Back to Journals » Journal of Experimental Pharmacology » Volume 12
Hypoglycemic, Anti-Hyperglycemic and Anti-Hyperlipidemic Effects of Bersama abyssinica Fresen (Melianthaceae) Leaves’ Solvent Fractions in Normoglycemic and Streptozotocin-Induced Diabetic Mice
Authors Kifle ZD , Anteneh DA , Atnafie SA
Received 11 August 2020
Accepted for publication 29 September 2020
Published 13 October 2020 Volume 2020:12 Pages 385—396
DOI https://doi.org/10.2147/JEP.S273959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Zemene Demelash Kifle,1 Demssie Ayalew Anteneh,2 Seyfe Asrade Atnafie1
1University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology, Gondar, Ethiopia; 2Department of Hospital Clinical Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Zemene Demelash Kifle
University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology, Gondar, Ethiopia
Tel +251918026724
Email [email protected]
Background: The leaves of Bersama abyssinica are used by traditional healers and the community for the treatment of diabetes mellitus. Thus, the current study intended to evaluate the hypoglycemic, anti-diabetic, and anti-hyperlipidemic effects of Bersama abyssinica.
Methods: The solvent fractions of Bersama abyssinica leaves were prepared. A total of 132 healthy, male Swiss albino mice weighing 20– 28 grams (age of 6– 10 weeks) were used. The antidiabetic activity of all the doses (100, 200, and 400 mg/kg) of Bersama abyssinica solvent fractions was evaluated by animal models: normoglycemic mice, oral glucose loaded mice, and diabetic mice. Diabetes was induced by a single intraperitoneal injection of streptozotocin (150mg/kg body weight). The effect of the plant extract on body weight and serum lipid levels were measured in diabetic mice. Statistical analysis was performed by using statistical package for social sciences version 24. The analyses were carried out using one-way ANOVA, followed by Tukey’s multiple comparison tests. The result was considered significant when p < 0.05.
Results: The solvent fractions of B. abyssinica at all tested doses (100, 200, and 400 mg/kg) exhibited significant (P< 0.05– 0.001) BGL reduction in all animal models. In hypoglycemic activity, the percentage reduction of baseline BGL was 25.90%, 26.36%, 38.43%, 30.96% and 49.42% for EAF200mg/kg, AQF200mg/kg, EAF400mg/kg, AQF400mg/kg, and GLC 5mg/kg, respectively. B. abyssinica at the dose of AQF 200 mg/kg (p< 0.05), AQF 400mg/kg (p< 0.001), EAF 200mg/kg (p< 0.01), and GLC 5mg/kg (p< 0.001) significantly reduced the BGL following 1-hour post-treatment as compared to the negative control. Likewise, the higher doses of the solvent fractions (400mg/kg) significantly (p< 0.001) reduced the BGL following 2- and 3-hours post-treatment as compared to the negative control. Daily administration of aqueous fraction of B. abyssinica caused a maximum reduction in fasting BGL at the fourteenth day of administration by 33.29%, 38.59%, 52.71%, and 59.66%, respectively, for AQF100, AQF200, AQF400, and GLC 5mg/kg. The aqueous fraction of B. abyssinica and the glibenclamide significantly (p< 0.05) prevent loss of body weight and showed improvement in serum lipid levels.
Conclusion: The solvent fractions of Bersama abyssinica exhibit noticeable antidiabetic activity in all animal models (normoglycemic mice, oral glucose loaded mice, and diabetic mice) and showed improvement in body weight and serum lipid profile levels, which rationalizes the claimed use of the B. abyssinica in the treatment of diabetes mellitus.
Keywords: Bersama abyssinica, diabetes mellitus, streptozotocin, solvent fraction
Introduction
Diabetes mellitus is a widespread disease that leads to impairment of glucose homeostasis and causes different complications, for instance, angiopathy, nephropathy, retinopathy, neuropathy, and neurological disorders.1 The cause of DM can be genetic, but environmental factors play a significant role.2 The prevalence of diabetes mellitus has increased in developing countries than in developed countries. According to the world health organization report, half of the diabetes mellitus cases were from South-East Asia and Western Pacific Regions.3 According to the International diabetes federation, about five million deaths were reported due to diabetes mellitus in 2017.4
World health organization recommends plant-derived traditional products for the treatment of diabetes mellitus. The use of traditional medicines for the treatment of DM has increased because of several reasons, including the participation of different concerning bodies in the production and investigations of herbal-based medicines.5,6 Previously, several studies were conducted in elucidating the medicinal values of herbal medicines for the treatment of diabetes mellitus.7 Metformin is an effective hypoglycemic medication that was discovered by the traditional method, through the use of Galega officinialis.8
Amino acids, alkaloids, glycopeptides, terpenoids, peptidoglycan, glycosides, carbohydrates, hypoglycans, polysaccharides, galactomannan gum, guanidine, and steroids are some of the main active constituents derived from medicinal plants that have anti-diabetic activity.9 Anti-hyperglycemic and anti-dyslipidemia activities of these secondary metabolites are accredited by several mechanisms such as improving the function of pancreatic beta-cells.10
B. abyssinica belongs to the genus Bersama which contains 4 species.11 The two subspecies of Bersama abyssinica (B. abyssinica Fresen. subssp. abyssinica and B. abyssinica subsp. Paullinioides) were found in East Africa. They are distributed in a different part of Africa such as Zambia, Angola, Ethiopia, Mozambique, Nigeria, Uganda, Kenya, Tanzania, Sudan, and Zimbabwe.12
The antihyperglycemic effect of plant extracts is because of the existence of different secondary bioactive compounds such as lipids, alkaloids, anthraquinones, glycosides, flavonoids, terpenoids, peptides, steroid, and additional secondary metabolites.8,13,14 In the previous study, different parts of B. abyssinica contain secondary metabolites such as steroid, vitamin, terpenes, carotenoid, flavonoids, and fatty acids. These bioactive compounds possess antiseptic, antioxidant, antimicrobial, preservative, and antitumor activities.15 An additional study revealed the existence of other phytoconstituents (phenols, tannins, alkaloids, triterpene, flavonoids, anthraquinones, steroids, polysterols, coumarins, glycosides, and absence of saponins).16
B. abyssinica has been used for the treatment of varieties of illnesses such as malaria, diarrhea, cholera, intestinal worms, amoebiasis, leprosy, diabetes, hypertension, febrile illness, and typhoid in Africa including Ethiopia.17–21 The leaves of Bersama abyssinica has been used for the treatment of diabetes mellitus in a different part of Ethiopian.17,22 Significant in vitro alpha-amylase inhibition, free radical scavenging activities, and antidiabetic activity in single-dose diabetic mice, normoglycemic mice, and oral glucose loaded mice were reported in Ethiopia.23 However, there is no previous study in the hypoglycemic, anti-hyperglycemic, and anti-hyperlipidemic effects of Bersama Abyssinica Fresen (Melianthaceae) leaves solvent fractions in normoglycemic and streptozotocin-induced diabetic mice. Thus, this study aimed to evaluate the anti-diabetic, anti-hyperlipidemic activity, and effect on the bodyweight of the repeated doses of the solvent fractions of Bersama abyssinica in normoglycemic and streptozotocin-induced diabetic mice.
