Back to Journals » Clinical Ophthalmology » Volume 15
Incidence and Risk Factors of Ocular Hypertension/Glaucoma After Descemet Stripping Automated Endothelial Keratoplasty
Authors Elalfy M, Maqsood S , Soliman S, Hegazy SM , Hannon AA , Gatzioufas Z, Lake D, Hamada S
Received 25 December 2020
Accepted for publication 15 April 2021
Published 25 May 2021 Volume 2021:15 Pages 2179—2188
DOI https://doi.org/10.2147/OPTH.S299098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohamed Elalfy, 1– 3,* Sundas Maqsood, 1,* Shady Soliman, 3 Sherif Momtaz Hegazy, 3 Ahmed Abdou Hannon, 3 Zisis Gatzioufas, 1, 4 Damian Lake, 1 Samer Hamada 1
1Corneo Plastic Unit, Queen Victoria Hospital, East Grinstead, UK; 2Eye Department, Maidstone and Turnbridge Wells Hospital, Maidstone, UK; 3Research Institute of Ophthalmology, Giza, Egypt; 4Eye Department, University Hospital Basel, Basel, Switzerland
*These authors contributed equally to this work
Correspondence: Mohamed Elalfy
Queen Victoria Hospital, Holtye Road, East Grinstead, West Sussex, RH19 3DZ, UK
Tel +44 01342414560
Email [email protected]
Purpose: To evaluate the incidence, demographics, associated risk factors, management and clinical outcomes of ocular hypertension/glaucoma after Descemet stripping automated endothelial keratoplasty (DSAEK).
Methods: A cohort review of 81 DSAEK cases was performed at Queen Victoria Hospital, United Kingdom. Patients with pre-existing glaucoma, transient increased IOP within the first 48 hours post-graft, additional post-transplant surgery, or failed to complete one year follow-up were excluded from the study. Ocular hypertension was defined as intraocular pressure (IOP) elevation > 21mmHg or ≥ 6mmHg from baseline at any postoperative visit. The study looked at the incidence, risk factors, response to anti-glaucoma treatment, graft failure and best corrected visual acuity.
Results: The incidence of post-DSAEK ocular hypertension and glaucoma was 51.9% and 13.6%, respectively. Steroid-induced IOP elevation was the most frequent cause, with an incidence of 38.3%. Risk factors such as pseudophakia (p=0.024) and preoperative IOP> 16 (p=0.003) were found to be associated with post-DSAEK ocular hypertension. Preoperative IOP> 16 had 5.27 times risk of IOP elevation. Eyes with graft dislocation and/or detachment were significantly associated with post-DSAEK glaucoma (p=0.038). There was no negative effect of OHT on visual acuity and graft status.
Conclusion: Glaucoma and OHT are common postoperative complications of DSAEK. Although steroid-induced IOP elevation was the most frequent cause, there are other reasons associated with development of post-DSAEK glaucoma, including graft dislocation and detachment. Eyes with preoperative IOP> 16 mm Hg may require a close monitoring of IOP. In addition, management by medical treatment results in good visual acuity and graft clarity.
Keywords: Descemet’s stripping endothelial keratoplasty, DSAEK, glaucoma, ocular hypertension
Introduction
Corneal transplantation is one of the most frequently performed transplant surgeries in humans.1 The 2019–2020 NHSBT report confirmed the number of total corneal transplants performed in the UK was 4504 (1486 DSAEK operations) compared to total organ transplants of 4140.2 Despite its high success rate, there are several recognized complications. For example, poor graft centration and damage to surrounding ocular structures can occur intra-operatively; while complications such as infection, wound dehiscence, graft failure, graft rejection and ocular hypertension (OHT) can occur postoperatively.1
Over the past decade, partial thickness corneal transplant procedures have been developed, such as deep anterior lamellar keratoplasty (DALK), Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK).1,3 DSAEK has become a favorable procedure over traditional penetrating keratoplasty (PKP) due to its rapid healing, faster visual recovery, minimal refractive change, less risk of graft rejection and better corneal integrity.3,4 It is often chosen in patients with endothelial corneal disease to replace the damaged corneal endothelium and Descemet membrane.5 Compared to PKP, early reports showed less IOP changes after DSAEK;5 the incidence of post-DSAEK OHT was reported between 0% to 18%.5–8 Despite being the most frequent postoperative complication, the incidence rate of OHT after DSAEK has not been clearly established.
