Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 12
Hypoglycemic and Antihyperglycemic Activities of 80% Methanol Root Extract of Acanthus polystachyus Delile (Acanthaceae) in Type 2 Diabetic Rats
Authors Derebe D , Wubetu M , Alamirew A
Received 28 July 2020
Accepted for publication 11 September 2020
Published 25 September 2020 Volume 2020:12 Pages 149—157
DOI https://doi.org/10.2147/CPAA.S273501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Dagninet Derebe, 1 Muluken Wubetu, 2 Amare Alamirew 3
1Department of Pharmacy, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 2Department of Pharmacy, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 3Department of Community Health, ALKAN Health Science, Business & Technology College, Bahir Dar, Ethiopia
Correspondence: Dagninet Derebe Email [email protected]
Background: The morbidity and mortality rate from diabetic mellitus are increasing in the world especially in low- and middle-income countries; hence, it is necessary to evaluate the efficacy and safety of medicinal plants to support existing drugs in treating diabetes mellitus. Therefore, the aim of this study was to evaluate the hypoglycemic effect of 80% methanol root extract of Acanthus polystachyus in normoglycemic, hyperglycemic, and streptozotocin–nicotinamide induced diabetic rats.
Methods: Male albino Wistar rats were divided into five groups (n=6) in all three models. In all models, group one rats served as a negative control and were received vehicle (10mL/kg distilled water), whereas group two (APRE100), three (APRE200), and four (APRE400) were treated with 100, 200, and 400mg/kg of extract, respectively, and group five were treated with glibenclamide (5mg/kg) and served as a positive control. Blood glucose levels were measured at different time points by taking blood from their tails. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test to carry out comparisons between and within-group and P < 0.05 was considered as statistically significant.
Results: The root of Acanthus polystachyus reduces peak blood sugar levels significantly after the loading of oral glucose at all tested doses. In streptozotocin–nicotinamide-induced type 2 diabetic rats, the daily oral administration of the crude extracts showed a significant reduction of blood glucose level at all tested doses compared to the negative control group. However, the extract did not reduce blood glucose levels in normoglycemic rats at all tested doses compared to both negative and positive control.
Conclusion: From this study, it can be concluded that the root extract of Acanthus polystachyus showed an antihyperglycemic effect in hyperglycemic and diabetic rats but lack hypoglycemic effect in normoglycemic rats. Hence, the plant root may be a good candidate for the development of new antidiabetic drugs.
Keywords: Acanthus polystachyus, antihyperglycemic, type 2 diabetes, streptozotocin, nicotinamide
Background
Diabetes Mellitus is one of the most common metabolic disorders characterized by hyperglycemia due to lack of insulin secretion or/and action.1–3 As a result of persistent hyperglycemia, there could be micro-and macrovascular complications that result in significant morbidity and mortality. It is considered as one of the five leading causes of death worldwide.4
There are a lot of pathogenic processes that are involved in the development of diabetes. Based on this pathogenic process diabetes is classified into four types; type 1 Diabetes Mellitus due to immune-mediated β-Cell destruction, type 2 Diabetes Mellitus due to insulin resistance, other specific types of diabetes, and gestational Diabetic Mellitus. Diabetes type 2 is the most common which accounts 90–95% of the total Diabetic Mellitus patients.1,5,6
It is estimated that the global prevalence of diabetes will increase from 382 to 592 million people between 2013 and 2035 because of factors such as aging, sedentary lifestyle, eating habits, and obesity. Obesity is associated with the increased production of reactive oxidative species, and it is one of the main risk factors for the development of type 2 diabetes, which is again characterized by insulin resistance and imbalanced glycemic homeostasis.7–9
The currently available oral hypoglycemic and antihyperglycemic drugs for type-II diabetes have their limitations, adverse effects, and secondary failures. Therefore, to reduce their cost, limitation, and adverse effects, the focus has been shifted towards the medicinal plants for safe and effective use. Recently, a lot of medicinal plants are being investigated for their role in the pharmacotherapy of diabetes.10
It was estimated that more than 1000 plant species are traditionally being used for the treatment of Diabetic Mellitus. Studies have shown that the antidiabetic activity of medicinal plants is mainly due to the presence of alkaloids, phenolic compounds, flavonoids, and terpenoids.10–15 The methanol extract of Acanthus polystachyus Delile (Acanthaceae) roots also contain secondary metabolites like flavonoids, polyphenols, and terpenoids which are known to have blood-glucose-lowering activity according to previous preliminary phytochemical studies.16,17 In addition, another plant (root of Acanthus ilicifolius) with the specious had shown to have antidiabetic activity.18 Hence, the present study was planned to evaluate the hypoglycemic and antihyperglycemic effect of 80% methanol root extract of Acanthus polystachyus in streptozotocin–nicotinamide-induced type 2 diabetic rats.