Methods
Drugs, Reagents, and Instruments
Glibenclamide (Sanofi-aventis, France), glucose solution (Reyoung pharmaceuticals, China), 2, 2-Diphenyl-1-picrylhydrazyl, 3.5 (Sigma Aldrich, Germany), Streptozotocin (Fisco Research laboratories, India), deep freezer (Labfreez instrument group, Germany), Hot air oven (Medit-Medizin Technik, Germany), automated chemistry analyzer (Shenzhen Mindray Bio-medical Electronics Co., Ltd, China), pH meter (Bante Instruments, UK), desiccators, and digital electronic balance (EPH-400 Abron Exports) were used in the study.
Plant Materials
The Fresh leaves of Bersama abyssinica Fresen were collected from Tara Gedam, South Gondar zone, Amhara region in December 2018. The botanical identification and authentication of Bersama abyssinica Fresen were done by a botanist and the voucher specimen (001-A/A/A) was deposited in the Biology Department, University of Gondar, Ethiopia.
Preparation of Plant Extract and Solvent Fractionation
The leaves of Bersama abyssinica was thoroughly washed with distilled water to remove dirt and dried at room temperature (25–27°C). The plant material was coarsely grounded into powder by the electrical mill. Then, the powdered plant material was macerated separately in methanol for about 72 hrs., then the plant material was filtered via Whatman filter paper. Likewise, fresh methanol was used to re-macerate the marc, and then the filtrate of each successive maceration was concentrated by using a rotary evaporator. Lastly, the semi-dried residue was frozen in the refrigerator and dried using a lyophilizer (Lab freez, China) to entirely confiscate the remaining solvent.24,25 Solvent fractionation of the leaves extract of Bersama abyssinica was carried out using water, ethyl acetate, and chloroform. Briefly, the leaves extract of Bersama abyssinica was dissolved in 400 mL of distilled water and this solution was transferred to a separating funnel. An equal volume of chloroform was added to it and was shaken vigorously. The mixture was separated into two layers, and then chloroform was removed. The partition with chloroform was repeated two times. The chloroform layer was combined and subjected to evaporation using a hot air oven set at 40°C to get the chloroform fraction. To the separating funnel containing aqueous layer, 400 mL of ethyl acetate was added. The mixture was separated into two layers, and then the ethyl acetate was separated and the procedure was repeated two times. The ethyl acetate layer was pooled and concentrated using a hot air oven set at 40°C to obtain the ethyl acetate fraction. The remaining aqueous layer was concentrated using a hot air oven set at 40°C and frozen in a refrigerator overnight and then, concentrated in a lyophilizer to remove the water. After drying, the solvent fractions were stored in a desiccator until used for the experiment.26,27
Experimental Animals
A total of 132 healthy, male Swiss albino mice weighing 20–28 grams (age of 6–10 weeks) were purchased from Ethiopian Public Health Institute, Adds Ababa. The mice were contained in a plastic cage and kept under standard laboratory conditions with 12 h light and dark cycles. Free access to standard pellets and water ad libitum were allowed to all groups of mice. Before starting the study, all mice were acclimatized to the laboratory condition of the department of pharmacology for about seven days. Animal handling and care were carried out according to international laboratory animal guidelines.28 At the end of each experiment, all animals were sacrificed using halothane followed by cervical dislocation.
