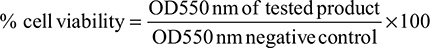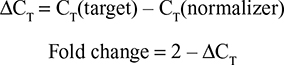Back to Journals » Medical Devices: Evidence and Research » Volume 11
In vitro study of RRS® Silisorg CE Class III medical device composed of silanol: effect on human skin fibroblasts and its clinical use
Authors Deglesne PA, Arroyo R , Fidalgo López J, Sepúlveda L , Ranneva E, Deprez P
Received 2 March 2018
Accepted for publication 24 April 2018
Published 7 September 2018 Volume 2018:11 Pages 313—320
DOI https://doi.org/10.2147/MDER.S167078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
In vitro study of silanol on skin fibroblasts
Views: 1793
Pierre-Antoine Deglesne,* Rodrigo Arroyo,* Javier Fidalgo López,* Lilian Sepúlveda,* Evgeniya Ranneva, Philippe Deprez
Research and Development, Skin Tech Pharma Group, Castelló d’Empúries, Spain
*These authors contributed equally to this work
Introduction: Silanol (organic silicon) has been used for decades in the treatment of skin photoaging as it stabilizes and maintains skin structures through hydrogen bonding electrostatic interaction with extracellular matrix (ECM) proteins or glycosaminoglycans. Organic silicon-based products are often presented as silanol derivatives which are currently associated to other structural molecules such as orthohydroxybenzoate, carboxymethyl theophylline alginate, ascorbate, acetyltyrosine, sodium lactate or mannuronate. Consequently, organic silicon formulations may differ substantially between the medical devices available on the market, which may result in additional effect on the skin. Therefore, there is a real need for a better characterization of the products in terms of their action on human skin and in vitro skin model.
Materials and methods: In this in vitro study, the effect of RRS® Silisorg was analyzed. RRS® Silisorg is a dermal implant (CE Class III medical device) containing monomethylsilanol mannuronate associated to an antioxidant resveratrol. Skin fibroblast viability and capacity to induce the production of key ECM genes were evaluated in the presence of different concentrations of RRS® Silisorg. The key ECM genes selected were collagen type I, elastin and hyaluronan synthase type 2 (HAS2), which is the cellular enzyme responsible for high-molecular weight hyaluronic acid (HA) production. Viability was evaluated through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and expression was quantified by quantitative polymerase chain reaction.
Results: RRS® Silisorg increased fibroblast gene expression of HAS2 in the first 24 hours, 25 times in the presence of 1 mg/mL of solution, followed by a collagen type I gene expression (4.7 times) and elastin expression (2.5 times) increase after 48 hours.
Conclusion: These results demonstrate that the silanol-based medical device RRS® Silisorg sustains HA, collagen and elastin production in human skin fibroblasts in vitro.
Keywords: dermal implant, mesotherapy, hyaluronic acid, collagen, organic silicon, photoaging
Introduction
Silicon is the second most abundant element on earth, after oxygen. Silicon occurs as silicon dioxide (SiO2) or the corresponding silicic acids formed by the hydration of the oxide. Orthosilicic acid (OSA [Si(OH)4]) is the simplest acid and it is accepted as being the natural biological form of silicon in humans.1 Other forms of water-soluble organic derivates are available, and are known as silanols or organic silicons.
