Back to Journals » Infection and Drug Resistance » Volume 12
In vitro antibacterial effect of deconex and sodium hypochlorite against bacterial taxa isolated from dental units
Authors Amin M, Ardaneh M, Hashemzadeh M , Asarehzadegan Dezfuli A , JafarZadeh E
Received 20 December 2018
Accepted for publication 18 February 2019
Published 11 April 2019 Volume 2019:12 Pages 805—814
DOI https://doi.org/10.2147/IDR.S197988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Mansour Amin, 1, 2 Marieh Ardaneh, 1 Mohammad Hashemzadeh, 1, 2 Aram Asarehzadegan Dezfuli, 1 Elham JafarZadeh 3
1Department of Microbiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 2Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 3Laboratory of Taleqhani Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Purpose: Dental unit’s environment and relevant instruments are a major source of infectious diseases caused by a variety of microorganisms. The application of various disinfectants is one of the most effective methods for reducing or eliminating microbial contamination. The objective of this study was to evaluate the antibacterial effects of deconex and sodium hypochlorite against bacterial taxa isolated from dental unit’s environment of Ahvaz Jundishapur University of Medical Sciences, southwest of Iran.
Methods: In order to evaluate the quality of disinfection, sampling was performed from different parts of 100 clinical units. For bacterial recovery and isolation, samples were enriched and cultured onto different microbiological culture media. Species identification was carried out using phenotypic and molecular methods ( 16S rDNA sequence analysis). In vitro activity of sodium hypochlorite and deconex were determined by the broth micro-dilution method.
Results: According to conventional techniques, Bacillus spp (48%) was the most frequently encountered isolates, followed by staphylococcus spp (26%). By using both techniques, Bacillus subtilis was the most frequently encountered species (n=23, 21%), followed by Bacillus licheniformis (n=8, 7.4%), Streptococcus pneumonia (n=8, 7.4%), Staphylococcus epidermidis (n=8, 7.4%), Staphylococcus saprophyticus (n=8, 7.4%) and Staphylococcus warneri. The highest levels of contamination were observed in oral medications. The deconex had lower minimum inhibitory concentration (MIC) concentration in comparasion to sodium hypochlorite, which showed that deconex was a much more potent disinfectant.
Conclusion: In conclusion, the results of the present in vitro study showed that deconex had promising results for decontamination of the tested microorganism, and it is recommended for disinfecting of dental units and environment. In this study, the high percentage of dental unit’s contamination showed the need to improve disinfection procedures, sterilization systems, and the use of an appropriate concentration of deconex and sodium hypochlorite for dental units decontamination .
Keywords: 16S rDNA gene, deconex and sodium hypochlorite, dental unit
A Letter to the Editor has been published for this article
A Response to Letter has been published for this article
Introduction
Dentist, dental materials and dental laboratories are potential sources for transmission of infectious diseases.1 The oral cavity is a providing medium for bacterial growth, which can be transferred to the instruments and clothing during dental procedures, with a high risk for cross infection.2 Cross-contamination and cross-infection can be occurred by infectious microorganism between dental office, from the patient to dental health care, and also through the hands of the dental team. Infection control in dental laboratories was first recommended by the American Dental Association (ADA) within the recommendations and guidelines of the Centers for Disease Control.3 Clinical contact surfaces that are frequently touched (eg, switch of dental light, dental stool, handle drawer), can act as reservoirs of microorganisms. When these surfaces are touched, microorganisms can be transferred from one resident to another through the use of commonly shared equipment, or to the nose, mouth or eyes of health care workers or patients.4 Depending on the patient’s susceptibility, a patient could be infected through a contaminated surface/instrument located in the dental practice.5–7
The use of disinfectants in the dental unit’s environment is one of the most effective procedures that can be applied to the infection control.8 Despite the effectiveness of using disinfectants, there are limitations to using them. Prolonged application of a biocide may pose a danger to staff, patients and enhance tolerance or biocidal resistance in microorganisms.9 Although these biocide treatments eliminate most surface contamination, some microorganisms percolate into the inner parts of these casts, hence making disinfection arduous.10 Proper decontamination of dental units surfaces depends on the type of biocide and appropriate methods according to international recommendations. Disinfection is performed by physical and chemical agents, however, none of the disinfectants succeeded completely. Deconex is Propanol-based alcoholic disinfectant agent and the predominant agent used in our dental units for many years. Regarding some reports on inappropriate decontamination, finding challenges the reliability in preventing cross infection11,12 and Assessment of decontamination procedure used in our faculty, the objective of this study was to evaluate the antibacterial effects of deconex and sodium hypochlorite against bacterial taxa isolated from dental unit’s environment of Ahvaz Jundishapur University of Medical Sciences, southwest of Iran.