Acute Toxicity Study
The acute oral toxicity of B. abyssinica was conducted in the previous work,23 according to the Organization for Economic Cooperation and Development (OECD) guideline No. 425.29 Single female Swiss albino mice fasted for four hours on the first day of the test then; 2000 mg/kg of the extract was given by oral route using oral gavage and the mice were observed for the manifestation of behavioral and physical changes and special attention was given during the first four hours. Depend on the results from the first mice, the next 4 females’ animals were employed and fasted for about four hours and then a single dose of 2000 mg/kg of the extract was given orally and followed firmly in the same manner. The observation was sustained daily for a total of fourteen days.29
Induction of Experimental Diabetes
After overnight fasting of all groups of male mice, the body weight and the BGL were measured before diabetes mellitus induction by streptozotocin solution. Then, the streptozotocin solution at a dose of 150 mg/kg was injected through the intraperitoneal route. Following diabetes induction, five percent glucose was given to all groups of mice after 6 hours of the streptozotocin solution administration (to avoid death secondary to hypoglycemic shock). Seventy-two hours later, the mice were screened for DM. Those mice having > 200 mg/dl blood glucose levels were contained within the experiment.30
Grouping and Dosing
The animals were randomly assigned into different groups of six animals per group to perform hypoglycemic, oral glucose tolerance test, and antihyperglycemic activity test. Dose selection was based on, acute toxicity study and a previous study of the plant extract,23 as well as pilot experiments. The middle dose was one-tenth of the limit dose, the higher dose was twice the middle dose, and the lower dose was calculated as half of the middle dose.29 The study was conducted using the oral route of administration since the plant materials are traditionally used by people via the oral route.17,22
Administration of the Solvent Fractions to Normoglycemic Mice
Mice were divided into eight groups (six test groups and two control groups), each group comprising a minimum of six mice (n=6). The mice were starved overnight before performing the experiment. The negative control group (Group 1) received distilled water, while the positive control group (Group 2) received glibenclamide, and the test groups, (Group 3–5) received an aqueous solvent fraction of B. abyssinica at doses of 100, 200, 400 mg/kg body weight, and (Group 6–8) received ethyl acetate solvent fraction of B. abyssinica at doses of 100, 200, 400 mg/kg body weight. The extract was administered orally by using oral gavage. The effects of the solvent fractions were compared with the control groups. Blood sample from the control and test animals was collected after 0, 1, 2, 4, and 6 hours following the solvent fractions, standard drug, and water administration. Blood glucose level was measured using glucometer on blood drawn from the tail of the mice.31
Administration of the Solvent Fractions to Oral Glucose-Loaded Mice
Overnight fasted mice were grouped into eight groups (six test groups and two control groups), of six mice each. Then, Group 1 received distilled water (10 mL/kg) and served as a negative control, Group 2 received glibenclamide 5 mg/kg and served as a positive control, Group 3–5 received aqueous solvent fraction of B. abyssinica at doses of 100, 200, 400 mg/kg body weight, and Group 6–8 received ethyl acetate solvent fraction of B. abyssinica at doses of 100, 200, 400 mg/kg body weight. Then, following 30 min post solvent fractions, standard drug, and water administration all the mice were fed with glucose (2000 mg/kg). Blood samples were collected from the tail vein before dosing and 30, 60, and 120 minutes after administration of glucose to evaluate their blood glucose level. The blood glucose level was analyzed using glucose-oxidase peroxide reactive strips.32
Repeated Administration of the Solvent Fraction to Streptozotocin-Induced Diabetic Mice
Overnight fasted mice were grouped into six groups (three test groups and three control groups), of six mice each. Streptozotocin-induced diabetic mice were administered (orally) 100mg/kg, 200mg/kg, and 400mg/kg of the aqueous solvent fraction of B. abyssinica for test groups; 5mg/kg glibenclamide for the positive control group; and 1 mL of distilled water for the negative control group. There was also a normal control group that received 1 mL of distilled water orally. Aqueous solvent fraction, standard drug, and water were administered once daily for 14 days. Blood glucose level and body weight were measured on 0-day, 7th day, and 14th day following the induction. The blood glucose level was measured using glucometer blood drawn from the tail of the mice.
Effect of the Solvent Fraction on Serum Lipid Level of Diabetic Mice
On day 15, blood samples were collected in a sterile tube by cardiac puncture under halothane anesthesia from the overnight fasted diabetic mice. The blood samples were left at room temperature for 2h and then centrifuged. The supernatant was immediately separated from the pellet to prepare serum samples to determine the level of triglyceride, total cholesterol, high-density lipoprotein, and low-density lipoprotein using an automated chemistry analyzer.33,34
Statistical Analysis
The data that was obtained from the experiments were expressed as mean ± SEM. Statistical analysis was done using statistical package for social sciences (SPSS) version 24. Between and within-group analyses were carried out by using one-way ANOVA, subsequently Tukey’s multiple comparison tests. Finally, the findings were considered significant when p-value < 0.05.
Results
The Percentage Yield of Plant Material Extraction
Among solvents tested, aqueous fraction resulted in the highest extraction yield (70.62%), followed by chloroform fraction (11.44%), and ethyl acetate fraction (9.0%).
Acute Toxicity Test
After a single oral administration of 2000 mg/kg of B. abyssinica, the mice did not show any signs of toxicity (lacrimation, hair erection, convulsion, coma, and death) during 24 hours as well as during the fourteen days of observation. This finding confirms that the LD50 of B. abyssinica is > 2gm/kg.
Hypoglycemic Activity of Solvent Fractions of B. abyssinica in Normoglycemic Mice
The blood glucose level was measured every 30 minutes for the first 1 h and then at 2nd, 4th, and 6th hours following administration of the three doses (100, 200, and 400 mg/kg) of the solvent fractions, glibenclamide 5mg/kg, and distilled water to each group of mice. In this study, a statistically significant difference in BGL was not noticed in the first 1 h after administration of the solvent fractions. A statistically significant BGL reduction was noticed at two hours following administration of EAF400 mg/kg (p<0.05), AQF400 mg/kg (p<0.01), and GLC5mg/kg (p<0.001), compared with baseline blood glucose level. However, the lower doses (100 mg/kg) and the middle doses (200 mg/kg) of the solvent fractions did not display a statistically significant variance in BGL at 2 h as compared to baseline blood glucose level. At 4th h, EAF 200 mg/kg and 400mg/kg (p<0.05, for both), AQF 200 and 400mg/kg (p<0.01, for both), and GLC (p<0.001) revealed a significant BGL drop as compared with the negative control. Furthermore, at 6th hour after administration of the solvent fractions, EAF 200 and 400mg/kg (p<0.01, for both), AQF 200 mg/kg (p<0.01), 400 mg/kg (p<0.001), and GLC (p<0.001) revealed a statically significant BGL drop as compared to the negative control. Similarly, AQF and EAF at doses of 200 and 400 mg/kg exhibited a significant (p<0.001) blood glucose decrement as compared to the baseline BGL at 6th h of administration of the solvent fractions. However, the lower dose (100mg/kg) of the solvent fractions didn`t display a significantly (p>0.05) BGL reduction as compared to the negative control group and baseline BGL at all points of time. The percentage reduction of baseline blood glucose levels was 25.90%, 26.36%, 38.43%, 30.96% and 49.42% for EAF200mg/kg, AQF200mg/kg, EAF400mg/kg, AQF400mg/kg, and GLC 5mg/kg, respectively (Table 1).