Silicon is a natural trace element of the mammalian diet, which has been reported to be important for the normal health of bone and the connective tissues.2–4 Indeed, dietary Si deprivation in growing animals appears to cause abnormal growth and defects in the connective tissues.5,6 On the contrary, high levels of Si are reported to be associated with healthy connective tissues (eg, aorta, bone, trachea, tendon, etc.), and especially their connective tissue components (ie, collagen and elastin), compared to non-connective tissues (eg, liver, kidney, spleen, etc.).7,8 A four to five times difference in Si levels between connective and non-connective tissues has been reported in adult rats, and serum Si levels have been demonstrated to be a serum marker of collagen type I turnover.9 Finally, the Si/collagen molar ratio has been found to be 1:6 in bone rats independently of the age, which supports a structural role for Si(OH)4.10 Experimental models confirm that OSA interacts with collagen through electrostatic hydrogen bonding, this interaction being maximum at pH 5.11
Organic silicons have been used for decades, alone or in combination, in mesotherapeutic treatments and, more recently, as food complements, with both utilizations claiming a beneficial action on skin dermis and other connective tissues.12 When added for nutritional purposes to food as a supplement, it often takes the form of monomethylsilanetriol as source of bioavailable silicon. The typical daily dietary silicon intake for an adult is estimated to be 17–40 mg/day on average.13
The clinical effect of the use of organic silicon was also emphasized by two randomized placebo-controlled double-blind clinical studies in women, aged 40–65 years, with clear clinical signs of photoaging on facial skin. These studies demonstrated a positive effect of oral choline-stabilized OSA supplementation on skin condition, hair morphology, hair tensile strengths and brittleness of hair and nails.14,15 In clearance studies, it was observed that 20% of the injected Si was retained by the tissues, with high levels in skin and bone.16,17 Studies show a direct involvement of Si in extracellular matrix (ECM) stabilization, particularly for collagen which is the main structural protein of connective tissues.4,18
RRS® Silisorg is a CE Class III medical device dermal implant containing monomethylsilanol mannuronate associated to an antioxidant resveratrol. RRS® Silisorg is indicated for the treatment of dermal and subcutaneous tissue depressions in the case of ultraviolet (UV)-induced skin laxity (photoaging and its consequences). In an exhaustive previous bibliographic review on mesotherapy/biorevitalization products for skin rejuvenation purposes, we showed that only a very few numbers of scientific reports are available in that field.34 Nevertheless, the number of aesthetic procedures currently using mesotherapy products is increasing and there is a real need to better characterize the action of those products as most of them are sold as medical devices for their ability to fill dermis. Those commercial products are usually constituted of hyaluronic acid (HA), and/or organic silicon or other biopolymers and may have an additional effect on skin cells, especially on the dermis compartment where there are injected.
In this study, we evaluated the effect of RRS® Silisorg (Skin Tech Pharma Group, Castelló d’Empúries, Spain) on human fibroblast cell viability, on hyaluronan synthase type 2 (HAS2) enzyme, and on collagen type I and elastin genes expression. These in vitro studies were performed by an independent, external and certified laboratory (Eurofins BioPharma Product Testing Spain SLU).
Materials and methods
Cell culture and MTT assay
Human skin fibroblasts purchased from ATCC (ATCC-CRL-2522) were seeded at 15,000 cells/well in 24-well cell culture plates and allowed to grow for 24 hours at 37°C with 5% CO2. The cell culture medium was Dulbecco’s Modified Eagle’s Medium (D-MEM) plus 10% fetal bovine serum (FBS), and cells underwent starvation in a serum-free medium for 6 hours before treatment. Next, cells were exposed to the tested sample at the specified concentrations (0.2 and 1 mg/mL) in a fresh medium without FBS and incubated for 24 or 48 hours. Cells treated with the medium alone were used as a negative control and those treated with human insulin (hu-insulin) at a 50 μg/mL concentration were used as a positive control. All dilutions were tested in triplicate.
After treatment, cell survival was determined using a MTT assay. Briefly, 300 μL of the MTT solution was added in each well and incubated for 3 hours at 37°C. The medium was removed, the precipitated blue formazan dye was dissolved in isopropanol or dimethyl sulfoxide (300 μL per well) and the absorbance was quantified spectrophotometrically at 550 nm. The plate was shaken on a gyratory plate shaker, ensuring that all the crystals were dissolved and formed a homogeneous solution. Three independent experiments were done and the absorbance for each experiment was measured per triplicate on a microplate reader (Tecan Sunrise remote), with background clearing. Results were expressed as follows:
|
RNA extraction, reverse transcription polymerase chain reaction and quantitative polymerase chain reaction
Human skin-derived normal fibroblasts purchased from ATCC (ATCC CRL-2522) were seeded at 10,000 cells/well in 24-well cell culture plates and allowed to grow for 24 hours at 37°C with 5% CO2. The cell culture medium was D-MEM (minimum essential medium) + 10% FBS, and cells underwent starvation in a serum-free medium for 6 hours before treatment. Next, cells were exposed to scalar dilutions of the tested products (0.2 and 1 mg/mL) in a serum-free fresh medium. Untreated cells were used as a negative control; cells treated with hu-insulin (50 μg/mL) were used as a positive control, and all experiments were performed in triplicates.
