pharmacokinetics and pharmacodynamics, with the potential to influence the drug therapy safety and effectiveness.1,2
One of the senility processes designs, characterized by a gradual and pathological decline in all body systems functioning, has determined the highest frequency of frailty and comorbidities and, by consequence, the polypharmacy in the older adults. The long-lived population is one of the largest consumers of drugs3 and studies have shown that the use of inappropriate medications is prevalent in older adults patients, as well as adverse reactions and drug interactions.4
Thus, the peculiarities of the older adults organism referring to pharmacotherapy become them more exposed to Drug-Related Problems – DRP and to Negative Results Associated with Medication – NMR, both responsible for a significant number of clinical admissions.5,6
In this context, Potentially Inappropriate Medicines (PIM) for the older adults arise. It is characterized by increasing the risk of having adverse reactions compared to younger patients or also when they have no evidence-based indication.6,7 According to Beer’s criteria, the PIM are defined as drugs with higher risk of intolerance related to adverse pharmacodynamics or pharmacokinetics or drugs-disease interactions REF, and it is classified into three categories, those that should be avoided in the older adults regardless of the clinical condition; those that should be avoided in the older adults with certain diseases, which can be aggravated by such drugs; and those that should be used with care/caution in the older adults.8
Several countries have been elaborated lists containing PIM for the older adults attempting to favor the prescription and rational use of medicines in geriatric practice9 e to keep them updated through constant reviews.10 Among these lists are the classification criteria of the American Geriatric Society (AGS) for the BEERS Criterion, version 2019;11 Screening Tool of Older Person’s Prescriptions (STOPP) Criterion Screening Tool to Alert to Right Treatment (START), 2015 version (STOPP/START-2015);12,13 the list of Germany; PRISCUS/201014 and the EU (7) -PIM List/2015,15 FORTA (fit for the Aged) List/EURO-FORTA- 2018,16 Taiwan-PIM/2019,17 McLeod and IPET-Improving Prescribing in the older adults/2008.18
In Brazil, the content validation of the Beers Criteria 2012 and STOPP 2006 resulted, in 2016, in the Brazilian Consensus on Potentially Inappropriate Medicines for the Older adults (BCPIM/2016).19
The present study aims to assess the selectivity of potentially inappropriate medications (PIM) to the older adults for the Brazilian criterion according to the convergences with the international lists under the specialists’ validation.
Materials and Methods
Study Design
The study has a mixed method.20 It is qualitative research once the modified Delphi technique21 was used to establish a strategy for systematized analysis of expert opinions and for the consensus on the drugs inclusion in BCPIM/2016,19 which were absent in this list, but were contained in the criteria North American and European internationals. It is also quantitative, cross-sectional study,22 due to the use of frequencies and agreement indicator concerning the concomitant presence of PIM in BCPIM/2016 with international lists.
Procedures
The research design is shown in Figure 1 and was carried out in six stages.
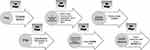 |
Figure 1 Experimental study design. |
1st Stage – Selection of International Lists of Drugs Potentially Inappropriate for the Older Adults
In addition to the Brazilian Consensus on Potentially Inappropriate Medicines for the Older adults, 2016 (BCPIM/2016) REF, the criteria (Figure 1) identified by the literature as the best known and most used in the United States and Europe today, applicable to regulatory studies, were selected, in their most current versions,7 as follows: the EU (7) – PIM List15 and the AGS Beers criteria.11 The Screening Tool of Older Person’s Prescriptions (STOPP), Screening Tool to Alert to Right Treatment (START) and the German list PRISCUS/2010 (14) criteria were also included (12, 13) as these are recent criteria and constantly pointed out in the literature as tools used to assess the PIM prescription and factors associated with the use of PIM. This process was supported by a comprehensive literature search that occurred in the first half of 2019. As a tool to identify the guiding lists of PIM that would be used in this study, we consulted the following bibliographic platforms for scientific research: PubMed, Lilacs, Scielo and Scopus. For searching in this database, the next descriptors were used in Portuguese, English and Spanish languages: “List of Potentially Inappropriate Medicines”, “Older adults”, “Aging”, “Side Effects”, “Drug-Related Adverse Reactions”.
