Back to Journals » International Medical Case Reports Journal » Volume 13
Iatrogenic Cushing’s Syndrome Following Intra-Articular Triamcinolone Injection in an HIV-Infected Patient on Cobicistat Presenting as a Pulmonary Embolism: Case Report and Literature Review
Authors Alidoost M, Conte GA, Agarwal K, Carson MP , Lann D, Marchesani D
Received 25 March 2020
Accepted for publication 26 May 2020
Published 9 June 2020 Volume 2020:13 Pages 229—235
DOI https://doi.org/10.2147/IMCRJ.S254461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Marjan Alidoost, Gabriella A Conte, Khushboo Agarwal, Michael P Carson, Danielle Lann, Diane Marchesani
Department of Medicine, Hackensack Meridian Health Jersey Shore University Medical Center, Neptune, NJ 07753, USA
Correspondence: Marjan Alidoost Email [email protected]
Background: Iatrogenic Cushing’s syndrome (ICS) typically develops after long-term exposure to corticosteroids, but it can occur after a single dose in patients treated with cobicistat or ritonavir for HIV. We present a patient who developed ICS due to the interaction between cobicistat and triamcinolone, a review of the literature, and what to our knowledge is the first case of ICS presenting as a pulmonary embolism.
Case Presentation: A 55-year old male with a past medical history of human immunodeficiency virus, undetectable for 15 years and currently on elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, received 2 intra-articular injections of triamcinolone one month apart for a Baker’s cyst in his right knee. He used nasal fluticasone for 9 days in-between the injections. After his second knee injection, he developed easy bruising and friable skin. Over the coming months, he experienced weight gain and Cushingoid facies. Four months after the knee injections he developed a pulmonary embolism and deep vein thrombosis treated with warfarin. The Cushingoid facies prompted an evaluation and diagnosis of ICS along with hypothalamic pituitary adrenal axis suppression.
Conclusion: This case demonstrates the need to monitor patients on pharmacological boosters with any exposure to corticosteroids, whether it be injected, inhaled, topical, oral or intravenous, as it can lead to profound adrenal suppression and ICS.
Keywords: human immunodeficiency virus, iatrogenic Cushing’s syndrome, adrenal suppression, case report
Introduction
Iatrogenic Cushing’s syndrome (ICS) is an adverse systemic complication of excessive exposure of exogenous corticosteroid administration. Exogenous glucocorticoids can inhibit corticotropin-releasing hormone (CRH) and cause bilateral adrenocortical atrophy resulting in decreased plasma adrenocorticotropic hormone (ACTH), and therefore decreased levels of cortisol leading to hypothalamic pituitary adrenal (HPA) axis suppression along with ICS.1 The most common cause of ICS is oral prednisone treatment of nonendocrine diseases, but it has also been associated with injected, topical and inhaled glucocorticoids, as well as progestins with intrinsic glucocorticoid activity such as megestrol acetate and high-dose medroxyprogesterone.2–5 There have also been multiple cases of patients on pharmacological boosters for human immunodeficiency virus (HIV) developing ICS. Pharmacological boosters for HIV cause a drug-drug interaction (DDI) by inhibiting cytochrome P450 (CYP450) and causing accumulation of exogenous steroids in the serum, which can occur even after one, with any modality of exposure. The HIV pharmacological boosters in question are Ritonavir (RTV) and Cobicistat. They are both used to treat HIV and enhance protease inhibitors (PIs) or elvitegravir (EVG), an integrase inhibitor, via their CYP450 inhibition.6 Cobicistat is a structural analogue of RTV and can be co-formulated with other antiviral medications but does not have any inherent antiviral activity like RTV does.6 In our review of the literature, we found 63 cases of patients on pharmacological boosters for HIV who developed ICS after non-systemic steroid exposure, as seen in Tables 1 and 2. However, the vast majority of cases involved RTV and, to our knowledge, we are presenting the third case of ICS in a patient on Cobicistat.
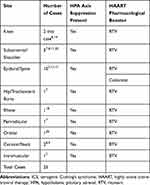 |
Table 1 Summary of Cases of Patients Who Developed ICS While on HAART with Pharmacological Boosters After They Received Steroid Injections |
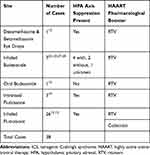 |
Table 2 Summary of Cases of Patients Who Developed ICS While on HAART with Pharmacological Boosters After They Had Other Non-Systemic Exposure to Steroids |
Additionally, our patient presented with a pulmonary embolism (PE), and in our literature review we could not find one other such case presenting as a PE. As intranasal and inhaled corticosteroids are becoming widespread in use for conditions such as allergic rhinitis, asthma, and chronic obstructive pulmonary disease (COPD), and are even available over the counter, it is vital that clinicians be aware that patients who do not have systemic steroid exposure may develop ICS. This case report and review will highlight the importance of early recognition of ICS and increase awareness regarding the pathophysiology, differential diagnosis and management of the syndrome.