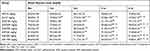 |
Table 1 Hypoglycemic Activity of Solvent Fractions in Normoglycemic Mice |
Effect of B. abyssinica Solvent Fractions on OGTT in Normal Mice
The effects of the solvent fractions of B. abyssinica on OGTT are shown in Table 2. The BGL of all groups before solvent fraction, GLC, and distilled water administration didn`t show a significant difference as compared to each other. However, after 2000 mg/kg glucose administration all groups of mice showed a significant (p < 0.001) elevation in blood glucose level at thirty minutes as compared to the baseline fasting blood glucose level irrespective of the treatments received, this indicates the induction of hyperglycemia by glucose administration. Administration of B.abyssinica at a dose of AQF 200 mg/kg (p<0.05), AQF 400mg/kg (p<0.001), EAF 200mg/kg (p<0.01), and GLC 5mg/kg (p<0.001) significantly reduced the BGL following 1-hour post-treatment with the solvent fractions and the standard drug as compared to the negative control. Likewise, the higher doses of the solvent fractions (400mg/kg) significantly (p<0.001) dropped the BGL following 2- and 3-hours post-treatment as compared to negative control groups.
 |
Table 2 Effect of Solvent Fractions on OGTT in Normal Mice |
Within a group analysis revealed that oral glucose challenge caused a significant drop in BGL at 1 and 2 hours as compared to the respective blood glucose level at 30 minutes post 2mg/kg glucose solution administration in all groups (Table 2).
Anti-Hyperglycemic Activity of the Repeated Doses of B. abyssinica Solvent Fraction
The antihyperglycemic activity of aqueous fraction of B. abyssinica on fasting BGL of STZ-induced diabetic mice was determined weekly in all groups of mice. Following the induction of diabetes by streptozotocin, all groups of diabetic mice exhibited significant (p < 0.001) variances in BGL as compared to normal mice across all time intervals. However, all diabetic mice didn`t show significant variance in baseline fasting blood glucose levels. Treatment with all doses (100 mg/kg, 200 mg/kg, and 400 mg/kg) of AQF displayed a significant (p < 0.001) reduction in BGL as compared to diabetic and normal control groups on the seventh and fourteenth days of treatment. Likewise, the glibenclamide treated group showed a significant (p < 0.001) drop in blood glucose level as compared to diabetic and normal control groups on the seventh and fourteenth days of treatment.
Within a group analysis showed that the aqueous fraction of B. abyssinica treated groups produced a significant (P < 0.001) drop in the blood glucose level on the seventh and fourteenth days as compared to the baseline BGL. Similarly, GLC 5mg/kg significantly (P < 0.001) dropped the blood glucose level on the seventh and fourteenth days as compared to the baseline BGL. As revealed in Figure 1, repeated treatment by aqueous fraction of B. abyssinica at doses of 100mg/kg, 200mg/kg and 400mg/kg produced a higher reduction in fasting BGL at the 14th days, 33.29%, 38.59%, 52.71%, and 59.66%, respectively for AQF100, AQF200, AQF400, and GLC 5mg/kg (Table 3).
 |
Table 3 Anti-Hyperglycemic Activity of the Repeated Doses of Solvent Fraction in STZ-Induced Diabetic Mice |
 |
Figure 1 Effect of repeated daily doses of Bersama abyssinica on blood glucose level of diabetic mice. |
Effect of the Repeated Daily Doses of the Aqueous Fraction on the Bodyweight
The effect of the repeated daily doses of the solvent fraction of B. abyssinica on the bodyweight of both in the diabetic and normal mice are summarized in Table 4. Before the indication of diabetes with streptozotocin, all groups of mice didn`t show a significant difference in body weight. However, Streptozotocin showed a significant (P < 0.001) decrement in the bodyweight of the diabetic control group on the seventh and fourteenth days of treatment as compared to the normal control group. Following treatment of diabetic mice with an aqueous fraction of B. abyssinica for 2 weeks improved weight loss in STZ-induced diabetic mice as compared to the diabetic control group. Only AQF400 mg/kg and GLC 5mg/kg improved weight loss significantly (P < 0.01) compared with the diabetic control group after 7th days of treatment. In contrast, AQF100 mg/kg and AQF200 mg/kg failed to improve weight loss significantly after the 7th day of treatment. However, the three aqueous fraction doses AQF100 mg/kg (P < 0.05), AQF 200mg/kg (P < 0.001), and AQF400 mg/kg (P < 0.001) and GLC5 mg (P < 0.001) improved weight loss significantly as compared with diabetic control group following fourteen days of administration of the solvent fractions and the standard drug.
 |
Table 4 Effect of the Repeated Daily Doses of Solvent Fraction on Body Weight of Diabetic Mice |
Effect of Repeated Daily Doses of the Solvent Fraction on Serum Lipid Level
Statistically significant (p<0.001) increment in serum concentrations of very-low-density lipoprotein cholesterol, serum total cholesterol, serum triglyceride, and low-density lipoprotein cholesterol with a progressive decrease in serum high-density lipoprotein cholesterol were observed within the diabetic control group compared with the normal control group.