After 24 and 48 hours of exposure, total RNA was extracted using a guanidine thiocyanate-based reagent according to manufacturer’s instruction (TRI Reagent®, Sigma-Aldrich Co., St Louis, MO, USA). After precipitation and centrifugation (30 minutes, 120,000 rpm, 4°C), RNA was resuspended in 20 µL of sterile water and its concentration determined spectrophotometrically at 260 and 280 nm. Then, 300 ng of total RNA was retro-transcribed into cDNA using random primers at 37°C for 2 hours in a thermal cycler following manufacturer’s instruction (Applied Biosystems, Foster City, CA, USA). Changes in the gene expression profile were analyzed by quantitative polymerase chain reaction (qPCR) technology using an SYBR green-based chemistry. Primers pair sequences used for HAS2, collagen type I and elastin gene amplification as well as the normalizer actin were designed by Eurofins. Three independent experiments were performed for each condition, and for each experiment duplicate reading was performed. The calculation of the fold change was done in two steps, after measuring CT (threshold cycle) according to the following formulas:
|
Statistical analysis
An unpaired t-test was used to determined p-value and statistical significance. GraphPad software was used for the graphic representation of the data and statistical analysis.
Results
RRS® Silisorg clinical survey
RRS® Silisorg is a CE Class III medical device dermal implant containing monomethylsilanol mannuronate associated to an antioxidant resveratrol. RRS® Silisorg is indicated for the treatment of dermal and subcutaneous tissue depressions in the case of UV-induced skin laxity (photoaging and its consequences). RRS® Silisorg is recommended to treat the skin of face/neck/décolletage and body using dermal injections. To perform injections, a 30 G or 32 G needle or cannula can be used. Following instructions for use, 5 mL of RRS® Silisorg is injected per treatment. Figure 1 presents the results for the use of RRS® Silisorg.
  | Figure 1 Results before and after RRS® Silisorg, courtesy of Dr Katarzyna Luch. |
A post-market survey according to EN ISO 13485 has been performed properly since 2013 till 2018; claims related to safety issues of RRS® Silisorg were not registered, and any serious adverse effect was not declared.
RRS® Silisorg does not affect human fibroblast viability
In vitro experiments were all carried out on primary human fibroblasts as they represent the main cellular population of the human dermis skin compartment. Indeed, dermal fibroblasts ensure the production of the ECM components composing the dermal connective tissue responsible for the skin’s tensile strength and mechanical properties.19
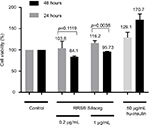
In this study, we first evaluated the effect of RRS® Silisorg on human fibroblast viability. Cell viability evaluation was performed using the MTT assay on resting synchronized serum starved human fibroblasts. The assay was done in the presence of two different concentrations of the RRS® Silisorg (0.2 and 1 mg/mL) and compared to two control conditions, one lacking RRS® Silisorg (non-stimulated control) and the other activated with 50 µg/mL of hu-insulin (stimulated control). Two different time points were evaluated at 24 and 48 hours posttreatment with RRS® Silisorg.
The results demonstrated that RRS® Silisorg was not toxic (p-value >0.05) to human fibroblasts in vitro at the highest dose (1 mg/mL) and at the posttreatment time points (24 and 48 hours). A slight increase in cellular viability 16.2±3.9% (p-value <0.05) was observed after 24 hours in the presence of 1 mg/mL of RRS® Silisorg. Nevertheless, this increase in viability was not sustained after 48 hours (Figure 2).