Ocular hypertension is the most significant risk factor following endothelial transplants that can lead to glaucoma. This can pose serious impact on patients due to its difficult diagnosis, monitoring for progression, complexity of management and the risk of compromising vision in the presence of other corneal comorbidities.9,10 It may lead to irreversible visual loss by damaging optic nerve11 and donor corneal endothelium, leading to graft failure.12
Recent DSAEK studies have been largely focused on IOP elevation in eyes with pre-existing glaucoma and Fuch’s endothelial dystrophy, with limited data on the risk factors in secondary glaucoma development, or pseudophakic bullous keratopathy.13 There is also lack of data in current literature on the efficacy of different treatment regimens;14 and this can often be due to the absence of postoperative management protocols and physicians’ tailored treatment plans.3
Therefore, the aim of this work is to describe incidence, demographics, associated risk factors, management and clinical outcomes of OHT or glaucoma after DSAEK.
Methods
A review of case records of 120 DSAEK cases performed by two experienced surgeons using standardized surgical technique and postoperative regime. All DSAEK procedures were performed at the tertiary center Queen Victoria Hospital, United Kingdom. Cases were identified from eye bank records according to diagnosis codes. All patient notes reviewed had a minimum of one year follow up. The study was registered with local institutional review board and adhered to the tenets of the Declaration of Helsinki.
Ocular hypertension was defined as raised IOP of ≥ 21mmHg14 or an increase in IOP from preoperative value ≥ 6mmHg at any post-operative examination. All eyes with postoperative elevated IOP were then categorized into: steroid responders defined as eyes in which the normalized IOP was ≤21mmHg or back to preoperative levels (if was included due to raised IOP ≥ 6mmHg) when the steroid treatment ended,14 and post-DSAEK glaucoma defined as documented increase in the cup-to-disc (CD) ratio of ≥0.7 compared to pre-operative appearance and/or glaucomatous visual field defect at any postoperative visits that was not present before surgery.
Patient who had prior history of glaucoma, transient increased IOP within the first 48 hours post-graft, additional post-transplant surgery, or failed to complete one year follow-up were excluded from the study.
The clinical data collected preoperatively for each patient included age, sex, ethnicity, other relevant past ocular history including family history, uncorrected distance visual acuity (UDVA), distance corrective visual acuity (CDVA), central corneal thickness (CCT), risk factors for glaucoma and indication for surgery. Details of surgery including additional procedures or intraoperative complication were also collected.
Post-operative complications and management, potency and dosage of steroid use and IOP measurements were reviewed and recorded. With regards to IOP elevation, the following data was also noted: the timing of postoperative ocular hypertensive events, IOP management including change in potency of steroid drops and additional glaucoma medication, development of characteristic glaucomatous optic disc and visual field changes, further surgery for glaucoma and date of controlled IOP. Last appointment details were also reviewed for each patient for last follow up date, post-operative UDVA, post-operative CDVA, episodes of graft rejection and failure, ongoing glaucoma medication, measurement of IOP, CCT, optic disc appearance and visual fields. Intraocular pressure measurements were obtained by Goldmann applanation tonometry.
Surgical Technique
All cases were performed under sub tenon blocks, with patients receiving additional sedation or general anesthesia according to patient indications. Descemet’s membrane was stripped up to 9mm diameter with AC maintainer in place. An 8.5mm pre-cut donor graft tissue of 80–100 microns thickness was transplanted through a 4.5mm wound using Busin glide and forceps and two paracentesis in place. The graft was secured firstly with a full air fill. The air was left in the AC for 10 mins while the dilating drops were instilled (tropicamide 1%, phenylephrine 2.5% and cyclopentolate 1%). After 10 mins the air was released down to 90% of anterior chamber filling by introducing intracameral cefuroxime (0.1mL/1mg). One 10/0 nylon suture was placed at the main incision. Subconjunctival dexamethasone (0.4mL - 4mg/mL) and cefuroxime (0.1mL/1mg) injected as the last step of the procedure. Patients were reviewed 60mins following procedure for further air release down to 60–70% if IOP of >25mmHg. All patients had oral acetazolamide 500mg stat, topical cyclopentolate 1% three times a day for a week and dexamethasone 1% for 3 months and chloramphenicol 0.5% four times a day for four weeks.