2nd Stage – Survey of Drugs Potentially Inappropriate for the Older Adults Contained in the Lists
We determined which medications were potentially inappropriate for the older adults selected on the 1st Stage. The drugs were listed in a database following the drug classification according to Anatomic Therapeutic Chemical (ATC).23
3rd Stage – Agreement Indicator (BCPIM)
An analytical contrast measure was adopted in the relationships between the PIM contained in the five criteria selected in the 1st Stage through the agreement indicator (I). Thus, after identifying the total number of drugs listed in the different criteria, we verified in what proportion two lists of drugs observed agreed among themselves, within the set of all drugs present in both lists. Such indicator was obtained from the following formula:
where
I= Agreement indicator between criteria a and b;
i(La, Lb) = Intersection between the criteria for PIM’s a (La) and b (Lb);
La = Number of drugs present in the criterion a;
Lb = Number of drugs present in the criterion b.
The agreement indicator is measured by a value ranging from 0 to 1. Thus, the indicator assumes zero if the lists in the criteria are totally different, having no medicine in common. On the other hand, the closer to 1, the more similar the lists assessed will be.
To define the sum of the total number of drugs present in two PIM criteria subtracted from the intersection between two criteria, the variable Є was established, as described below:
where:
ϵ = Discordance between two lists;
I= Agreement indicator between criteria a and b;
i(La, Lb) = Intersection between the criteria for PIM’s a (La) and b (Lb);
La = Number of drugs present in the criterion a;
Lb = Number of drugs present in the criterion b.
4th Stage – Determination of PIM Use in Older Adults in the Lists Selected in Step 1 with the Possibility of Being Included in BCPIM/2016
We verified how many and which were the drugs commercialized in Brazil that were concurrently included in the other lists studied and were not part of BCPIM/2016. We consulted the website of the National Health Surveillance Agency (ANVISA) to find out which of these drugs were marketed in the country.24
5th Stage – Criteria for Selection of Specialists, Adapted Ferhing Scale
With the analysis of PIM absences in BCPIM/2016, the casuistry was questioned and the etiology of these absences was investigated according to the experts’ knowledge. Then, experts from different regions of the country were sought, based on curriculum analysis, in a research on the National Council for Scientific and Technological Development - CNPq website. Those who reached a score of >5 were eligible according to the Fehring scale adapted.25 After accepting to participate voluntarily to the study, they answered all research-related forms. The exclusion criteria of this group of experts were not to reaching a score >5, according to the adapted Fehring scale,25 or failing to answer any of the forms.
6th Stage – Application of Method Delphi for Content Validation Regarding the Reasons for Non-Inclusion of MPI Use in Older Adult at BCPIM/2016
Two questionnaires were prepared: the first to investigate the experts’ sociodemographic profile; and, the second, containing a list of PIMs that were not included in BCPIM/2016, even though they were commercialized in Brazil and were part of any of the lists investigated. This instrument was applied through an interactive process in Google forms, containing a semi-structured questionnaire and the Informed Consent Form, according to the modified Delphi technique26 This technique recommends obtaining the greatest consensus in a group of people, carefully selected, on a given topic and can occur in several rounds.26 In our study, two rounds were carried out.
The first question was intended to consult experts about their inclusion or not in BCPIM/2016. In the second round, experts were asked to justify the drugs that they choose to not include in the consensus. After obtaining these justifications, a content analysis was carried out, defined by Rocha as a set of investigation techniques in the study of communication analysis, to understand the objectives of prescription practices in the field of science, choosing directions that ensure legitimacy, leading us to observe an assumption, a conception of science, without losing its heterogeneity,27 in order to reflect why the questioned drugs are not BCPIM/2016.19 According to the articulation of these elements, which characterize the content analysis approach the meaning production refers to a deduction, that is, to reach a significance about the non-inclusion of these drugs. In Content Analysis, the answers written means the subject’s expression, where the research seeks to categorize the units of text that are repeated, inferring an expression that represents them.29 For that, we categorized the answers given by the specialists and counted the repetitions of the same.
Ethical Aspects
This research was approved by the Research Ethics Committee of the Faculty of Ceilândia at the University of Brasília, under protocol number 3.317.495.