Case Presentation
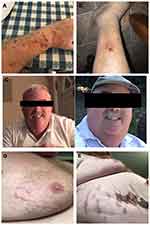
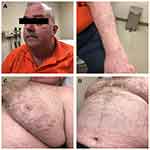
The patient is a 55-year-old-male with a past medical history of HIV with an undetectable viral load on highly active antiretroviral therapy (HAART) for over 15 years. He previously took elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (Stribild ®) but a recent change in the regimen included only an alternate formulation of the tenofovir: tenofovir alafenamide (Genvoya ®). Both regimens still contained cobicistat, a potent CYP450 inhibitor. Six months prior to presentation to endocrinology, he developed a Baker’s cyst of his right knee confirmed via doppler ultrasound and magnetic resonance imaging (MRI) of the right knee. He underwent two triamcinolone acetonide injections over the next two months. Between the knee injections, he used fluticasone nasal spray for nasal congestion. In the weeks after his second knee injection he noticed easy bruising (Figure 1A), a bruise on his left leg became infected and progressed to a slow-healing ulcer (Figure 1B), and over the next 2 months he experienced significant weight gain, cushingoid facies and facial plethora (Figure 1C). Five months later he developed sudden-onset shortness of breath and was found to have a PE via Computed Tomography Angiography (CTA) of the chest and a deep vein thrombosis (DVT) via doppler ultrasound of the lower extremities. At this time violaceous striae were noted on his abdomen (Figure 1D and 1E). The day after he was discharged home, he used his fluticasone nasal spray intermittently for 9 days. After this hospitalization his infectious disease physician referred him to endocrinologist for workup of ICS which was diagnosed along with hypothalamic pituitary adrenal (HPA) axis suppression with results as follows: Morning cortisol was undetectable at <0.5 mcg/dL (normal 4.0–22.0 mcg/dL), ACTH was also undetectable <5 pg/mL (normal 6–50 pg/mL), DHEA-sulfate was low at 12 mcg/dL (normal 38–313 mcg/dL), and urinary triamcinolone acetonide was elevated (1.3 mcg/dL, normal < 0.10). He was prescribed hydrocortisone 20mg PO in the morning and 10mg PO in the late afternoon; his HIV regimen was subsequently changed from elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide to an unboosted regimen with bictegravir/emtricitabine/tenofovir alafenamide. At several months follow-up, his urine studies show undetectable level of triamcinolone and his 8 am labs, with hydrocortisone held, were as follows: ACTH 25.4, cortisol 6.1, aldosterone 2.9, renin 0.776, sodium 141, and potassium 4.1. Additionally, his symptoms – namely his easy bruising, wide violaceous striae on the chest and abdomen, Cushingoid facies and facial plethora have improved, as seen in Figure 2, and he reports feeling much better. His steroids have been down titrated to 10 mg PO twice a day with the eventual goal of weaning off completely.