All data were entered into a spreadsheet, and statistical analysis was performed with SPSS 22.0 for Windows (SPSS, Chicago, IL). Descriptive statistics were expressed as range (mean ± standard deviation). Snellen visual acuities were converted to the logarithm of the minimum angle of resolution (LogMar) for statistical analysis. Univariate analysis included Chi-square test or Fisher’s exact test in categorical comparisons. Logistic regression was also performed to analyze the statistically significant variables in the univariate analysis. Differences were considered statistically significant when P values were less than 0.05.
Results
On the basis of the inclusion and exclusion criteria, 81 eyes qualified for the study. All participants were Caucasians, 32 (39.5%) were males and 49 (60.5%) were females. Age ranged between 41 to 94 years (77±10.365 years). The average follow-up period was 27±13.71 months, with a range of 12 to 66 months.
Table 1 summarizes the demographic data and baseline characteristics including the main indications for corneal transplantation, graft status and medical comorbidities. Other relevant history, such as family history of glaucoma was found in 3 patients (3.3%).
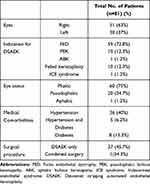 |
Table 1 Results of Demographics and Baseline Characteristics |
More than half of the cohort underwent DSAEK with additional surgical procedures: 38 eyes (86.3%) had cataract surgery, 3 eyes (6.8%) had anterior vitrectomies with IOL exchange, 2 eyes (4.5%) had IOL exchange and 1 eye (2.3%) had anterior chamber (AC) tap. The mean preoperative UDVA and CDVA were 1.0 ± 0.58 logMAR units and 0.7±0.66 logMAR units respectively. The average preoperative IOP was 15.05 ± 3.542 mmHg in a range of 6 to 26 mmHg. The mean pre-graft and post-graft CCT was 674.97± 97.61 μm and 667.97±85.76 μm respectively.
None of the patients developed any intra-operative complications. Twenty patients (24.7%) developed postoperative complications: 13 (16%) eyes had DSAEK detachment (partial), 5 (6%) eyes had graft dislocation and 2 (2.5%) eyes developed microbial keratitis. Graft detachment and dislocation were successfully reattached and repositioned in all complicated cases.
Table 2 shows the incidence of OHT and post-DSAEK glaucoma. Forty-two patients (51.9%) developed post-DSAEK IOP elevation between one week and 32 months (6.5 ±6.83 months) postoperatively. The average IOP at time of diagnosis of OHT was 26 ± 7.79 mmHg, ranging between 22 to 59 mmHg. All ocular hypertensive eyes were treated medically within one week to 40 months (5.61± 7.13), and follow up was lost in 2 patients. The first step of OHT management was either tapering of Dexamethasone or switch to a less potent steroid; switch was made either to Loteprednol (if diagnosed before 6 months post-operative period) or Fluorometholone (if diagnosed after 6 months post-operative period). The incidence of steroid-induced raised IOP was 16 eyes (19.8%). The mean daily frequency of Dexamethasone 0.1% at the time of IOP elevation was four times per day. The time it took to develop steroid-induced OHT ranged from one week to 16.03 months (5.7 ± 4.04 months) postoperatively. The mean of steroid-induced IOP elevation was 28 ± 6.20 mmHg, (range of 22 to 52 mmHg). The incidence of OHT only where ocular hypertensive eyes neither grouped into steroid responders nor post-DSAEK glaucoma was 18.5%.
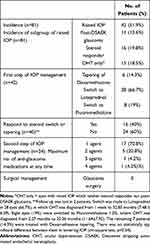 |
Table 2 Management of Post-Operative Intraocular Pressure |
The incidence of post-DSAEK glaucoma was 11 (13.6%) eyes, occurring from 6.5 weeks to 32.85 months postoperatively (mean 9.37 ± 9.68 months). In this group, IOP ranged from 22 to 59 mmHg (31.09±11.21mmHg). In 11 eyes of post-DSAEK glaucoma, IOP were controlled medically from one week to 20 months (5.19±5.38 months). None of the patients required glaucoma surgery to control IOP.
After the initial management, a total of 16 eyes (19.8%) were identified to be steroid responder where their IOP normalized with steroid change or tapering, meeting our inclusion criteria. IOP in steroid responders was controlled from 3 weeks to 20 months (6.56±5.47 months). The remaining 24 ocular hypertensive eyes (60%) that did not respond to steroid, required second stage of treatment by using anti-glaucoma medications to control IOP, and the maximum number of medications used were detailed in Table 2. As expected, there was a positive correlation between the amount of IOP drop and the number of anti-glaucoma medication used (P<0.0001, ANOVA). Among the whole cohort, 17 eyes (21%) were still using anti-glaucoma medications at the last follow up.