Materials and methods
Ethics statement
The study was approved by the Research Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences (No: IR.AJUMS.REC.1395.1074), Ahvaz, Iran.
Sample collection and preparation
This study was conducted at the dental faculty of Ahvaz Jundishapur University of Medical Sciences (AJUMS) from October to November 2017. One hundred samples were collected from different parts of dental faculty units including pediatric, prosthetic, endodontic, radiology, oral medicine, operative dentistry, restoration, periodontal, oral maxillofacial surgery, root canal therapy, orthodontic and surgical units. After submission of the preliminary proposal, necessary permission for sample collection was granted. The sample was taken at the end of the working day after daily disinfection with deconex dental BB (Borer Chemic, Geneva, Switzerland; Contact time 15 mins). In accordance with the claim of the infection control officer the disinfection is carried out is based on the standard protocol. Each sample have been taken by a sterile swab, in a width of 2 cm and a length of 10 cm, from one part of each unit including the switch of dental light, armrest, turbine handpiece tie-in, handle drawer, dental stool, inductive air locked rotatory arm system, dental tree way syringe, saliva ejector, automatic cup filler and headrest. The swab was placed into a transport medium containing 5 mL of Tryptic Soy Broth (TSB) and the test tube was closed. The samples were immediately transferred to the microbiological laboratory in the cold box for 10 mins (Igloo USA) and incubated in the atmosphere of 5% CO2 at 37°C for 18–24 hrs. A loop full (0.01 mL) of each sample were cultured on standard culture media including blood agar and MacConkey agar (Merck, Germany) and incubated at 37ºC for 24 hrs. The bacterial isolates were subjected to early phenotypic tests such as hot dyeing, morphology, catalase and oxidase reaction and all isolates were categorized into the appropriate genera.13 The pure bacterial colonies were inoculated onto the medium containing 1.5 mL of sterile TSB containing glycerol (20%) and stored at −20°C for further investigation.
Molecular identification
DNA extraction
Genomic DNA extracted from pure colonies using the simple boiling method as described elsewhere.14, In brief, a few bacterial colonies were removed from fresh overnight culture on Mueller-Hinton agar medium and dissolved in 500 μL of TE buffer, boiled for 10 mins and placed at −20°C for 5 mins. After centrifugation at 14,000×g for 10 mins, the supernatant was used as a template for the PCR amplification. The concentration of extracted DNA was determined at 260 nm, using a Nano-drop instrument (Thermo Scientific, USA).
Identification of bacteria with amplification of gene encoding 16S rDNA
For definitive identification at the species level, an approximately 1500 bp fragment of the 16S rDNA gene was amplified and sequenced by two specific primers 27F (5′- AGA GTC CAT CMT GGC TCA A-3′) and 1525 (5′-AAG GGG AGG TGW TCC ARC G-3′) as previously described.15,16 The composition of PCR mixture was: 500 mM KCl, 200 mM Tris-HCl (PH=8.4), 1.5 mM MgCl2, 0.2 Mm dNTPs, 0.4 µM each primer, 1 unit of Taq DNA polymerase, DW 30.3 µL, DNA Template 81 ng/µL. The final volume was 50 µL. The PCR condition was initial denaturation one cycle of 95°C for 2 mins, followed by 30 cycles of 95°C for 30 s, annealing of 53.5°C for 30 s, an extension of 72°C for 1.30 mins and the final extension at 72°C for 10 mins. PCR amplifications for studied genes were conducted on a thermal cycler 5530 (Eppendorf master, Germany). The amplified PCR products of the 16S rDNA gene for each isolate were purified and the sequences of the products were determined using an ABI PRISM_ 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the standard protocol of the supplier. The sequences of the 16S rDNA gene for each isolate were aligned separately and compared with all existing relevant sequences of recovered from the GenBank database using the JPhydit program.17 Percentages of similarity between sequences of each gene were determined by comparing sequences to an in-house database of 16S rDNA sequences.