Discussion
It is often thought that inhaled, intranasal and intra-articular steroids do not have the same side effects as systemic steroids, but lately it has been shown that in certain populations, this is not the case.7 Patients who are on medications that inhibit CYP450 metabolism in the liver – such as RTV and cobicistat – are at higher risk to develop ICS. In such patients it has been documented that they have devolved ICS associated with a single dose of non-systemic steroids.8 Many of these patients develop ICS that is accompanied by HPA axis suppression, as seen in our patient.8 Other agents that inhibit CYP450 include macrolides, azoles, and diltiazem.9,10 Co-administration of these medications with an exogenous corticosteroid such as triamcinolone acetonide (TCA) can increase the likelihood of systemic adverse effects. In our review of the literature we found 2 cases of patients on pharmacological boosters who received joint injections while receiving azoles who subsequently developed ICS.7,11
In addition to injections and inhalations, use of corticosteroid eye drops have caused ICS.12 Our search of the literature review identified 24 cases of TCA injection, besides our own case, leading to ICS (Table 1), and all resulted in HPA axis suppression. 10 were epidural injections, 5 were subacromial injections, and we found only 2 other cases of an intraarticular knee injection resulting in ICS (Table 1). Interestingly, we also found one case of an intramuscular injection, administered for dermatitis, that resulted in ICS.7
HPA axis suppression has been documented to occur after 5 days of high-dose glucocorticoid treatment or even a single dose of intramuscular TCA.13 TCA has a half-life of 3 hours, but intra-articular injections can be absorbed over a period of 3 weeks.14 Most inhaled corticosteroids have a half-life of under 2.5 hours, however fluticasone has a half-life of 7.8 hours due to its high volume of distribution.10 In terms of inhaled corticosteroid exposure, 29 cases report fluticasone-associated ICS (Table 2), 3 of which were intranasal, the rest were inhaled and the intranasal fluticasone exposure was associated with a slower onset of symptoms vs inhaled. A total of 8 cases describe budesonide-mediated ICS, 7 of which were inhaled (Table 2). There was one case reported by Yeoh et al of a patient on RTV and oral budesonide, which is clinically significant as budesonide has a high first-pass metabolism rate and is thought to have lower likelihood of systemic effects than other oral steroids.15
The majority of cases we found of ICS with HAART were with RTV rather than cobicistat. In our review we found 62 cases of ICS due to steroid-pharmacological booster interactions, outlined in Tables 1 and 2. 24 cases were due to steroid injections and 38 cases were from other exposures to non-systemic steroids. Of those 62 cases, two were associated with cobicistat rather than RTV.16,17 Of those two cases, only one, by Wassner et al described a patient who received a steroid injection.17 The patient reported by Wassner et al was a 49-year-old woman who was taking EVG, as was our patient, and received two injections of TCA a couple months apart. Of note, when she received her first injection, she was taking RTV but her regimen was changed to cobicistat when she received her second injection. She presented with a DVT, Cushingoid facies, weight gain, and skin thinning. After her diagnosis, her HAART regimen was changed to an un-boosted regimen with EVG and her symptoms resolved. The other case involving cobicistat was reported by Monge et al described a 49-year-old man who presented with abdominal striae and Cushingoid facies. He had been taking cobicistat for one year and began using inhaled fluticasone for COPD three months prior to presentation and began noticing symptoms two months prior to presentation.
Another unique aspect of our case is that our patient presented with a PE. In our review of the literature, we did not find another case of a patient who presented with a PE but we did find a case of a patient whose course was complicated by a PE.18 The case, reported by John et al, described a 51-year-old man on RTV who received 2 TCA injections in his bilateral elbows and initially complained of a pruritic rash. He was eventually diagnosed with ICS and prescribed hydrocortisone. However, his symptoms and evidence of HPA axis suppression persisted and he remained on steroids for a year after his diagnosis. Shortly after he was taken off of hydrocortisone he was diagnosed with a PE and DVT. More commonly described signs and symptoms include increased appetite, weight gain, facial plethora and cushingoid facies, which were present in our case as well.17 Less commonly patients reported were worsening hypertensive control, worsening diabetic control, bruising, insomnia and irritability.10 Given that some of the prominent and more common presenting symptoms were weight gain and Cushingoid facies, which can overlap with HIV-associated lipodystrophy, it is important to differentiate between the two. Lipodystrophy commonly causes a “buffalo hump” at the base of the neck as well as increased abdominal girth. HIV-associated lipodystrophy, however, does not result in abdominal striae or facial plethora as seen in Cushing’s Syndrome (CS).14
To evaluate for ICS and HPA axis suppression, first a morning cortisol level should be measured. Classically in CS, the morning and random cortisol levels are elevated but in ICS the morning cortisol is low. Next, ACTH levels should be obtained and cosyntropin stimulation test can be performed. ICS and HPA axis suppression are diagnosed with a low morning cortisol, a low ACTH, and failure of cosyntropin to stimulate cortisol secretion from the adrenals.10 Additionally, urinary tests can be used to detect synthetic glucocorticoids to confirm the diagnosis, as they were in our patient.