Univariate analysis was performed to identify individual risk factors for post-DSAEK OHT, details as shown in Table 3, Table 4. In this analysis, age, sex, the indications for keratoplasty, combined surgery, medical comorbidities and family history of glaucoma were not found to be significant risk factors for post-operative OHT (chi-square test, p values 0.52 to 1.00). In contrast, the following factors were found to be significant risk factors for IOP elevation after DSAEK, preoperative IOP>16 mmHg (p=0.003) and pseudophakia (p=0.024).
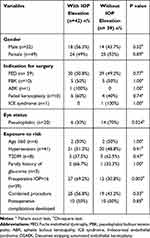 |
Table 3 Comparison of Demographic Characteristics, IOP and Graft Status Between Patients with and without IOP Elevation After DSAEK |
 |
Table 4 Comparison of CDVA Between Eyes with and without OHT |
Significant risk factors, ie, preoperative IOP>16 mmHg and pseudophakia were then subjected to logistic regression analysis, only preoperative IOP>16 [OR 5.27; 95% CI, 1.89–13.64; p=0.001] was found to be associated with postoperative OHT with 5.27 times risk. In contrast, the odds ratio for pseudophakia [OR 0.3; 95% CI, 0.1–0.88; p=0.028] was less than one, which had less risk of developing post-DSAEK OHT when compared to preoperative IOP>16 mmHg.
With regards to post-DSAEK glaucoma, eyes that developed postoperative complication such as graft dislocation and/or detachment were found to be statistically significant associated with post-DSAEK glaucoma (Fisher’s exact test, p=0.038).
Preoperative UDVA ranged from less than 6/60 to 6/9.5, (1.07 ± 0.59 logMAR units) (Table 4). Paired sample t-test revealed that there was an improvement from preoperative (1.07 ± 0.59 logMAR units) to postoperative UDVA (0.63, ± 0.56 logMAR units) (p<0.0001). Details of UDVA and CDVA are presented in Figure 1 and comparison of CDVA in eyes with and without OHT is shown in Figure 2.
 |
Figure 1 Percentage comparison of preoperative and post-operative distance corrected visual acuity (CDVA) and uncorrected distance visual acuity (UDVA). |
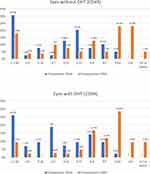 |
Figure 2 Comparison of distance corrected visual acuity (CDVA) between eyes with and without ocular hypertension (OHT). |
None of the patients developed any intra-operative complications. Twenty patients (24.7%) developed postoperative complications: 13 (16%) eyes had DSAEK detachment, 5 (6%) eyes had graft dislocation and 2 (2.5%) eyes developed microbial keratitis. Graft detachment and dislocation were successfully reattached and repositioned in all complicated cases.
We reported on graft status at the last follow up among the whole cohort and in ocular hypertensive eyes. Episodes of graft rejection occurred in 3 eyes (3.7%), and in 6 eyes (7.4%) corneal graft has failed. In eyes with IOP elevation, there were 2 (4.8%) graft failures and none developed graft rejection. All eyes diagnosed with post-DSAEK glaucoma achieved clinically significant better CDVA (p<0.001) and a clear graft at last follow up visit.
Discussion
To date, the incidence of OHT or glaucoma after DSAEK has been inconsistent in the literature, this is because of the heterogeneity in the definition of OHT, inclusion criteria, follow-up duration, and steroid regime. This study not only evaluate the incidence of elevated IOP after DSAEK but also the incidence of post-DSAEK glaucoma, the associated risk factors, management and clinical outcomes of OHT after DSAEK.
Elevated IOP and glaucoma are the most frequent postoperative complications after both PKP and DSAEK.10,15–18 Sharma and associates demonstrated that post procedure IOP control requiring treatment after PKP is significantly difficult compared to post DSAEK patients.19 Compared to the above mentioned studies, we found a higher percentage of IOP elevation after DSAEK of 51.9%; in addition, it was even higher than eyes with preexisting glaucoma after DSAEK, which is reported to be 43% to 45%.18 However, our finding is comparable to reported incidence of 51% OHT in the study including cases with and without preexisting glaucoma.17 The inconsistency of findings on incidence could be a result of the difference in definition of OHT; if we used IOP greater than or equal to 24mmHg as a cutoff value, as defined in Vajaranant and associates, the incidence would be 36 out of 81 eyes (44.4%).