Between-group analysis showed that all tested doses of the aqueous fraction (AQF100 mg/kg, AQF200 mg/kg, and AQF400 mg/kg) revealed a statically significant improvement in serum lipid profiles: low-density lipoprotein cholesterol (p<0.001); serum total cholesterol (p<0.001); and serum triglyceride (p<0.01, p<0.01, p<0.001, respectively for the three doses); high-density lipoprotein cholesterol (p<0.01, p<0.001, p<0.001, respectively); and only AQF400 mg/kg displayed a significant (p<0.05) improvement in serum lipid profile of very-low-density lipoprotein cholesterol when compared with the diabetic control group. Thus, treatment with leaf aqueous fraction of B. abyssinica and glibenclamide 5 mg/kg to diabetic mice significantly and dose-dependently improved TC, TG, and LDL-c, and VLDL-c levels as compared to the diabetic control group. Although considerably increase in serum HDL-cholesterol level was shown in the aqueous fraction of B. abyssinica treated groups compared to the diabetic control group (Table 5).
 |
Table 5 Effect of Repeated Daily Doses of Solvent Fraction on Serum Lipid Level of Diabetic Mice |
Discussion
The study was conducted to investigate the hypoglycemic, anti-diabetic, and anti-hyperlipidemic activity of the solvent fractions of Bersama abyssinica in normoglycemic and diabetic mice with the view to validating its traditional use in the management of DM in humans. DM is a medical condition that is characterized by a chronic increment of blood glucose level secondary to a relative/absolute shortage of insulin/resistance to the action of insulin.35
The streptozotocin-induced animal model can be explained as a valuable animal model to investigate the activity of anti-diabetic agents such as glibenclamide against DM. STZ is well-known to induce diabetes, hyperinsulinemia, or hyperglycemia by damaging the pancreatic β cells.36 Streptozotocin gets into β-cell using GLUT2 transporter and resulting in the alkylation of DNA, thereby brings the activation of poly ADP ribosylation. Poly ADP-ribosylation leads to depletion of cellular NAD+ and ATP.37 Abnormalities in lipid profiles are common complications in diabetes mellitus.38 Following the administration streptozotocin solution (150mg/kg) to all groups of mice, diabetes mellitus was effectively induced. This was validated with sustained elevation of blood glucose level after 72 hours of injection of streptozotocin.
In the normoglycemic model, the mice were treated with a single dose ranging from 100–400mg/kg of aqueous solvent fraction, ethyl acetate solvent fraction, and GLC 5mg/kg for each group containing six mice showed a significant drop in BGL of normoglycemic mice. In a previous study, both the crude and its solvent fractions (chloroform, ethyl acetate, and aqueous) showed a statically significant alpha-amylase inhibition activity at different tested doses.23 This hypoglycemic effect of the solvent fractions could be because of the inactivation of the α-amylase activity. In the previous experimental study, GLC can cause hypoglycemia effect through stimulation of insulin secretion from the pancreatic β cells.39 Similarly, the solvent fractions of B. abyssinica could possess an insulin-like effect/stimulate insulin secretion from the pancreas. However, the lower doses of the ethyl acetate solvent fraction and the aqueous solvent fraction of Bersama abyssinica leaves did not show any significant hypoglycemic effect after the solvent fractions were administered. The current findings are in agreement with previously reported similar studies.40–42
After oral administration of B. abyssinica solvent fractions at all tested doses (100mg/kg, 200mg/kg and 400mg/kg) to glucose challenged mice exhibited a significant (p <0.001) drop in blood glucose level at 1- and 2-hours post-treatment as compared to the negative control. Likewise, GLC 5mg/kg showed a significant reduction in blood glucose levels early from the 30 minutes onwards (Table 2). This indicates that the solvent fractions can effectively control the elevated blood glucose level, showing its possible benefit to lower postprandial hyperglycemia and related complications of DM. Regulator control of postprandial hyperglycemia is the most common approach for the management of DM. OGTT measures the body’s ability to utilize glucose and it is seen as the “gold standard” in diagnosing diabetes mellitus.43 The observed activity may fairly be a due reduction of glucose absorption into the bloodstreams via inhibition of α-amylase as a previous study supports the α-amylase inhibitory activity of B. abyssinica.23 Furthermore, the observed activity might be due to other possible mechanisms such as decreased gluconeogenesis, stimulation of glycogenesis in the liver, and enhanced tissue glucose utilization.32
The other possible anti-hyperglycemic effect of B.abyssinica on the OGTT might be due to the attendance of phytoconstituents such as flavonoids,44 since flavonoids can regenerate pancreatic β-cells, inhibiting the glucose transporter activity from the intestine, and increasing the peripheral utilization of glucose.45
In the repeated daily doses, the solvent fractions of B. abyssinica and the standard drug have shown a significant reduction in fasting blood glucose levels as compared to the diabetic control group. All the tested doses of the solvent fractions (100mg/kg, 200mg/kg and 400mg/kg) caused a maximum drop in fasting BGL at the 14th days, 33.29%, 38.59%, 52.71%, and 59.66%, respectively for AQF100, AQF200, AQF400, and GLC 5mg/kg (Table 3). The current finding is in agreement with previous similar studies.41,46 This indicates that the solvent fractions showed its effect in a dose-dependent manner, the difference between the three doses might be because of the higher doses of the solvent fractions contains higher amounts of phytoconstituents responsible for more reduction of the fasting BGL than the lower doses of the solvent fractions. Similarly, glibenclamide reduces the fasting BGL by blockage of ATP sensitive K+ (KATP) channels, leads to membrane depolarization, activate voltage-gated Ca2+ channels, a rise in cytosolic (Ca2+), and then release of insulin in the pancreas.47
Oxidative stress plays a key role in the pathogenesis and progression of DM. β-cells are more sensitive to different free radicals which can be produced by streptozotocin.48,49 In the previous study, the leaf crude and solvent fractions of B.abyssinica revealed potent antioxidant activity.23 This indicates that the β-cell protective effect of the solvent fractions of B. abyssinica may be contributed to its anti-hyperglycemic activity in streptozotocin-induced diabetic mice.