In a retrospective study, Song et al analyzed 11 patients who developed ICS with HPA axis suppression as a result of TCA injections and found that discontinuation of RTV had a more significant effect on recovery compared to those who continued RTV (3–4 months versus 2.5–6 months respectively).9 Upon discontinuation of RTV, CYP450 is no longer inhibited, normal metabolism is therefore recovered, and the HPA axis can more easily return to homeostasis. Notably, in patients whom RTV was discontinued, there was a faster resolution of symptoms.9 Along with changing HIV patients’ antiretroviral regimens from an RTV-antiretroviral based regimen to an alternate regimen, patients’ full medication lists should also be reviewed for other medications that can inhibit the CYP450 pathway. Steroids should also be avoided in such patients. However, it is important to not stop steroids suddenly. Patients being weaned off steroids should be monitored for signs and symptoms of sudden adrenal insufficiency as it can have catastrophic effects. For patients who cannot avoid steroids, such as patients with COPD who require inhaled steroids, it is best to avoid fluticasone as it has the longest half-life of all of the inhaled corticosteroids.10 Mometasone has a half-life of 5.8 hours and should also ideally be avoided.10 For patients whose HAART regimens cannot be changed and require inhaled steroids, Beclomethasone is recommended as it does not rely on CYP450 for its metabolism, and is therefore considered safe for patients on CYP450 inhibitors.19 It is also important for clinicians to monitor patients on RTV carefully for ICS, including monitoring for more subtle signs, such as worsening diabetic/hypertensive control, insomnia, and osteoporosis, to prevent delayed diagnosis.20 Thorough medication checks and clear communication between not only healthcare providers but also with patients is key to prompt diagnosis and treatment of ICS as well as other drug-drug interactions.
Conclusion
RTV and cobicistat are commonly used HIV medications, used to enhance the efficacy of other antiretrovirals and enable reduced dosage to improve ease of use for patients. However, through their potent CYP450 inhibition, they can prolong the half-life of other co-prescribed medications. Our paper focused on the interaction between cobicistat and triamcinolone. Even at low doses of exposure, such as intra-articular injections, patients can experience systemic effects of steroids, namely ICS and/or HPA axis suppression. Clinicians should be aware of the potential side effects of RTV and cobicistat and that ICS can develop even with locally administered steroids. Pharmacological booster-associated ICS requires a change in the antiviral regimen, if possible and if a change is not possible, then monitor closely for development of adrenal insufficiency. Patients with adrenal insufficiency should be treated with oral glucocorticoid replacement, such as hydrocortisone in 2–3 doses per day or prednisolone daily in the morning along with stress-dosed steroids during times of physiologic stress.21
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgment
The authors gratefully acknowledge the support of the Departments of Infectious Disease and Internal Medicine at Jersey Shore University Medical Center, Hackensack Meridian Health. We thank our patient who kindly gave his consent for this publication.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
1. Quddusi S, Browne P, Toivola B, et al. Cushing syndrome due to surreptitious glucocorticoid administration. Arch Intern Med. 1998;158(3):294.
2. Weber SL. Cushing’S syndrome attributable to topical use of lotrisone. Endocrine Pract. 1997. www.ncbi.nlm.nih.gov/pubmed/15251475.
3. Nutting CM, Page SR. Iatrogenic Cushing’s syndrome due to nasal betamethasone: a problem not to be sniffed at! Postgrad Med J. 1995;71(834):231–232. doi:10.1136/pgmj.71.834.231
4. Hughes JM, Hichens M, Booze GW, Thorner MO. Cushing’s syndrome from the therapeutic use of intramuscular dexamethasone acetate. Arch Int Med. 1986;146(9):1848.
5. Mann M, Koller E, Murgo A, et al. Glucocorticoid-like activity of megestrol. a summary of food and drug administration experience and a review of the literature. Arch Intern Med. 157(15):1651–6.
6. Marzolini C, Gibbons S, Khoo S, et al. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016;71(7):1755–1758. doi:10.1093/jac/dkw032
7. Sadarangani S, Berg ML, Mauck W, et al. Iatrogenic cushing syndrome secondary to ritonavir-epidural triamcinolone interaction: an illustrative case and review. Interdiscip Perspect Infect Dis. 2014;2014:849432. doi:10.1155/2014/849432
8. Yombi JC, et al. Iatrogenic Cushing’s syndrome and secondary adrenal insufficiency after a single intra-articular administration of triamcinolone acetonide in HIV-infected patients treated with ritonavir. Clin Rheumatol. 2008. www.ncbi.nlm.nih.gov/pubmed/18827959.
9. Song Y, Schroeder JR, Bush LM, et al. Iatrogenic cushing syndrome and secondary adrenal insufficiency related to concomitant triamcinolone and ritonavir administration: a case report and review. J Int Assoc Prov AIDS Care. 2014;13(6):511–514.
10. Foisy MM, Yakiwchuk E, Chiu I, et al. Adrenal suppression and cushing’s syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9(6):389–396.