Statistical Analysis
To perform the data analysis, we used the software Statistical Package for the Social Sciences (SPSS), version 22. The categorical variables data were presented in absolute and/or relative frequencies. For discrimination of comparison measures, the relative prevalence were observed.
Results
Eighteen specialists agreed to participate in the study, being: eight geriatricians, six clinical pharmacists and four nurses. Regarding the degree, ten were specialists, three were masters and four PhD. As for the geographical distribution by residence, three were from the Northeast, four from the Southeast, six from the Midwest and five from the South Brazil.
The Agreement Indicator between BCPIM/2016 and the other criteria established in this study for Potentially Inappropriate Medications for the older adults ranged from 0.2 to 0.3 (Table 1).
 |
Table 1 Indicator of Agreement Between BCPIM/2016 and the Criteria for BEERS/2019, STOPP/START/2015, PRISCUs2010, EU (7)-PIM/2015 |
A total of 337 Potentially Inappropriate Medicines were found for use in the older adults in the five criteria, excluding repetitions. We observed that eight of these drugs were common in all the studied criteria, and, according to Anatomic Therapeutic Chemical (ATC),23 they belong to the nervous system (n=4), cardiovascular system (n=2), digestive and metabolic systems (n=2) classes. The reasons for which they were inserted, as well as precautions for use and therapeutic alternatives are described (Table 2).
We found that a total of 144 drugs, present in at least one of the international criteria surveyed, were not part of the criteria of BCPIM/2016. Of these, 78 drugs were not commercialized in Brazil. Among the 66 drugs marketer in the country that could be included in BCPIM/2016 once they are described in at least one of the other international criteria- 12 have action on the digestive and metabolic system, four on the hematological system, 18 on the cardiovascular system, four on the genital-urinary system and sex hormones, seven in the skeletal muscle system, 18 in the central nervous system and three in the respiratory system (Table 3).
Health experts agreed on a percentage of 60% or more regarding the inclusion of these 66 PIM in BCPIM/2016 (Figure 2).
There was 100% validation among experts regarding the inclusion of 24 of these drugs in the BCPIM/2016 (Table 4).
 |
Table 4 Medicines with 100% Validation by Experts Regarding Their Inclusion in the BCPIM/2016 |
In the content analysis, we stratified seven categories of different justifications most cited for the non-inclusion of PIM in BCPIM/2016, as follows: the low frequency of PIM use among the older adults (n = 5); the prescription could be carried out depending on the clinical condition (n = 25); more scientific evidence would be required for drug inclusion (n = 5); ADR is independent of age (n = 19); the low frequency of RAM (n = 11); present low side effect (n = 8); and it is not a medication for continuous use (n = 5).
Discussion
Worldwide, European and North American lists containing medications potentially inappropriate for the older adults have been used by prescribers to subsidize them in pharmacotherapy.30 This difference in health determinants may explain the fact that the international lists assessed in this work show a convergence of, at most, 30% in relation to BCPIM-2016.
Although BCPIM-2016 was based on the Beers/2012 and STOPP/2006 Criteria, there are 262 discrepant drugs with Beers/2019 and 245 discrepant drugs with STOPP/START/2015. Part of these differences occurred due to the use, in this research, of updated versions of the lists and because few of the PIMs are not marketed in Brazil. These stand out need for constant review of PIM lists.