For PKP, corneal disease,20 BPK, and trauma have been reported as high risk factors for OHT.21,22 Our study confirms that such surgical indications do not influence the risk of IOP elevation after DSAEK, which is in agreement with previous reported literature.21,23
A majority of studies have been done to compare the incidence of OHT after DSAEK between non-preexisting and preexisting glaucoma eyes; consistent results supported that preexisting glaucoma predicts IOP elevation after DSAEK,3,14,17,18 which we eliminated in our study. The risk of OHT has been reported to be increased in eyes with goniosynechiolysis, postoperative procedures and complications, age<60 years and glaucoma in fellow eye with 3.3, 2.9, 2.4 and 3.2 times respectively.13 In this work, we excluded cases with additional postoperative procedures, and we did not find age<60 to be a risk factor of postoperative IOP elevation.
Our results agreed with Ozeki et al24 finding that combined surgery was not associated with OHT after DSAEK, although there was a significant difference between pseudophakic and phakic eyes in IOP elevation after DSAEK (p=0.024). On the other hand, another significant risk factor in this study was preoperative IOP>16mmHg (p=0.003), which had 5.27 times risk of developing OHT after DSAEK. With respect to the time of onset of OHT after DSAEK, we found the average time period of postoperative 6.5 months, comparable to reported literature.13
There are a few proposed causes of increased IOP after DSAEK, including the use of topical steroids, retained viscoelastics, inflammation, peripheral anterior synechiae, iatrogenic damage to trabecular meshwork and distortion of the angle.10,23,25 These causes are responsible for IOP elevation at different postoperative time periods; and this study excluded any cases with immediate postoperative IOP elevation, such as pupillary block glaucoma after DSAEK.
In steroid-induced OHT, Francois reported the time of onset was dependent on the potency of corticosteroid drops.26 We found that all eyes with steroid-induced IOP elevation were on the highest potency of steroid drop, Dexamethasone 0.1%, and the earliest onset of IOP elevation was seen within a week. Steroid-induced IOP elevation could normally be controlled by tapering steroids, changing steroids or glaucoma medication,14 where all steroid-induced OHT eyes were controlled medically on average of 6.5 months in our work.
Recent studies reported that Loteprednol is less likely to cause IOP elevation than Prednisolone acetate in steroid responders.27,28
The management of steroid-induced OHT can be complicated; it is vital to achieve a balance treatment between inflammation and the use of steroid drops in steroid responders, as inflammation itself is also a risk factor for corneal graft failure.27 There is some evidence which shows that the cessation of steroid drops will help return IOP to normal or baseline level within four weeks’ time.29,30 Loteprednol have been used as an alternative rather than tapering steroid to manage IOP elevation in steroid responders. It has also been suggested that Loteprednol possesses adequate anti-inflammatory effects when it is used as a second-line therapy for prophylaxis of graft rejection. However, the long-term effects of Loteprednol still remain unknown.27
Uncontrolled IOP increases the risk of poor visual outcomes and is associated with graft failure after PKP and DSAEK.17,31 This study showed the overall rate of graft failure to be 7.4%, which is within range of reported rates of failure in other DSAEK studies (4–14% at 3 years).13,31 In addition, there was no significant difference in graft failure rates between eyes with or without post-DSAEK glaucoma (p=0.42)
Apart from the heterogeneity in cutoff value for IOP, there is a huge variability in the definition of OHT and glaucoma. In 2009, a report from the American Academy of Ophthalmology (AAO) summarized literatures on DSAEK in that year, concluded that incidence of glaucoma after DSAEK varied between 0% to 15% with 3 to 18 months of follow up in 23 studies.4,13 This study reports the incidence of post-DSAEK glaucoma to be 13.6%, which is slightly higher than the incidence of 11.9% reported previously.14
This is a non-randomized study; although we excluded eyes with preexisting glaucoma as a known risk factor for causing IOP elevation after DSAEK, it is possible that some patients with undiagnosed OHT were enrolled in the study. In addition, more participants would make the results more representative. In terms of measuring visual acuity outcome after DSAEK, postoperative UDVA and CDVA were not recorded at fixed time-points which could be useful to compare the trend of changes in visual acuity between eyes with and without postoperative OHT throughout postoperative period. Because of the added thickness of the donor graft, the central corneal thickness after DSAEK would increase compromising the accuracy of IOP measurements. Clemmensen and associates have demonstrated that this increase in corneal thickness does not affect corneal rigidity and thus bear no relationship with IOP measurements in post DSAEK patients.32 A good correlation when measuring IOPs by three different devices (i Care, Pascal dynamic contour tonometer, GAT) in post DSAEK patients has also been reported.33
Despite the above limitations, the study is one of the few study to presents both the incidence of OHT and glaucoma after DSAEK and the associated risk factors. Eyes with pre-existing glaucoma and OHT needs close monitoring post DSAEK as they are at higher risk of developing IOP elevation.