Determination of minimal inhibitory concentration (MIC) of deconex and sodium hypochlorite by broth micro-dilution method
The MIC of deconex dental BB and sodium hypochlorite against some opportunistic pathogens (Streptococcus pneumonia, Enterococcus faecalis, Klebsiella pneumonia, Staphylococcus epidermidis, etc.) were determined by broth micro-dilution method according to the Clinical and Laboratory Standards Institute procedures.18 In brief, the primary stock solution of deconex and sodium hypochlorite were diluted at the final tested concentrations ranged from 0.5 to 256 mg/mL. According to the product data sheet, the initial dilution of deconex (Borer Chemie AG, Switzerland) and sodium hypochlorite (Laboratory chemical, Iran) was 2.5 mg/mL and 5 mg/mL, respectively. 50 µL of each dilution was added to 96-well microtiter plates containing 50 µL Luria-Bertani (LB) broth. Each well was inoculated with 50 µL of the bacterial sample and mixed gently, yielding the final bacterial concentration of approximately 1×106 colony forming unit (CFU/mL). The microplate trays were covered with aluminum foil and placed in plastic bags and incubated at 37°C for 24 hrs. The MIC was defined as the lowest concentration of the tested agent that resulted in the complete inhibition of visible growth in LB broth. The experiments were repeated 3 times and the results were constant in all tests.
Results
In the present study, a total of 100 samples were collected from different part of dental units. Conventional phenotypic methods showed that 91 (91%) samples had microbial contamination and 109 bacterial isolates were identified. Based on phenotypic tests, most isolates were identified up to the genus or complex level. For definitive identification, all 109 isolates were subjected to 16S rDNA gene sequencing. The results of 16S rDNA gene sequence analysis revealed that all isolates showed more than 99% homology with relevant species as shown in Tables 1–3. Distribution of bacterial contamination in the clinics and different parts of units are shown in Table 1. The greatest contamination of units was found in Oral medicine, Root canal therapy, Surgical units (all 10 units) followed by Operative dentistry, orthodontic, prosthetic, periodontal (9 units). Also, the most bacterial contamination was found on the handles drawer, dental stool, followed by the switch of dental light and armrest. According to conventional techniques and 16S rDNA gene sequence analysis, Bacillus spp (48%) was the most frequently encountered isolates, followed by staphylococcus spp (26%), and the other Gram-negative and Gram-positive bacteria. Relative frequency of bacterial species was shown with details in Table 2. By using both techniques, Bacillus subtilis was the most frequently encountered species (n=23, 21%), followed by Bacillus licheniformis (n=8, 7.4%), Streptococcus pneumonia (n=8, 7.4%), Staphylococcus epidermidis (n=8, 7.4%), Staphylococcus saprophyticus (n=8, 7.4%) and Staphylococcus warneri. The proportion and type of bacterial contamination in each clinic and related units are shown in Table 3. According to the table’s guide (top left), the contamination levels of each unit are shown, in which the yellow and red colors show the lowest and highest levels of contamination, respectively. It should be noted that oral medicine is also the highest in terms of both the diversity of isolated bacterial species and the pediatric section showed the lowest (Tables 1 and 3). There is a significant difference between the effect of deconex and sodium hypochlorite for all the mentioned microorganism (p<0.05) (Table 4). Based on Statistical analysis the mean of MIC for the deconox and the sodium hypochlorite was 2.5 and 25 mg/mL, respectively. This difference is statistically significant (P-value=0.005), which means deconex are much more potent in compare to Sodium hypochlorite.