11. Maviki M, Cowley P, Marmery H, et al. Injecting epidural and intra-articular triamcinolone in HIV-positive patients on ritonavir: beware of iatrogenic Cushing’s syndrome. Skeletal Radiol. 2013;42(2):313–315. doi:10.1007/s00256-012-1539-6
12. Molloy A, Matheson NJ, Meyer PA, et al. Cushing’s syndrome and adrenal axis suppression in a patient treated with ritonavir and corticosteroid eye drops. AIDS (London, England). 2011;25(10):1337–1339.
13. Sahıp B, Celik M, Ayturk S, et al. Iatrogenic cushing’s syndrome after topical steroid therapy for psoriasis. Indian J Dermatol. 2016;61(1):120. doi:10.4103/0019-5154.174094
14. Dubrocq G, Estrada A, Kelly S, et al. Acute development of Cushing syndrome in an HIV-infected child on atazanavir/ritonavir based antiretroviral therapy. Endocrinol Diabetes Metabol Case Rep. 2017;2017:17–0076. doi:10.1530/EDM-17-0076
15. Yeoh SW. Iatrogenic cushing syndrome from interaction between ritonavir and oral budesonide during direct acting antiviral Hepatitis C therapy. J Clin Exp Hepatol. 2016;6(3):246–249. doi:10.1016/j.jceh.2016.05.006
16. Monge E, et al. Iatrogenic Cushing syndrome due to drug interaction between inhaled fluticasone and cobicistat. Le infezioni in medicina. 2019;27(4):445–448.
17. Wassner C, Maiti S, Kodroff K, et al. “IATROGENIC Adrenal Insufficiency Secondary to Combination Therapy with Elvitegravir/Cobicistat/Tenofovir Disoproxil Fumarate/Emtricitabine and Interlaminar Triamcinolone Injection in an AIDS Patient. J Int Assoc Prov AIDS Care. 2017;16(6):535–539.
18. John G, Ollo D, Meyer P, et al. Pulmonary embolism and iatrogenic Cushing’s syndrome after co-administration of injected-triamcinolone and ritonavir. AIDS (London, England). 2013;27(17):2827–2828. doi:10.1097/QAD.0000000000000010
19. A V R. Inhalational Steroids and Iatrogenic Cushing’s Syndrome. Open Respir Med J. 2014;8(1):74–84. doi:10.2174/1874306401408010074
20. Hall JJ, Hughes CA, Foisy MM, et al. Iatrogenic cushing syndrome after intra-articular triamcinolone in a patient receiving ritonavir-boosted darunavir. Int J STD AIDS. 2013;24(9):748–752.
21. Elliot ER, Theodoraki A, Jain LR, et al. Iatrogenic Cushing’s syndrome due to drug interaction between glucocorticoids and the ritonavir or cobicistat containing HIV therapies. Clin Med. 2016;16(5):412–418. doi:10.7861/clinmedicine.16-5-412
22. Colpitts L, Murray TB, Tahhan SG, et al. Iatrogenic cushing syndrome in a 47-year-old HIV-positive woman on ritonavir and inhaled budesonide. J Int Assoc Prov AIDS Care. 2017;16(6):531–534.
23. Blondin M-C, Beauregard H, Serri O, et al. Iatrogenic cushing syndrome in patients receiving inhaled budesonide and itraconazole or ritonavir: two cases and literature review. Endocrine Pract. 2013;19(6):e138–e141.
24. Yoganathan K, David L, Williams C, et al. Cushing’s syndrome with adrenal suppression induced by inhaled budesonide due to a ritonavir drug interaction in a woman with HIV infection. Int J STD AIDS. 2012;23(7):520–521.
25. Frankel JK, Packer CD, Cushing’s syndrome due to antiretroviral-budesonide interaction. Ann Pharmacother. 2011;45(6):823–824. doi:10.1345/aph.1P731
26. McConkey HZR, Williams H, Kulasegaram R, et al. Orbital floor triamcinolone causing Cushing’s syndrome in a patient treated with Kaletra for HIV 1. BMJ Case Rep. 2013;2013(feb25 1):bcr0220125849. doi:10.1136/bcr.02.2012.5849
27. Gray D, Roux P, Carrihill M, et al. Adrenal suppression and Cushing’s syndrome secondary to ritonavir and budesonide. S Afr Med J. 2010;100(5):296–297. doi:10.7196/samj.3848
28. Kedem E, Shahar E, Hassoun G, et al. Iatrogenic Cushing’s syndrome due to coadministration of ritonavir and inhaled budesonide in an asthmatic human immunodeficiency virus infected patient. J Asthma. 2010;47(7):830–831. doi:10.3109/02770903.2010.485666
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.