Abbreviations
ABK, aphakic bullous keratopathy; AC Tap, anterior chamber tap; CDVA, distance corrected visual acuity; CCT, central corneal thickness; CD ratio, cup-to-disc ratio; DSAEK, Descemet’s stripping automated endothelial keratoplasty; FED, Fuchs endothelial dystrophy; IOP, intraocular pressure; OHT, ocular hypertension; PBK, pseudophakic bullous keratopathy; PKP, penetrating keratoplasty; UDVA, uncorrected distance visual acuity.
Ethics Approval and Informed Consent
The study was approved as prospective audit by local institutional review board at Queen Victoria Hospital, East Grinstead, UK and adhered to the Declaration of Helsinki.
All participants provided informed consent for participation in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boynton G, Woodward M. Evolving techniques in corneal transplantation. Curr Surg Rep. 2015;3(2):2. doi:10.1007/s40137-014-0079-5
2. Activity report NHSBT UK. Organ donation and transplantation activity report 2019/2020; 2020. Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19220/activity-report-2019-2020.pdf.
3. Nguyen P, Khashabi S, Chopra V, et al. Descemet stripping with automated endothelial keratoplasty: a comparative study of outcome in patients with preexisting glaucoma. Saudi J Ophthalmol. 2013;27(2):73–78. doi:10.1016/j.sjopt.2013.02.002
4. Lee WB, Jacobs DS, Musch DC, et al. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818–1830. doi:10.1016/j.ophtha.2009.06.021
5. Espana EM, Robertson ZM, Huang B. Intraocular pressure changes following Descemet’s stripping with endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2010;248(2):237–242. doi:10.1007/s00417-009-1199-y
6. Terry MA. Precut tissue for Descemet stripping automated endothelial keratoplasty: complications are from technique, not tissue. Cornea. 2008;27(6):627–629. doi:10.1097/01.ico.0000611356.66299.c1
7. Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty: a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology. 2008;115(7):1179–1186. doi:10.1016/j.ophtha.2007.09.005
8. Nieuwendaal CP, van der Meulen IJ, Lapid-Gortzak R, Mourits MP. Intraocular pressure after Descemet stripping endothelial keratoplasty (DSEK). Int Ophthalmol. 2013;33(2):147–151. doi:10.1007/s10792-012-9665-7
9. Erdurmus M, Cohen EJ, Yildiz EH, et al. Steroid-induced intraocular pressure elevation or glaucoma after penetrating keratoplasty in patients with keratoconus or Fuchs dystrophy. Cornea. 2009;28(7):759–764. doi:10.1097/ICO.0b013e3181967318
10. Huber KK, Maier AK, Klamann MK, et al. Glaucoma in penetrating keratoplasty: risk factors, management and outcome. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):105–116. doi:10.1007/s00417-012-2065-x
11. Dada T, Aggarwal A, Minudath K, et al. Post-penetrating keratoplasty glaucoma. Indian J Ophthalmol. 2008;56(4):269–277. doi:10.4103/0301-4738.41410
12. Wilson SE, Kaufman HE. Graft failure after penetrating keratoplasty. Surv Ophthalmol. 1990;34:325–356.
13. Chan EW, Wong TT, Htoon HM, et al. De novo ocular hypertension after Descemet stripping endothelial keratoplasty: comparative 3-year incidence, risk factors, and outcomes. Clin Ophthalmol. 2013;7:1829–1841. doi:10.2147/OPTH.S50584
14. Maier AK, Klamann MK, Torun N, et al. Intraocular pressure elevation and post-DSEK glaucoma after Descemet`s stripping endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1191–1198. doi:10.1007/s00417-012-2203-5
15. Olson R, Kaufman H. A mathematical description of causative factors and prevention of elevated intraocular pressure after keratoplasty. Invest Ophthalmol Vis Sci. 1977;16(12):1085–1092.