 | Table 1 A summary of bacterial species isolated from different parts of dental unit in different clinics (all isolates identified by combination of conventional techniques and sequence analysis) |
 | Table 2 Frequency of bacterial isolates from various dental units/clinics identified by combination of conventional techniques and sequence analysis |
 | Table 3 The proportion and type of bacterial contamination in each clinic and related units |
 | Table 3(Continued). |
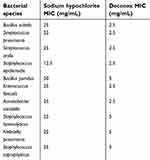 | Table 4 A summary of antimicrobial activity of deconex and sodium hypochlorite against some clinically important bacterial isolates |
Discussion
Infection control and the safety of dental clinics have been a high profile issue in dental health professionals and for which regularly procedures to protect both patients and dental team have been used. Although some studies have reported that washing the impression materials with running water could reduce about 40–90% of bacteria.19,20 Disinfecting all patients’ impressions materials is recommended by the International Dental Federation and the ADA. Nonetheless, single-use devices, pre-sterilization and cleaning of re-usable instruments in general dental practice have been a usual practice in dentistry.21–23 In this study we followed three aims; First, identifying dental unit bacterial diversity at the end of working day after daily disinfection with deconex, second, in vitro evaluating the bacterial effect of deconex and sodium hypochlorite against bacterial isolates and the third, assessment of decontamination procedure used in our faculty. In our study, microbial contamination was detected in 91% of the examined dental units with opportunistic and pathogenic microorganisms. Willams et al. also have been recorded the higher contamination at the end of daily work as compare to initial time.24 In our study the most contamination has been seen in dental stool and handle drawer (Table 1) probably due to direct touch and ignore it by dental staff during sterilization, the same results have been declared in previous studies.25,26
In the current study, Bacillus subtilis (n=23, 21%), Bacillus licheniformis (n=8, 7.4%), Streptococcus pneumonia (n=8, 7.4%), Staphylococcus epidermidis (n=8, 7.4%), Staphylococcus saprophyticus (n=8, 7.4%) and Staphylococcus warneri (n=8, 7.4%) were the most frequently recovered species from dental units using combination of conventional technique and 16S rDNA sequence analysis (Table 2). These results are similar to those are reported in some similar studies.25–27 Most of the microorganisms identified in this study do not represent a risk to public health, but are considered as opportunistic microorganisms which can be associated with important infections in pregnant women, the elderly patients, cancer patients, AIDS patients and other immunocompromised disorders. These patients are much more susceptible to being infected by opportunistic pathogens.28 Although this low frequency in isolation medically important bacteria in dental units including Legionella and Mycobacterial species could be related to the lake of appropriate methods for recovering these taxa. The proportion and type of bacterial contamination in each clinic and related units are shown in Table 3. According to this, contamination was high intense during oral medicine and root canal therapy. The oral microbiome is comprised of over 600 prevalent taxa at the species level.29 Although the possible unit’s contamination with microorganisms from the patients’ mouths and oral bacteria are not usually present in our study. It seems the main reason for this objective is the use of anti-retraction valves and sterile handpieces, which control the suction of these microorganisms. In contrast to our study, Coelho et al showed that Gram-negative bacteria were the most common isolates in dental clinics instrument.2 Among the many physical methods that can improve the microbiological quality of the clinical environment and the instrument, the use of disinfectants is the most effective way to ensure that the decontamination process is effective.30
The MIC of deconex dental BB and sodium hypochlorite against some opportunistic pathogens were determined by broth micro-dilution method. It seems that the use of these clinical species is somewhat more suitable because they may be more resistant in compare to references strains. The results of this study showed that deconex was more strong to eliminate these microorganisms, has been confirmed in previous studies.31,32 In order to evaluate the performance of every disinfectant it is important to study the time of decimal decreasing (D-value).2,33 Based on the findings of the study done by Ghasemi et al, deconex has the highest capacity when it is used different impression materials and it eradicates all microorganisms in both 5 and 10 mins. In other study done by Bagheri et al, a significant decrease was seen in the number of bacterial colonies in the restoration, pediatric, orthodontic and diagnosis units.23,33
In conclusion, the results of the present in vitro study showed that deconex revealed a promising effect on bacterial contamination of the dental environment, and it is recommended for disinfecting of dental units and environment. Considering the promising in vitro effect of deconex, along with high percentage of contamination of dental units, it seems that some technical errors are happened by the dental technician in decontamination procedure. Therefore, it is necessary to re-inspection and improve the methods of decontamination and the use of appropriate concentrations of this product. In fact, according to the results obtained in this study, the quality of decontamination procedures by the personnel is doubtful. Furthermore, our study underlines that microbial monitoring could represent an important element to detect alert values which indicate the presence of risk factors and require the adoption of control measures.