Materials and Methods
Plant Material
The root of Acanthus polystachyus was collected from around Bahir Dar, the capital city of the Amhara Regional Governmental State. The city is 560Km Northwest of Addis Ababa, Central Ethiopia. The authenticity of the plant material was confirmed by the National Herbarium, Department of Biology, Addis Ababa University, where a voucher specimen (with a voucher number of DD002) was deposited.
Reagents and Drugs
Methanol, Streptozotocin, Glibenclamide, Nicotinamide, and all other regents with analytical grade were purchased from Ethiopian Pharmaceutical Supply Agency, Addis Ababa-Ethiopia.
Experimental Animals
Albino Wistar rats (150–200gm) and Swiss albino mice (20–30mg) of either sex used in this study were purchased from the animal department of Ethiopian Public Health Institute and acclimatized for a week before commencing the experiment. They were kept under standard conditions (at a temperature of 22 ± 2°C, humidity 50 ± 15%, and with 12 hr light/12 hr dark cycle) and the animals are freely accessed to standard pellets and water ad libitum. The care and handling of the animals were performed according to the internationally accepted standard guideline for the use of laboratory animals.19
Extract Preparation
The fresh root of the plant material was thoroughly washed with distilled water to remove dirt, soil, and dried under a shade with optimal ventilation for 2 weeks. The dried root was further chopped into small pieces and reduced to powder using an electronic miller. The coarsely powdered root (100g) was subjected to a maceration extraction procedure using 80% methanol. The plant and solvent mixtures were placed on an orbital shaker (at 160rpm) for 72 hours at room temperature. Then, each sample was filtered out using a Whatman number #1 filter paper and the mark was macerated three times. The filtrates were combined and dried in an oven at a temperature not exceeding 40ºC. Finally, the weights of the dry extract were measured to determine the percentage yield of each extract, and all the dry extracts were transferred into a vial and kept in a desiccator until use as shown in Figure 1.
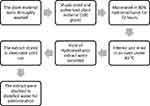 |
Figure 1 Schematic diagram of extraction. |
For this study, 80% of methanol, instead of water, was used to get a greater percentage of extract yield based on previous studies conducted. Importantly, 80% methanol is more efficient in the cell wall and seed degradation as well as having low or no enzyme activity as compared to water. Additionally, the methanol extract of plant materials contains a wide variety of polar (and moderately non-polar) compounds.20,21
Preliminary Phytochemical Screening
The 80% methanol root extract of Acanthus polystachyus was screened for the presence of alkaloids, flavonoids, polyphenols, tannins, saponins, terpenoids, glycosides, and anthraquinones, and steroidal compounds.22
Acute Oral Toxicity Test
Acute oral toxicity studies were performed according to OECD 425 guideline. Five Swiss Albino mice were used. After overnight fasting, one animal was given a single dose of 2000mg/kg root extract of Acanthus polystachyus orally by using oral gavage. The animal was closely observed for any behavioral and physical abnormality for 30 minutes and every four hours for 24 hours, then regularly for 14 days. Based on the results from the first mouse, additional four mice were taken and fasted for 3 hours, administered a single dose of 2000 mg/kg, and followed similarly.23
Measurement of Blood Glucose Level
Blood samples were withdrawn from the tail vein of each animal aseptically, and the blood glucose level was measured using glucometer. In all cases, blood glucose level measurement was done in triplicate and the average value was taken.
Hypoglycemic Activity of the Extract in Normoglycemic Rats
Before commencing the experiment, the rats have been fasted for 18 hours with free access to water. Then, 30 non-diabetics rats were randomly divided into five different groups (n=6). Group I: normal control rats received distilled water; group II–IV: rats treated with 100, 200, and 400mg/Kg body weight of root extract of Acanthus polystachyus orally respectively; group V: rats received glibenclamide (5mg/kg). The blood glucose level of each rat was measured just before treatment (at 0 hr) as a baseline, and then at 1, 2, 4, and 6 hours after treatment.