16. Chien AM, Schmidt CM, Cohen EJ, et al. Glaucoma in the immediate postoperative period after penetrating keratoplasty. Am J Ophthalmol. 1993;115(6):711–714. doi:10.1016/S0002-9394(14)73636-0
17. Allen MB, Lieu P, Mootha VV, et al. Risk factors for intraocular pressure elevation after Descemet stripping automated endothelial keratoplasty. Eye Contact Lens. 2010;36(4):223–227. doi:10.1097/ICL.0b013e3181e6ae30
18. Vajaranant TS, Price MO, Price FW, et al. Visual acuity and intraocular pressure after Descemet’s stripping endothelial keratoplasty in eyes with and without preexisting glaucoma. Ophthalmology. 2009;116(9):1644–1650. doi:10.1016/j.ophtha.2009.05.034
19. Sharma RA, Bursztyn L, Golesic E, et al. Comparison of intra ocular pressures post penetrating keratoplasty vs Descemet’s stripping endothelial keratoplasty. Can J Ophthalmol. 2016;51(1):19–24. doi:10.1016/j.jcjo.2015.09.014
20. Goldberg DB, Schanzlin DJ, Brown SI. Incidence of increased intraocular pressure after keratoplasty. Am J Ophthalmol. 1981;92(3):372–377. doi:10.1016/0002-9394(81)90527-4
21. Karadag O, Kugu S, Erdogan G, et al. Incidence of and risk factors for increased intraocular pressure after penetrating keratoplasty. Cornea. 2010;29(3):278–282. doi:10.1097/ICO.0b013e3181b6eb9e
22. Kirkness CM, Ficker LA. Risk factors for the development of postkeratoplasty glaucoma. Cornea. 1992;11(5):427–432. doi:10.1097/00003226-199209000-00012
23. Al-Mohaimeed M, Al-Shahwan S, Al-Torbak A, Wagoner MD. Escalation of glaucoma therapy after penetrating keratoplasty. Ophthalmology. 2007;114(12):2281–2286. doi:10.1016/j.ophtha.2007.08.043
24. Ozeki N, Yuki K, Shiba D, et al. Intraocular pressure elevation after Descemet’s stripping endothelial keratoplasty. Jpn J Ophthalmol. 2012;56(4):307–311. doi:10.1007/s10384-012-0149-0
25. Sihota R, Sharma N, Panda A, Aggarwal HC, Singh R. Post-penetrating keratoplasty glaucoma: risk factors, management and visual outcome. Aust N Z J Ophthalmol. 1998;26(4):305–309. doi:10.1111/j.1442-9071.1998.tb01334.x
26. Francois J. Corticosteroid glaucoma. Ann Ophthalmol. 1977;9(9):1075–1080.
27. Holland EJ, Djalilian AR, Sanderson JP. Attenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantation. Cornea. 2009;28(10):1139–1143. doi:10.1097/ICO.0b013e3181a3c52f
28. Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9(2):157–165. doi:10.1089/jop.1993.9.157
29. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (Lond). 2006;20(4):407–416. doi:10.1038/sj.eye.6701895
30. Weinreb RN, Polansky JR, Kramer SG, Baxter JD. Acute effects of dexamethasone on intraocular pressure in glaucoma. Invest Ophthalmol Vis Sci. 1985;26(2):170–175.
31. Ang M, Mehta JS, Lim F, et al. Endothelial cell loss and graft survival after Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2012;119(11):2239–2244. doi:10.1016/j.ophtha.2012.06.012
32. Clemmensen K, Hjortdal J. Intaocular pressure and corneal biomechanics in Fuch’s endothelial dystrophy and after posterior lamellar keratoplasty. Acta Ophthalmol. 2014;92(4):350–400. doi:10.1111/aos.12137
33. Achiron A, Blumenfeld O, Avizemer H, et al. Intraocular pressure measurement after DSAEK by i care, Goldman applanation and dynamic contour tonometry: Acomparitive Study. J Fr Ophtalmol. 2016;39(10):822–828. doi:10.1016/j.jfo.2016.09.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