Some limitations of this study can be considered. On the other hand, in the present study the microbial contamination has not been considered with some clinical variables such as the number of patients seen per day in every treated unit. Also, the samples were taken at the end of the working day after treatment with deconex while to compare the isolated bacteria and disinfectant effect, it was better to sample at the beginning of the working day, too. The other limitation of this study was that the anaerobic culture methods and special methods for isolation of Legionella and Mycobacteria species were not considered.
Acknowledgments
This work is a part of the M. Sc. thesis of Marieh Ardaneh, which has been approved by the Department of Microbiology of Ahvaz Jundishapur University of Medical Sciences. The authors thank the Department of Microbiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran and the Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, for financial support (grant number 96146). The study was also sponsored by the authors. The authors received no funding from any other individual or institution.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1.
2. Coelho VH, Venâncio GN, Cestari TF, de Almeida ME, Da Cruz CB. Microbial contamination of a University dental clinic in Brazil. Braz J Oral Sci. 2016;15(4):248–251.
3. Cottone JA, Terezhalmy GT, Molinari JA. Practical Infection Control in Dentistry.
4. Guida M, Gallã F, Di Onofrio V, et al. Environmental microbial contamination in dental setting: a local experience. J Prev Med Hyg. 2012;53(4):207–212.
5. Available from:
6. Laheij AM, Kistler JO, Belibasakis GN, Välimaa H, De Soet JJ;
7. Barton A. Patient safety and quality: an evidencebased handbook for nurses. Aorn J. 2009;90(4):601–602. doi:10.1016/j.aorn.2009.09.014
8. Bachman CE, White JM, Goodis HE, Rosenquist JW. American Academy Of Oral and Maxillofacial Radiology infection control guidelines for dental radiographic procedures. Oral Surg Oral Med Oral Pathol. 1992;73:248–249. doi:10.1016/0030-4220(92)90202-2
9. Walker JT, Bradshaw DJ, Fulford MR, Marsh PD. Microbiological evaluation of a range of disinfectant products to control mixed-species biofilm contamination in a laboratory model of a dental unit water system. Appl Environ Microbiol. 2003;69(6):3327–3332.
10. Sammy KC, Benjamin SN. Infection control mechanisms employed by dental laboratories to prevent infection of their dental technicians/technologists. J Oral Health Craniofac Sci. 2016;1:1-1.
11. Ganavadiya R, Shekar BC, Saxena V, Tomar P, Gupta R, Khandelwal G. Disinfecting efficacy of three chemical disinfectants on contaminated diagnostic instruments: A randomized trial. J Basic Clin Pharm. 2014;5(4):98–104. doi:10.4103/0976-0105.141946
12. Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665–690. doi:10.1128/CMR.00020-14
13. Forbest BA, Saham DF, Wisfeld AS. Bailey & Scott’s Diagnostic Microbiology.
14. Oliveira CF, Paim TG, Reiter KC, Rieger A, D’azevedo PA. Evaluation of four different DNA extraction methods in coagulase-negative Staphylococci clinical isolates. Rev Inst Med Trop Sao Paulo. 2014;56(1):29–33. doi:10.1590/S0036-46652014000100004
15. Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevantnocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37(1):99–102.