Hypoglycemic Effect on Oral Glucose Tolerance Test (OGTT)
After 18 hours of fasting, 30 normoglycemic rats were selected and randomly divided into five groups (n=6). Group I received distilled water (control), Group II (APRE200), Group III (APRE100), and Group IV (APRE400) dosed with 200, 300, and 400mg/kg of Acanthus polystachyus root extract, respectively. Group V received glibenclamide (5mg/kg) orally. Thirty minutes after the treatment, all the animals were loaded with 3 g/kg of glucose solution. Then blood glucose was measured at the baseline (0 minutes), 30, 60, 90, and 180 min after the glucose loading.
Induction of Type 2 Diabetes in Rats
Type 2 diabetes mellitus was induced by a single intraperitoneal (IP) injection of freshly prepared solution of streptozocin (65mg/kg) with 0.1 M citrate buffer (pH 4.5), after a 15-minute intraperitoneal injection of nicotinamide (250mg/kg) prepared in normal saline, in overnight fasted rats. Then, hyperglycemia was confirmed by measuring blood glucose level after 72 hours of streptozocin administration. When the plasma glucose levels persistently elevated by ≥250mg/kg via repeated measurements of blood glucose in the 7 and 14 days of streptozocin administration, rats were considered to be diabetic and included in the study.24–26
Antihyperglycemic Effect of the Extract in Diabetic Rats
After 18 hours of fasting, 30 experimentally induced diabetic rats were recruited and divided into five groups (n=6) randomly. Group I: normal control rats received of distilled water (10mL/kg); group II–IV: rats treated daily with 100, 200 and 400 mg/Kg body weight of Acanthus polystachyus root extract orally respectively; group V: Rats received glibenclamide (5 mg/kg) for 28days. A blood glucose level of each rat was measured just before treatment (at 0 days) as a baseline, and then at 7, 14, 21, and 28 days after treatment. Besides this, the body weight, lipid profiles such as total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL), and low-density lipoproteins (LDL) were determined on the 28th-day.
Lipid Profile Determination
The lipid profile was determined in overnight fasted diabetic rats. On day 28, the rats were euthanized with ether anesthesia and cardiac puncture was performed to collect the blood sample. The blood sample was put for 1 hour at room temperature and centrifuged at 1500 × for 15 min at 4°C to prepare. Then, the levels of serum TC, HDL-C, and TG were determined with an automated chemistry analyzer (humostar 80, Germany). LDL-C levels were calculated by the Friedewald formula, where LDL= TC−(HDL)−TG/5.27
Statistical Analysis
Data were analyzed using SPSS version 23 and expressed as means ± SD. Data between and within the group were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test. Significant levels were tested at p < 0.05.
Ethical Considerations
Animals were handled according to international animal care and welfare.19 The ethical clearance was requested and an ethical clearance letter with reference number HRTT/04/234/2019 was obtained from the research and ethics review committee of the Amhara Public health institute before commencing the actual work.
Results
Extraction
The percentage yield value of 80% methanol extract of the dried root of Acanthus polystachyus was found to be 18.82% (w/w). The extract was dark-brown at room temperature and solidified when kept in an oven at 40°C.
Phytochemical Screening
Qualitative phytochemical analysis of the crude extract confirmed the presence of tannins, flavonoids, saponins, polyphenols, terpenoids, glycosides, and anthraquinones, whereas alkaloids and steroids were not detected (Table 1).
 |
Table 1 Results of Phytochemical Screening of Methanolic Root Extract of Acanthus polystachyus |
Acute Toxicity Study
Acute toxicity study of the 80% methanol root extract of Acanthus polystachyus was done through oral administration of one dose of 2000mg/kg. The gross physical and behavioral observation failed to reveal any behavioral, neurological, autonomic, or physical changes such as alertness, motor activity, restlessness, convulsions, coma, diarrhea, and lacrimation throughout the observation time. Thus, the median dose (LD50) of the plant extract is alleged to be greater than 2000mg/kg, indicating a decent margin of safety.
Hypoglycemic Activity of the Extract in Normoglycemic Rats
The root extract of Acanthus polystachyus failed to significantly reduce blood sugar levels in normoglycemic rats at all tested dose levels at all observed times compared to negative controls. But, glibenclamide reduces blood sugar level significantly compared to the negative controls at 1 hour, 2 hours, 4 hours, and 6 hours post-treatment. The results were summarized in (Table 2).
 |
Table 2 In vivo Hypoglycemic Effect of Methanol Extract of the Root of Acanthus polystachyus Against Normoglycemic Rats (n=6) |
Hypoglycemic Effect on Oral Glucose Tolerance Test (OGTT)
A single dose of Acanthus Polystachyus root extract was given to non-diabetic rats to verify its antihyperglycemic activity by oral glucose tolerance test. The crude extract reduces the increase of blood glucose level at 30, 60, and 120 minutes after a loading dose of glucose as shown in (Table 3) compared to the negative control.