The ATC (24) Classification System have a proposal consistent with the Brazilian perspective, as in STOPP/START/2015 and Eu (7) PIM/2015. Thus, medicines are divided into different groups according with the organ or system on which they act, paying attention to physical, pharmacological and therapeutic properties. Thus, it is easy to monitor NRM (Negative results associated with the medication), PNRD (Prevention and resolution of negative drugs-associated results) and ADR (Adverse drug reactions). However, although the use of PIM is related to increased morbidity and mortality, the prescription of these drugs in older adults patients remains very common.31
Of the eight drugs that appeared on all the lists in our study, the predominant class was the Central Nervous System, which encompasses the highest percentage of drugs that theoretically cause the most negative results for patients, especially in the older adults.32 Brain aging causes structural and functional changes and there is a compromise in the preservation of brain white matter in senescence.33 This fact justifies the greater risk of delirium, cognitive decline34 and difficulties with postural reflexes in the older adults under the use of medications that have a sedative and anticholinergic action.35,36
Still, regarding the drugs in all the researched lists, we highlight those that are part of the cardiovascular system, such as digoxin and nifedipine,37 as they are associated with the risk of intoxication, ischemia and orthostatic hypotension.38 In the same way, it is possible to mention those drugs that act in the digestive and metabolic system, because they present a risk that the adverse effects become greater than the therapeutic ones in face of the pharmacokinetic alterations that occur in senescence, such as glibenclamide and metoclopramide.39,40
In this study, of the 66 drugs that were at least in one of the international lists surveyed, but were not included in BCPIM/2016, 16 were included in the National List of Essential Medicines-RENAME,40 with no reference to dealing with potentially inappropriate drugs for use in the older adults. A list of essential drugs, materialized through RENAME and the State Relations of Essential Medicines (RESME) and the Municipal Relations of Essential Medicines (REMUME), are guiding lists for the acquisition, prescription and use of medicines, especially in the Unified Health System (SUS).41
Since these lists contain PIM, their prescription and use could increase their risk for this age group. The increased risk of inadequate prescription may compromise the pharmacotherapy directed to the older adults, especially to those with low income, who depend exclusively on the Unified Health System (SUS). There is a need, therefore, to use evidence-based criteria for the elaboration of summaries with a lower percentage of PIM for the older adults in these essential drug lists.
When consulted, the experts unanimously agreed that of the 66 drugs on at least one of the international lists, 24 of them should be present at BCPIM/2016. It is important to highlight that the lowest percentage of agreement among the specialists about include the 66 drugs in the BCPIM/2016 was 66.7%.
Fiber supplements were the ones that obtained the lowest validation percentage among specialists for inclusion in CBPIM/2016 and, among the justifications, is that older adults patients often have constipation, and fiber is well indicated and can assist in the proper intestinal functioning, causing no potential damage. These factors are corroborated by the literature.42 Insoluble fibers have limited fermentation in the large intestine and are not soluble in water, which leads to an increase in the feces volume and activate the release of hormones involved in food intake regulation in the intestine.43 However, for fiber supplements, mentioned in the Stopp/Start criterion,44 they recommend discontinuity in case of prophylaxis, once soluble fibers are viscous and easily fermentable in the large intestine, which can delay gastric emptying and affect the secretion and insulin action.45
Adapting a list of medications to the national reality has unprecedented significance, as it makes possible to analyze the pharmacotherapy risks for the older adults in real time. Thus, after the experts’ analysis, we found that the number of drugs belonging to the classes of Cardiovascular System (n = 7) and Digestive and Metabolic Systems (n = 6) was higher compared with other classes when the agreement to be part of BCPIM/2016 was 100%. Experts reported that cardiovascular drugs can cause bradycardia, orthostatic hypotension, respiratory depression or its exacerbation, cognitive decline, and risk of falls. In the digestive and metabolic system experts claimed that the drugs can increase intestinal motility, aggravate intestinal dysfunction, and increase the risk of hypoglycemia, abdominal pain, dizziness, headaches and peripheral changes. All these findings are corroborated for the scientific literature.46
As for the drugs that act on the skeletal muscle system, the experts’ suggestions for inclusion in BCPIM/2016 cause adverse reactions including risk of bleeding, ulcerations or gastrointestinal perforations, severe adverse effects, therapeutic insecurity, blood dyscrasia, bone marrow depression, and situations corroborated by scientific evidence.47
Those who act on the respiratory system, experts emphasized that many antihistamines, with or without prescription, have potent anticholinergic properties, claiming that there are non-anticholinergic drugs as an option for older adults patients. Studies have shown that inhaled corticosteroids (IC) at low doses can produce good results, have few adverse systemic effects and are safe in the older adults, showing significantly positive changes in airway inflammation.48
Conclusion
This study aimed to contribute to the older adults healthcare by determining the PIM in the international criteria most cited in the literature compared to CBPIM/2016.