RRS® Silisorg promotes the synthesis of HAS2, collagen type I and elastin gene transcripts by human fibroblasts
The ECM of the dermis is largely composed of collagen, elastic fibers and HA. Dermal collagen represents by far the most abundant ECM protein and constitutes the bulk of skin (90% dry weight).20,21 Among the five types of collagen present in the skin, collagen type I represents 80% of the total content and is therefore the main structural collagen type in the skin.22 Another 10%–15% is represented by collagen type III.23 The elastic fiber network in the ECM gives it the required resilience to recoil after stretching and is composed of elastin proteins. Finally, HA, which is a non-sulfated glycosaminoglycan, not only ensures essential moisturizing of the dermis but is also involved in cell signaling through receptors such as the CD44.24
We evaluated the gene expression of collagen type I, elastin and the HAS2 enzyme responsible for the production of high-molecular chain HA. Gene expression was assessed by qPCR as described in the “Materials and methods” section, in synchronized human fibroblasts in the presence of different concentrations of RRS® Silisorg (0.2 and 1 mg/mL) at two different time points (24 and 48 hours). One negative control (lacking RRS® Silisorg) and a positive control (cultured in the presence of 50 µg/mL of hu-insulin) were used for subsequent comparisons.
Results were expressed as a fold increase in gene transcript expression between the activated condition and the control condition. RRS® Silisorg was able to significantly increase the expression of mRNA of HAS2, collagen type I and elastin gene. Nevertheless, the expression pattern of HAS2 was different from collagen type I and elastin gene. Indeed, the maximum expression of HAS2 gene was reached at 24 hours with a fold increase of 10.3±2.3 and 25.5±4.6 at a dose of 0.2 and 1 mg/mL, respectively (p-value <0.05). At 48 hours, HAS2 mRNA expression started to decrease with a fold increase of 8.5±1.1 for 0.2 mg/mL and 10.5±2.3 for 1 mg/mL (p-value <0.05). In the case of collagen type I and elastin gene, the maximum increase was obtained at 48 hours following the activation of fibroblasts by RRS® Silisorg. Collagen type I RNA expression reached its maximum expression at 48 hours with a fold increase of 2.61±0.39 and 4.73±1.06 at an RRS® Silisorg concentration of 0.2 and 1 mg/mL, respectively (p-value <0.05). For elastin gene, the maximum fold increase was obtained at 48 hours and was 2.57±0.18 for an RRS® Silisorg concentration of 1 mg/mL (p-value <0.05; Figure 3).
Discussion
In this study, the RRS® Silisorg activity was tested on human fibroblasts, which is justified by the fact that the product is directly injected in the dermis compartment where it comes in contact with dermal fibroblasts. Viability data obtained in this in vitro study on human fibroblasts are in line with preclinical tests that were realized with the RRS® Silisorg for the delivery of the CE Class III certificate. Those tests, described in Table 1, demonstrate the biocompatibility and lack of toxicity of the RRS® Silisorg medical device, based on studies successfully performed on cell lines, animals and bacteria. They are part of the CE Class III medical device evaluation and are done to ensure patient safety.
  | Table 1 Preclinical tests performed with RRS® Silisorg |
RRS® Silisorg greatly enhances the expression of HAS2 up to 25 times in human fibroblasts following 24 hours of contact in vitro. The transcript expression started to drop at 48 hours suggesting that the enzyme HAS2 was translated into the protein. As mentioned before, among the three enzymes responsible for HA synthesis, HAS2 is the one producing the longest chain length or, in other words, the HA with high molecular weight. The biological roles and cellular interactions of HA with cells are highly dependent on the HA chain length. Indeed, high-molecular weight HA is a large bioinert molecule, which serves to maintain a highly hydrated cellular environment, regulate osmotic balance and acts as a shock absorber. On the other hand, low-molecular weight HA is a potent pro-inflammatory molecule. Interestingly, typical signs of aging and photoaged skin are dependent on silicon and HA levels in connective tissues.24 Several topical actives have been tested in aged and photodamaged skins.25 At the molecular level, the human HAS2 gene was demonstrated to be a primary retinoic acid-responsive gene.26 In animal models, it was demonstrated that chronic UVB irradiation causes loss of HA from mouse dermis because of downregulation of HA synthases.27,28 Interestingly, HAS2 protects skin fibroblasts against apoptosis induced by environmental factors (UV radiation induced).29 To our knowledge, it is the first time that HAS2 expression is reported to be increased by organic silicon-based formulation, containing monomethylsilanol mannuronate combined with resveratrol (RRS® Silisorg), which could explain the beneficial clinical effects on skin photoaging.