The loss of body weight in diabetic mice is due to increased muscle wasting,50 and catabolism of tissue proteins.51 Similarly, in the current finding, streptozotocin produced a significant loss of weight in diabetic control. However, all tested doses (100mg/kg, 200mg/kg, and 400mg/kg) of the aqueous fraction treated groups have shown significant body weight gain on day 14 from the initial (0) day when compared with the diabetic control group. The ability of aqueous solvent fraction to prevent weight loss may be due to its ability to reduce the elevated blood glucose level which is the sign of suitable glucose utilization,52 preventing muscle wasting and controlling protein turn over and/or improvement in diabetes mellitus associated disorders.53 The current finding showed that all tested doses (100mg/kg, 200mg/kg, and 400mg/kg) of the aqueous fraction have shown a significant body weight gain on day 14 from the initial (0) day as compared to diabetic control. This result is consistent with previous similar studies.41,42,54
Abnormalities in lipid profiles are common complications in diabetes mellitus. In addition to its anti-hyperglycemic effect, an aqueous fraction of B. abyssinica was also able to alter the levels of serum lipid levels such as TC, TG, HDL, VLDL, and LDL cholesterol levels in diabetic mice suggesting a remarkable anti-hyperlipidemic effect. The serum lipid levels are typically raised in diabetic patients and such increments indicate a risk factor for CHD.55 Activation of hormone-sensitive lipase during insulin deficiency causes an increase in free fatty acid mobilization from adipose tissue. In addition, hyperglycemia is accompanied by a rise in TC, TG, LDL-C, and a fall in HDL-C.56
In this study, a significant (P<0.001) increment in the TC, TG, and LDL levels on diabetic mice when compared with normal control groups. This elevated TG level in diabetic mice could be because of the higher production of triglyceride-rich lipoprotein particles in the liver and diminished catabolism. Since insulin has a dominant inhibitory effect on lipolysis in adipocytes, insulin deficiency is linked with excess lipolysis and increased influx of free fatty acids to the liver.57 The increment of LDL and VLDL levels in the STZ diabetic mice could be because of the over synthesis of LDL and VLDL by the liver.58 Previous studies have revealed that the elevated TG level-rich lipoproteins might be due to the reduction of LPL activity as a result of its glycation.59
The highest reduction in TC, TG, VLDL, and LDL was attended at the higher dose (400mg/kg) of an aqueous fraction. This dose-dependent activity of the aqueous fraction of B. abyssinica might be attributed to the higher dose (400 mg/kg) contains a higher amount of secondary metabolites responsible for a significant reduction in serum STG, STC, LDL-C, and VLDL-C; and increment in HDL-C than the lower (100mg/kg) and the middle doses (200mg/kg). The reductions of TG, TC, and LDL level by an aqueous fraction of B. abyssinica might be due to the involvement of polyphenolic part of the extract in preventing the formation of AGEs in diabetic mice.60 The aqueous fraction of B. abyssinica fiber may delay the absorption of glucose and fatty acids, therefore providing fewer substrate for the production of triglycerides.61
HDL and plasma lectin cholesterol acyltransferase are believed to be involved in the transport of cholesterol from extrahepatic tissues to the liver for its excretion.62 The higher levels of cholesterol associated with HDL and the increase in the activity of plasma lectin cholesterol acyltransferase on the administration of aqueous fraction of B. abyssinica may result in a higher amount of cholesterol being removed from extrahepatic tissues which may contribute to the anti-hypercholesterolemia. Thus, the lowering in cholesterol levels of serum and tissues by the administration of aqueous fraction of B. abyssinica would seem to be mediated through its increased rate of degradation to bile acids and neutral sterols.
The secondary metabolites existing in the plants have a role in the treatment of various diseases.63 In the previous study, the extract of B. abyssinica contains phytoconstituents including, phenols, tannins, alkaloids, triterpene, flavonoids, anthraquinones, steroids, polysterols, coumarins, and glycosides.16 Flavonoids, tannin, and an alkaloid were some of the renowned compounds that were isolated from the extracts that are capable of reducing the elevated BGL.64–66 Therefore, the significant anti-diabetic and anti-hyperlipidemic activity of the solvent fractions of B. abyssinica could be because of the attendance of the aforementioned secondary metabolites in the plant.
Conclusions
In the present study, the administration of solvent fractions of B. abyssinica has shown significant antidiabetic activity in normoglycemic mice, oral glucose loaded mice, and streptozotocin-induced diabetic mice. The solvent fractions also improve the serum biochemical parameters such as lipid profile: total cholesterol, triacylglycerol, high-density lipoprotein cholesterols, low-density lipoprotein cholesterol, and very-low-density lipoprotein cholesterol. From the observed antidiabetic activity of solvent fractions of B. abyssinica, the study may support the use of B. abyssinica herb for the treatment of diabetes mellitus by a traditional healer. Further researches are needed to identify the lead compound (s) present in Bersama abyssinica Fresen (Melianthaceae) with its (their) molecular mechanism of action on PPAR, insulin sensitization, histological analysis, and other insulin targets based on the pathophysiology of diabetes mellitus.
Availability of Materials and Data
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
BGL, blood glucose level; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; STZ, streptozotocin; TC, total cholesterol; TG, triglycerides; VLDL, very low-density lipoprotein.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the research and ethics committee of Department of Pharmacology, School of Pharmacy, University of Gondar to with reference number (school of pharmacy 04-116-2011). The mice were used and sacrificed under the Guide for Use and Care of Laboratory Animals.
Acknowledgment
The authors acknowledge the University of Gondar for material support and for allowing to use of the laboratory facility.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Kristova V, Líšková S, Sotnikova R, Vojtko R, Kurtanský A. Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats. Physiological Research. 2008;57:3.