Drugs that are absent from the Brazilian list, but included in international criteria, can provide health professionals with a better evaluation of the risks and benefits when prescribing PIM in the older adults, identifying the causes of the PIM adverse effects, and seeking pharmacotherapeutic options for this age population. Thus, it will be possible to contribute to the prescription and responsible use of medicines by the Brazilian population of older adults, as well as to help other researchers to update the criteria of their own countries by sharing the methodology used in the present study.
The results allowed to reflect on the relevance of considering the specificities of pharmacotherapy for the older adults and the need for constant review of CBPIM/2016 in view of the knowledge generated by the research. Thus, we verified the possibility of expanding the Brazilian list by at least 24 PIM distributed in the several human body systems according with the unanimous consensus of specialists.
However, the clinical decision is the prerogative of the prescriber who, in agreement with the patient, defines the best therapy, respecting the individual response of each patient, as well as the various variables that can influence clinical outcomes. Thus, clinical judgment is fundamental in individualizing the medical prescription, according to the patient’s circumstances and treatment objectives.
We emphasize the importance that should be attributed to the health professional when making the prescription, assessing the risks and benefits for the older adults population. This knowledge that medication can bring to the patient must be very clear and having an appropriate list for the older adults Brazilian population is essential to health.
Acknowledgments
This work received support from the research’s members of University of Aging/University of Brasilia (UniSER/UnB), from Education and Human Aging Institute (IEEH) and from the research group – Human aging determinants from National Council for Scientific and Technological Development – CNPQ. We also thank Mr. Mateus de Castro for his contribution in the development of the third stage of this work.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Oliveira GSRD, Bressan L, Balarini F. Direct and indirect assessment of functional abilities in patients with Parkinson’s disease transitioning to dementia. Dement Neuropsychol. 2020;14(2):171–177. doi:10.1590/1980-57642020dn14-020011
2. Moreira FSM, Jerez-Roig J, Ferreira LM, et al. Use of potentially inappropriate medications in institutionalized elderly: prevalence and associated factors. Ciencia e Saude Coletiva. 2020;25:2073–2082. doi:10.1590/1413-81232020256.26752018
3. Stahl S, Boaventura AP. Polypharmacy in elderly hospitalized in the intensive care unit of a university hospital. VITTALLE-Revista de Ciências da Saúde. 2020;2:88–95.
4. Chowdhury TP, Starr R, Brennan M. A quality improvement initiative to improve medication management in an acute care for elders program through integration of a clinical pharmacist. J Pharm Pract. 2020;33(1):55–62. doi:10.1177/0897190018786618
5. Al-Azayzih A, Alamoori R, Ltawalbeh SMA. Potentially inappropriate medications prescribing according to Beers criteria among elderly outpatients in Jordan: a cross sectional study. Pharm Pract (Granada). 2019;17(2):1439. doi:10.18549/PharmPract.2019.2.1439
6. Chiapella LC, Montemarani Menna J, Marzi M, et al. Prevalence of potentially inappropriate medications in older adults in Argentina using Beers criteria and the IFAsPIAM list. Int J Clin Pharm. 2019;41:913–919. doi:10.1007/s11096-019-00858-8
7. Fialová D, Laffon B, Marinković V, et al. Medication use in older patients and age-blind approach: narrative literature review (insufficient evidence on the efficacy and safety of drugs in older age, frequent use of PIMs and polypharmacy, and underuse of highly beneficial nonpharmacological strat). Eur J Clin Pharmacol. 2019;75:451–466. doi:10.1007/s00228-018-2603-5
8. Miller MG, Kneuss TG, Patel JN, Armida G, Parala-Metz DEH. Identifying potentially inappropriate medication (PIM) use in geriatric oncology. J Geriatr Oncol. 2020;12:
9. Hanlon JT, Semla TP, Schmader KE, et al. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18. doi:10.1111/jgs.13807
10. Chandrasekhar D, Samjas M, Pattani D. Evaluation of potentially inappropriate medications among hospitalized geriatric patients in tertiary care referral hospital using STOPP/START criteria. Clin Epidemiol Glob Health. 2019;7:268–273. doi:10.1016/j.cegh.2018.10.008
11. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 updated AGS Beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694.