16. Osmanagaoglu O, Oral B, Kiran F, Evaluation of RFLP analysis of 16s rDNA (16s ARDRA) for inter-and intra-species differentiation of lactic acid bacteria. N Biotechnol. 2009;25:103. doi:10.1016/j.nbt.2009.06.691
17. Jeon YS, Chung H, et al. jPHYDIT: a JAVA-based integrated environment for molecular phylogeny of ribosomal RNA sequences. Bioinformatics. 2005;21(14):3171–3173. doi:10.1093/bioinformatics/bti463
18.
19. Al-Jabrah O, Al-Shumailan Y, Al-Rashdan M. Antimicrobial effect of 4 disinfectants on alginate, polyether, and polyvinyl siloxane impression materials. Int J Prosthodont. 2007;20(3):1–10.
20. Correia-Sousa J, Tabaio AM, Silva A, Pereira T, Sampaio-Maia B, Vasconcelos M. The effect of water and sodium hypochlorite disinfection on alginate impressions. Rev Port Estomatol Cir Maxilofac. 2013;54(1):8–12.
21. Turbi OO. Infection control for dental clinics. Acta Sci Dent Sci. 2018;2(10):48–56.
22. Campbell L, Barton A, Boyle R, Tully V. Improving the inspection and manual cleaning of dental instruments in a dental hospital. BMJ Open Qual. 2016;5(1):1–5.
23. Ghasemi E, Badrian H, Hosseini N, Khalighinejad N. The effect of three different disinfectant materials on polyether impressions by spray method. WJD. 2012;3(3):229–233. doi:10.5005/jp-journals-10015-1161
24. Williams HN, Singh R, Romberg E. Surface contamination in the dental operatory: a comparison over two decades. J Am Dent Assoc. 2003;134(3):325–330.
25. Khorakian F, Movahed T, Ghazvini K, et al. Evaluation of frequency of microbial contamination in clinical setting surface in Dental School of Mashhad University of Medical Sciences. J Mashhad Dent Sch. 2017;41(3):209–218.
26. Valian A, Shahbazi R, Farshidnia S, Sadat Tabatabaee F. Evaluation of the bacterial contamination of dental units in restorative and peridontics Departments of Dental School of Shahid Beheshti University of Medical Sciences. J Mashhad Dent Sch. 2014;37(4):345–356.
27. Monarca S, Grottolo M, Renzi D, et al. Evaluation of environmental bacterial contamination and procedures to control cross infection in a sample of Italian dental surgeries. Occup Environ Med. 2000;57(11):721–726.
28. Yamashita K, Ohara M, Kojima T, et al. Prevalence of drug-resistant opportunistic microorganisms in oral cavity after treatment for oral cancer. J Oral Sci. 2013;55(2):145–155.
29. Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. doi:10.1128/JB.00542-10
30. Patel M, Desai J, Owen PC. The efficacy of disinfectants in the decontamination of dental unit water lines: an in vitro laboratory study. BDJ Open. 2016;26(2):1–4.
31. Ghahramanloo A, Sadeghian A, Sohrabi K, Bidi A. A microbiologic investigation following the disinfection of irreversible hydrocolloid materials using the spray method. J Calif Dent Assoc. 2009;37(7):471–477.
32. Ezoddini Ardakani F, Zandi H, Mohammadi Z, Ayatollahi J, Ayatollahi F, Behniafar B. Comparing the disinfecting efficacies of micro 10, deconex, alprocid and microzid AF on the microorganisms on radiographic equipments. J Dent Res Dent Clin Dent Prospects. 2008;2(2):48–52. doi:10.5681/joddd.2008.010
33. Bagheri SM, Shooriabi M, Amin M, et al. Evaluation of disinfection quality of Dental Faculty Units of Ahvaz Jundishapur University of Medical Sciences, Southwest of Iran in 2017. J Mol Biol Res. 2018;8(1):88–94. doi:10.5539/jmbr.v8n1p88
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