2. Li W, Zheng H, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92(1):1–21. doi:10.1016/j.jep.2003.12.031
3. Organization WH. Global Report on Diabetes. World Health Organization; 2016.
4. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
5. García-Risco MR, Mouhid L, Salas-Pérez L, et al. Biological activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) plant extracts. Plant Foods Human Nutrition. 2017;72(1):96–102. doi:10.1007/s11130-016-0596-8
6. Gupta B, Sharma I, Kohli N, Sharma S, Rathi A, Sharma A. Preliminary clinical assessment and non-toxicity evaluation of an ayurvedic formulation BGR-34 in NIDDM. J Traditional Complementary Med. 2018;8(4):506–514. doi:10.1016/j.jtcme.2017.11.004
7. Jani DK, Goswami S. Antidiabetic activity of Cassia angustifolia Vahl. and Raphanus sativus Linn. leaf extracts. J Traditional Complementary Medicine. 2020;10(2):124. doi:10.1016/j.jtcme.2019.03.002
8. Grover J, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81–100. doi:10.1016/S0378-8741(02)00059-4
9. Prabhakar PK, Doble M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med. 2011;17(8):563.
10. Fatima A, Agrawal P, Singh PP. Herbal option for diabetes: an overview. Asian Pacific J Tropical Disease. 2012;2:S536S44. doi:10.1016/S2222-1808(12)60216-3
11. Hegazy M-E, Ngandeu F, Neguim G, et al. C-Glucoside xanthone from the stem bark extract of Bersama engleriana. Pharmacognosy Res. 2010;2(4):229. doi:10.4103/0974-8490.69110
12. Mikkelsen K, Seberg O. Morphometric analysis of the Bersama abyssinica Fresen. complex (Melianthaceae) in East Africa. Plant Systematics Evolution. 2001;227(34):157–182. doi:10.1007/s006060170046
13. Demissew S, Friis I, Awas T, et al. Four new species of Aloe (Aloaceae) from Ethiopia, with notes on the ethics of describing new taxa from foreign countries. Kew Bulletin. 2011;66(1):111–121. doi:10.1007/s12225-011-9263-2
14. Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutrition, Metabolism Cardiovascular Diseases. 2005;15(3):149–160. doi:10.1016/j.numecd.2005.05.001
15. Zekeya N, Chacha M, Shahada F, Kidukuli A. Analysis of phytochemical composition of Bersama abyssinica by gas chromatography-mass spectrometry. J Pharmacognosy Phytochemistry. 2014;3(4):246–252.
16. Mathewos Anza FW, Libsu S, Mamo F. Milkyas endale phytochemical screening and antibacterial activity of leaves extract of bersama abyssinica. J of Advanced Botany Zoology. 2015;3:2.
17. Amit L, Vikas G, Vaibhav T, Vikash K, Siddhartha G, Lather A. Phytochemistry and pharmacological activities of bersama engleriana guerke-an overview. Sexually Transmitted Diseases. 2010;11:12.
18. Edeoga H, Okwu D, Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. African j Biotechnol. 2005;4(7):685–688. doi:10.5897/AJB2005.000-3127
19. Birhanu Z, Endale A, Shewamene Z. An ethnomedicinal investigation of plants used by traditional healers of Gondar town, North-Western Ethiopia. J Medicinal Plants Studies. 2015;3(2):36–43.
20. Bekele G, Reddy PR. Ethnobotanical study of medicinal plants used to treat human ailments by Guji Oromo tribes in Abaya District, Borana, Oromia, Ethiopia. Universal J Plant Sci. 2015;3(1):1–8.
21. Esubalew ST, Belete A, Lulekal E, Gabriel T, Engidawork E, Asres K. Review of ethnobotanical and ethnopharmacological evidences of some ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiopian J Health Development. 2017;31(3):161–187.
22. Schmelzer G, Gurib-Fakim A. Plant Resources of Tropical Africa 11 (1). Medicinal Plants 1. Wageningen/Leiden: PROTA Foundation. Backhuys Publishers; 2008.
23. Kifle ZD, Enyew EF. Evaluation of in vivo antidiabetic, in vitro α-amylase inhibitory, and in vitro antioxidant activity of leaves crude extract and solvent fractions of bersama abyssinica fresen (melianthaceae). J Evid Based Integr Med. 2020;25:2515690x20935827. doi:10.1177/2515690X20935827
24. Satyajit D, Sarker ZL, Alexander I. Gray. Natural Products Isolation-Second Edition. Biotechnology™. United Kingdom: Humana Press Inc; 2006.
25. Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.). Cufod Leaves in Rats Frontiers Pharmacol. 2016;7:97.
26. Molla M, Gemeda N, Abay SM. Investigating potential modes of actions of Mimusops kummel fruit extract and solvent fractions for their antidiarrheal activities in mice. Evidence-Based Complementary Alternative Medicine. 2017;2017.
27. Kashimawo A, Kolawole J, Ahmadu A. Bioassay Guided Fractionation and Α-Amylase Inhibitory Activity of Lupeol from the Stem Bark of Faidherbia Albida Del. Mimosaceae. International Journal of Pharmaceutical Science Invention. 2017;6(6):29–32.
28. Care I, Animals U, Resources N. Guide for the care and Use of Laboratory animals. National Academies; 1985.
29. OCDE O. Acute oral Toxicity: up and Down Procedure. OECD Guideline Testing Chemicals. 2008;425:1–2.
30. Baquer NZ, Kumar P, Taha A, Kale R, Cowsik S, McLean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36(2):383–396. doi:10.1007/s12038-011-9042-0
31. Birru EM, Abdelwuhab M, Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2015;15(1):321.
32. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7(2):110–113.
33. Bowe JE, Franklin ZJ, Hauge-Evans AC, et al. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222(3):G13G25. doi:10.1530/JOE-14-0182
34. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;dmm:006239.
35. Krishnaveni M, Mirunalini S, Karthishwaran K, Dhamodharan G. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn. (Euphorbiaceae) on streptozotocin induced diabetic rats. Pak J Nutr. 2010;9(1):43–51. doi:10.3923/pjn.2010.43.51
36. Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman H-J. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61(4):356–360.
37. Budin SB, Othman F, Louis S, et al. Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. Rom J Morphol Embryol. 2009;50(1):23–30.