12. O’mahony D, O’sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi:10.1093/ageing/afu145
13. Lavan AH, Gallagher P, Parsons C, et al. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46:600–607.
14. Holt S, Schmiedl S, Thürmann PA. Potenziell inadäquate medikation für ältere menschen: die PRISCUS-liste. [Potentially inappropriate medication for the elderly: the PRISCUS list]. Dtsch Arztebl. 2010;107:543–551.
15. Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71:861–875. doi:10.1007/s00228-015-1860-9
16. Pazan F, Weiss C, Wehling M, et al. The EURO-FORTA (Fit fOR The Aged) list: international consensus validation of a clinical tool for improved drug treatment in older people. Drugs Aging. 2018;35:61–71. doi:10.1007/s40266-017-0514-2
17. Chang C, Lai HY, Hwang SJ, et al. The updated PIM-Taiwan criteria: a list of potentially inappropriate medications in older people. Ther Adv Chronic Dis. 2019;10:1–21. doi:10.1177/2040622319879602
18. Naugler CT, Brymer C, Stolee ZAA, Arcese ZA. Development and validation of an improving prescribing in the elderly tool. J Can de Pharmacol Clin. 2000;7(2):103–107.
19. Oliveira MG, Amorim WW, Oliveira CB, et al. Brazilian consensus of potentially inappropriate medication for elderly people. Geriatr Gerontol Aging. 2016. doi:10.5327/Z2447-211520161600054
20. Creswell JW. Research Project: Qualitative, Quantitative, and Mixed Methods. SAGE Publication; 2010.
21. Rocha-Filho CR, Cardoso TC, Dewulf NLS. Modified e-Delphi Method: A Guide for Validating Health Assessment Instruments. Brazil Publishing; 2019. doi:10.31012/978-65-5016-268-9
22. Sitta ÉI, Arakawa AM, de Caldana ML, et al. The contribution of cross-sectional studies in the area of language with a focus on aphasia. Revista CEFAC. 2010;12:1059–1066. doi:10.1590/S1516-18462010005000086
23. Zhou JP, Chen L, Guo ZH, et al. IATC-NRAKEL: an efficient multi-label classifier for recognizing anatomical therapeutic chemical classes of drugs. Bioinformatics. 2020;36:1391–1396. doi:10.1093/bioinformatics/btaa166
24. Brazilian Health Regulatory Agenc. ANVISA - Agência Nacional de Vigilância Sanitária. Available from: https://www.gov.br/anvisa/pt-br.
25. Fehring RJ. Methods to validate nursing diagnoses. Heart Lung. 1987;16:625–629.
26. Rowe G, Wright G. The Delphi technique as a forecasting tool: issues and analysis. Int J Forecast. 1999;15:353–375. doi:10.1016/S0169-2070(99)00018-7
27. Rocha D, Deusdará B. Content analysis and discourse analysis: approximation and departures in the (re) construction of a trajectory. ALEA. 2005;7:305–322. doi:10.1590/S1517-106X2005000200010
28. Davidoff AJ, Miller GE, Sarpong EM, et al. Prevalence of potentially inappropriate medication use in older adults using the 2012 beers criteria. J Am Geriatr Soc. 2015;63:486–500. doi:10.1111/jgs.13320
29. Miller GE, Sarpong EM, Davidoff AJ, et al. Determinants of potentially inappropriate medication use among community-dwelling older adults. Health Serv Res. 2017;52:1534–1549. doi:10.1111/1475-6773.12562
30. Lopez-Rodriguez JA, Rogero-Blanco E, Aza-Pascual-Salcedo M, et al. Potentially inappropriate prescriptions according to explicit and implicit criteria in patients with multimorbidity and polypharmacy. MULTIPAP: a cross-sectional study. PLoS One. 2020;15:e0237186. doi:10.1371/journal.pone.0237186
31. Matanović SM, Vlahovic-Palcevski V. Potentially inappropriate medications in the elderly: a comprehensive protocol. Eur J Clin Pharmacol. 2012;68:1123–1138. doi:10.1007/s00228-012-1238-1
32. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. doi:10.1080/03602530902722679
33. Luo N, Sui J, Abrol A, et al. Age-related structural and functional variations in 5967 individuals across the adult lifespan. Hum Brain Mapp. 2020;41:1725–1737. doi:10.1002/hbm.24905