In addition, the expression of collagen type I and elastin genes was also increased, although it was delayed and started to significantly increase at 48 hours in comparison to HAS2 gene. As RRS® Silisorg is composed of both actives, methylsilanol mannuronate (as organic silicon source) and resveratrol (an antioxidant), it is difficult to determine which is the main fibroblast activator in vitro. Nevertheless, organic silicon in the form of OSA was demonstrated to stimulate collagen type I synthesis in human osteoblast-like cells and skin fibroblasts in vitro. It appears that OSA did not directly alter collagen type I gene expression, but rather modulated the activity of prolyl hydroxylase, an enzyme involved in the production of collagen.18,30 However, resveratrol has been exhaustively described by several studies to inhibit collagen type I and elastin production by fibroblasts and is now implemented in some treatments of skin fibrosis and keloids.30–33 Therefore, the increase in collagen type I and elastin may be directly due to organic silicon stimulation, and retarded kinetic expression of both genes could be the result of the presence of resveratrol in the formulation.
In 2017, RRS® HA Injectable dermal implant (CE Class III medical device) was studied by using the same in vitro model and method. Results proved that RRS® HA Injectable enhances the transcription of collagen type I and elastin genes up to 10 and 15 times, respectively.34 However, RRS® HA Injectable showed no effect on the levels of HA in vitro (data not published). RRS® Silisorg results demonstrate that the expression of HAS2 gene is increased, which leads to extra HA production. Those two studies bring researchers to conclude that the combination of both products could lead to a more beneficial situation. In terms of ECM organization, RRS® Silisorg and RRS® HA Injectable seem to be complementary to each other, which could lead to better clinical results. Further clinical efficacy studies should be done to support this hypothesis.
Conclusion
Our results demonstrated that RRS® Silisorg promotes the expression of not only HAS2 but also collagen type I and elastin genes in human skin fibroblasts.
Acknowledgments
All in vitro experiments and collection of data corresponding to the experiments were performed by Eurofins BioPharma Product Testing Spain SLU, an external independent certified laboratory. Patient pictures were kindly provided by Dr Katarzyna Luch, esthetic medicine practitioner. The patient provided written informed consent for the publication of her images.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
All authors of the paper belong to the company Skin Tech Pharma Group. This paper has been revised by Eurofins BioPharma Product Testing Spain SLU to validate that all the data exposed conform with the results obtained. The authors report no other conflicts of interest in this work.
References
Carlisle EM. Silicon. In: O’Dell BL, Sunde RA, editors. Handbook of Nutritionally Essential Mineral Elements. New York, NY: Marcel Dekker; 1997:603–618. | ||
Nielsen FH. Nutritional requirements for boron, silicon, vanadium, nickel, and arsenic: current knowledge and speculation. FASEB J. 1991;5(12):2661–2667. | ||
Nielsen FH. Update on the possible nutritional importance of silicon. J Trace Elem Med Biol. 2014;28(4):379–382. | ||
Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging. 2007;11(2):99–110. | ||
Schwarz K, Milne DB. Growth-promoting effects of silicon in rats. Nature. 1972;239(5371):333–334. | ||
Elliot MA, Edwards HM Jr. Effect of dietary silicon on growth and skeletal development in chickens. J Nutr. 1991;121(2):201–207. | ||
Carlisle EM. Silicon as an essential trace element in animal nutrition. Ciba Found Symp. 1986;121:123–139. | ||
Schwarz K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc Natl Acad Sci U S A. 1973;70(5):1608–1612. | ||
Jugdaohsingh R, Watson AI, Pedro LD, Powell JJ. The decrease in silicon concentration of the connective tissues with age in rats is a marker of connective tissue turnover. Bone. 2015;75:40–48. | ||