38. Gibbons GF. Hyperlipidaemia of diabetes. Clin Sci. 1986;71(5):477–486. doi:10.1042/cs0710477
39. Gebreyohannis T, Shibeshi W, Asres K. Effects of solvent fractions of caylusea abyssinica (fresen.) fisch. and mey. on blood glucose levels of normoglycemic, glucose loaded and streptozotocin-induced diabetic rodents. J Natural Remedies. 2013;14(1):67–75.
40. Arya A, Nyamathulla S, Noordin MI, Mohd MA. Antioxidant and hypoglycemic activities of leaf extracts of three popular Terminalia species. E-J Chem. 2012;9.
41. Kifle ZD, Yesuf JS, Atnafie SA. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of hagenia abyssinica (rosaceae). J Exp Pharmacol. 2020;12:151–167. doi:10.2147/JEP.S249964
42. Alema NM, Periasamy G, Sibhat GG, Tekulu GH, Hiben MG. Antidiabetic activity of extracts of terminalia brownii fresen. stem bark in mice. J Exp Pharmacol. 2020;12:61. doi:10.2147/JEP.S240266
43. Etuk E. Animals models for studying diabetes mellitus. Agric Biol JN Am. 2010;1(2):130–134.
44. Beshir K, Shibeshi W, Ejigu A, Engidawork E. In-vivo wound healing activity of 70% ethanol leaf extract of Beciumgrandiflorum Lam. (Lamiaceae) in mice. Ethiopian Pharmarmaceutical J. 2016;32:117–130. doi:10.4314/epj.v32i2.3
45. Jadhav R, Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Group. 2012;1:100g.
46. Belayneh YM, Birhanu Z, Birru EM, Getenet G. Evaluation of in vivo antidiabetic, antidyslipidemic, and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae). J Exp Pharmacol. 2019;11:29. doi:10.2147/JEP.S192264
47. TOMAI F CREA, Gaspardone F, Versaci A, et al. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. American Heart Association. 1994;
48. Spinas GA. The dual role of nitric oxide in islet β-cells. Physiology. 1999;14(2):49–54. doi:10.1152/physiologyonline.1999.14.2.49
49. Šoltésová D, Herichová I. On the mechanisms of diabetogenic effects of alloxan and streptozotocin. Diabetologie, Metabolismus, Endokrinologie, Výživa. 2011;14:130–138.
50. Swanston-Flatt S, Day C, Bailey C, Flatt P. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33(8):462–464. doi:10.1007/BF00405106
51. Ravi K, Ramachandran B, Subramanian S. Protective effect of Eugenia jambolana seed kernel on tissue antioxidants in streptozotocin-induced diabetic rats. Biol Pharm Bull. 2004;27(8):1212–1217. doi:10.1248/bpb.27.1212
52. Pari L, Satheesh MA. Antidiabetic activity of Boerhaavia diffusa L.: effect on hepatic key enzymes in experimental diabetes. J Ethnopharmacol. 2004;91(1):109–113. doi:10.1016/j.jep.2003.12.013
53. Oyedemi S, Bradley G, Afolayan A. Antidiabetic activities of aqueous stem bark extract of Strychnoshenningsii Gilg in streptozotocin-nicotinamide type 2 diabetic rats. IJPR. 2012;11(1):221.
54. Hammeso WW, Emiru YK, Ayalew Getahun K, Kahaliw W. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. Evidence-Based Complementary Alternative Medicine. 2019;2019.
55. Sahib NG, Anwar F, Gilani AH, Hamid AA, Saari N, Alkharfy KM. A potential source of high-value components for functional foods and nutraceuticals-a review. Phytotherapy Research. 2013.
56. Gao D, Li Q, Li Y, et al. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan‐induced diabetic rats. Phytother Res. 2009;23(9):1257–1262. doi:10.1002/ptr.2603
57. Enkhmaa B, Ozturk Z, Anuurad E, Berglund L. Postprandial lipoproteins and cardiovascular disease risk in diabetes mellitus. Curr Diab Rep. 2010;10(1):61–69. doi:10.1007/s11892-009-0088-4
58. Samatha P, Venkateswarlu M, Siva Prabodh V. Lipid profile levels in type 2 diabetes mellitus from the tribal population of Adilabad in Andhra Pradesh, India. J Clin Diagnostic Res. 2012;6(4):590–592.
59. Puddu A, Sanguineti R, Mach F, Dallegri F, Viviani GL, Montecucco F. Update on the protective molecular pathways improving pancreatic beta-cell dysfunction. Mediators Inflamm. 2013;2013.
60. Jia Q, Liu X, Wu X, et al. Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2009;16(8):744–750. doi:10.1016/j.phymed.2008.12.012
61. Jelodar G, Mohsen M, Shahram S. Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced diabetic rats. African J Traditional, Complementary Alternative Medicines. 2007;4(3):299–305. doi:10.4314/ajtcam.v4i3.31223
62. Shiju TM, Pragasam V. Lipoprotein modification: a hallmark in the progression of diabetic nephropathy. Webmedcentral. 2012.
63. Egga E, Adeyanju O, Agyeno O. Preliminary phytochemical, antimicrobial and proximate analysis of tender leaves of psidium guajava l in jos, plateau state, nigeria. Asian Review of Environmental and Earth Sciences. 2014.
64. Carlson TJ, King SR. From plant to patient: an ethnomedical approach to the identification of new drugs for the treatment of NIDDM. Diabetologia. 1997;40:614–617. doi:10.1007/s001250050724
65. Ragavan B, Krishnakumari S. Antidiabetic effect ofT. arjuna bark extract in alloxan induced diabetic rats. Indian J Clin Biochem. 2006;21(2):123. doi:10.1007/BF02912926
66. Miura T, Koike T, Ishida T. Antidiabetic activity of green tea (Thea sinensis L.) in genetically type 2 diabetic mice. J Health Science. 2005;51(6):708–710. doi:10.1248/jhs.51.708
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