34. Forgerini M, Schiavo G, Lucchetta RC, et al. Drug interactions for elderly people with mental and behavioral disorders: a systematic scoping review. Arch Gerontol Geriatr. 2020;93:104283. doi:10.1016/j.archger.2020.104283
35. Motter FR, Fritzen JS, Hilmer SN, et al. Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria. Eur J Clin Pharmacol. 2018;74:679–700. doi:10.1007/s00228-018-2446-0
36. Lavareda Baixinho C, Dos Anjos Dixe M, Madeira C, et al. Falls in institutionalized elderly with and without cognitive decline A study of some factors. Dement Neuropsychol. 2019;13:116–121. doi:10.1590/1980-57642018dn13-010014
37. Ivanova I, Elseviers M, Wauters M, et al. European repository of explicit criteria of potentially inappropriate medications in old age. Geriatr Gerontol Int. 2018;18:1293–1297. doi:10.1111/ggi.13331
38. Oliveira J, Firmo A, Peixoto SV, et al. Health behaviors and hypertension control: the results of ELSI-BRASIL Comportamentos em saúde e o controle da hipertensão arterial: resultados do ELSI-BRASIL Comportamientos de salud y control de la hipertensión arterial: resultados de ELSI-BRASIL. Cadernos de saude publica. 2019. doi:10.1590/0102-311X00091018
39. Oliveira ALM, Nascimento MM, Castro-Costa É, et al.Study of trend in the use of benzodiazepines among elderly residents in the municipality of Bambuí, Minas Gerais. Revista Brasileira de Epidemiologia. 2020. doi:10.1590/1980-549720200029
40. BRASIL. Ministério da Saúde. Secretaria de Ciência Tecnologia Inovação e Insumos em saúde. Departamento de Assistência Farmacêutica e Insumos Estratégicos. Ministry of Health. Secretariat of Science, Technology, Innovation and Health Supplies. Pharmaceutical Assistance and Strategic Inputs Department. National List of Essential Medicines - RENAME 2020 within the scope of the Unified Health System (SUS). 2020.
41. Brazilian Health Regulatory Agency. ANVISA. Conhecer a Relação de Medicamentos Essencias do SUS — Português (Brasil). [To Know the Essential Drug List of Health Universal System]. Available from: https://www.gov.br/pt-br/servicos/conhecer-a-relacao-de-medicamentos-essencias-do-sus.
42. Moraes Ferreira-Nunes P, Justina Papini S, Eduardo Corrente J. Eating patterns and nutrient intake for older people: analysis with different methodological approaches. Ciencia Saude Coletiva. 2018. doi:10.1590/1413-812320182312.28552016
43. Maria da Silva GI, Bronzi Durante ÉI, de Assumpção DI, et al. High prevalence of inadequate dietary fiber consumption and associated factors in older adults: a population-based study Elevada prevalência de inadequação do consumo de fibras alimentares em idosos e fatores associados: um estudo de base populacional. Revista Brasileira de Epidemiologia. 2019. doi:10.1590/1980-549720190044
44. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857–866. doi:10.2147/CIA.S80280
45. Muhlack DC, Hoppe LK, Saum KU, et al. Investigation of a possible association of potentially inappropriate medication for older adults and frailty in a prospective cohort study from Germany. Age Ageing. 2019;49:20–25. doi:10.1093/ageing/afz127
46. Muhlack DC, Hoppe LK, Stock C, et al. The associations of geriatric syndromes and other patient characteristics with the current and future use of potentially inappropriate medications in a large cohort study. Eur J Clin Pharmacol. 2018;74:1633–1644. doi:10.1007/s00228-018-2534-1
47. Akkawi ME, Mohamed MHN. Influence of hospitalization on potentially inappropriate prescribing among elderly patients in a Malaysian community. Trop J Pharma Res. 2018;17:151–160. doi:10.4314/tjpr.v17i1.21
48. Braman SS. Asthma in the elderly. Clin Geriatr Med. 2003;19:57–75. doi:10.1016/S0749-0690(02)00052-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.