Jugdaohsingh R, Pedro LD, Watson A, Powell JJ. Silicon and boron differ in their localization and loading in bone. Bone Rep. 2014;1:9–15. | ||
Miñones J, Fernández SG, Iribarnegaray E, Pedrer PS. The interaction of silicic acid with protein monolayers: effect of pH and ionic strength of substrate. J Colloid Interface Sci. 1973;42(3):503–515. | ||
Herreros FOC, Cintra ML, Adam RL, De Moraes AM, Metze K. Remodeling of the human dermis after application of salicylate silanol. Arch Dermatol Res. 2007;299(1):41–45. | ||
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). Scientific opinion on the safety of organic silicon (monomethylsilanetriol, MMST) as a novel food ingredient for use as a source of silicon in food supplements and bioavailability of orthosilicic acid from the source. EFSA J. 2016;14(4):4436. | ||
Barel A, Calomme M, Timchenko A, et al. Effect of oral intake of choline-stabilized orthosilicic acid on skin, nails and hair in women with photodamaged skin. Arch Dermatol Res. 2005;297(4):147–153. | ||
Wickett RR, Kossmann E, Barel A, et al. Effect of oral intake of choline-stabilized orthosilicic acid on hair tensile strength and morphology in women with fine hair. Arch Dermatol Res. 2007;299(10):499–505. | ||
Adler AJ, Etzion Z, Berlyne GM. Uptake, distribution, and excretion of 31 silicon in normal rats. Am J Physiol. 1986;251(6 Pt 1):E670–E673. | ||
Berlyne GM, Shainkin-Kestenbaum R, Yagil R, Alfassi Z, Kushelevsky A, Etzion Z. Distribution of (31)silicon-labeled silicic acid in the rat. Biol Trace Elem Res. 1986;10(2):159–162. | ||
Reffitt DM, Ogston N, Jugdaohsingh R, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32(2):127–135. | ||
Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. | ||
Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61(5):427–434. | ||
Li Y, Lei D, Swindell WR, et al. Age-associated increase in skin fibroblast-derived prostaglandin E(2) contributes to reduced collagen levels in elderly human skin. J Invest Dermatol. 2015;135(9):2181–2188. | ||
Diegelmann RF. Collagen metabolism. Wounds. 2001;13:177–182. | ||
Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24(3):371–378. | ||
Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinology. 2012;4(3):253–258. | ||
Lundin A, Berne B, Michaëlsson G. Topical retinoic acid treatment of photoaged skin: its effects on hyaluronan distribution in epidermis and on hyaluronan and retinoic acid in suction blister fluid. Acta Derm Venereol. 1992;72(6):423–427. | ||
Saavalainen K, Pasonen-Seppänen S, Dunlop TW, Tammi R, Tammi MI, Carlberg C. The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J Biol Chem. 2005;280(15):14636–14644. | ||
Meyer LJ, Stern R. Age-dependent changes of hyaluronan in human skin. J Invest Dermatol. 1994;102(3):385–389. | ||
Dai G, Freudenberger T, Zipper P, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol. 2007;171(5):1451–1461. | ||
Wang Y, Lauer ME, Anand S, Mack JA, Maytin EV. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J Biol Chem. 2014;289(46):32253–32265. | ||
Carlisle EM, Berger JW, Alpenfels WF. A silicon requirement for prolyl hydroxylase activity. Fed Proc. 1981;40:886. | ||
Li X, Li J, Wang L, et al. The role of metformin and resveratrol in the prevention of hypoxia-inducible factor 1α accumulation and fibrosis in hypoxic adipose tissue. Br J Pharmacol. 2016;173(12):2001–2015. | ||
Mamalis A, Jagdeo J. The combination of resveratrol and high-fluence light emitting diode-red light produces synergistic photobotanical inhibition of fibroblast proliferation and collagen synthesis: a novel treatment for skin fibrosis. Dermatol Surg. 2017;43(1):81–86. | ||
Li P, Liang ML, Zhu Y, et al. Resveratrol inhibits collagen I synthesis by suppressing IGF-1R activation in intestinal fibroblasts. World J Gastroenterol. 2014;20(16):4648–4661. | ||
Deglesne PA, Arroyo R, Ranneva E, Deprez P. In vitro study of RRS® HA injectable mesotherapy/biorevitalization product on human skin fibroblasts and its clinical utilization. Clin Cosmet Investig Dermatol. 2016;9:41–53. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.