 |
Table 3 In vivo Antihyperglycemic Effect of Methanol Extract of the Root of Acanthus polystachyus Against Oral Glucose Tolerance Test (n=6) |
Antihyperglycemic Effect of the Extract
Before commencing the experiment, the blood glucose levels did not show significant difference (P>0.05) between the control and test groups. After the administration of the crude extract, the plant extract reduces blood sugar levels significantly compared to the negative control groups as shown in Table 4.
 |
Table 4 Antihyperglycemic Effect of Root Crude Extract Acanthus polystachyus Against Streptozocin–Nicotinamide-Induced Diabetic Rats |
Effect of Extract on Body Weights of Diabetic Rats
Diabetic Mellitus causes loss of body weight, thus evaluating the effect of the extract on weight is very important. As indicated in Table 5, before extract administration, there was no considerable bodyweight difference across all groups. It was revealed that all three doses of the extract showed significant improvement in body weight on the 28thday of treatment compared to the negative control and the result is comparable to the standard drug glibenclamide.
 |
Table 5 Effect of Root Crude Extract Acanthus polystachyus Against on Body Weight of Streptozocin–Nicotinamide-Induced Diabetic Rats |
Effect of the Extract on Serum Lipid Profile of Diabetic Rats
It is also rational to test the dyslipidemic effect of the extract, as dyslipidemia is one of the most common complications and causes of dyslipidemia. As shown in (Table 6), the crude extract of all three-dose of the extract has significant antidyslipidaemic effects.
 |
Table 6 Effect of Root Crude Extract Acanthus polystachyus on Lipid Profile of Diabetics Rats (n=6) |
Discussion
Diabetes Mellitus, particularly type 2, is among world’s common public health problems. During these days, its prevalence is increasing alarmingly in both developed as well as developing countries.2 The currently available medications have numerous limitations like:-not curative, have a varied adverse effect, expensive and modest efficacy. Therefore, there is a desire to hunt for safer, more effective, and less expensive treatment regimens. Hence, evaluating plant-derived compounds for Diabetic Mellitus is an important research area as they are believed to be safe and simply accessible to the public.4,11
The current study aimed at to investigating the hypoglycemic and antihyperglycemic effect of the root extract of Acanthus polystachyus. It was performed in three models i.e in normoglycemic, glucose loaded, and streptozocin–nicotinamide-induced type 2 diabetes rats. The root extract was also tested for its antihyperlipidemic effect in diabetic rats. Streptozotocin–nicotinamide-induced type 2 diabetes in rats is a known and well-documented model of experimental diabetes. Streptozocin induce a selective cytotoxic effect on the pancreatic beta cells in mammals, which mimics type 1 Diabetic Mellitus. Nevertheless, when it preceded with cytoprotective nicotinamide (230mg/kg, IP) 15min before streptozocin (65mg/kg, IP) was found to develop moderate and stable hyperglycemia without any significant change in plasma insulin level that mimics type 2 diabetes. The streptozocin-nicotinamide model of type 2 diabetes has the following features like stable moderate hyperglycemia, reduction of β-cells (≈40%), glucose intolerance, presence of glucose – stimulate insulin secretion, polyphagia, and polydipsia.26,28–30 To compare the antidiabetic activity of the root extract, glibenclamide was used as a standard drug like other similar studies done previously.31–37
This study revealed that the root extract Acanthus polystachyus at all tested doses (100 mg/kg, 200mg/kg, and 400mg/kg) did not show significant hypoglycemic effect in normoglycemic rats, unlike the standard drug glibenclamide. This may be due to the plant’s different mechanism of action with the standard drug. Lack of hypoglycemic effect is very important because hypoglycemia is one of the most serious complications in the management of diabetics.38 This finding is similar to studies.31,39
To determine the effect of the extract on glucose utilization, oral glucose tolerance tests were performed.40 This study revealed that Acanthus polystachyus treated groups had significantly lowered blood glucose levels after 30 minutes of glucose loading at all tested doses (100mg/kg, 200mg/kg, and 400mg/kg) compared to the negative control. This indicates that extract-treated rats had better glucose utilization compared to the negative control. The effect of the extract on postprandial hyperglycemia may be due to delay absorption, decrease glucose production from the liver, or increased peripheral glucose utilization.41
In the present study, daily administration different dose (100mg/kg, 200mg/kg, and 400mg/kg) of the extract in streptozocin–nicotinamide-induced type 2 diabetes for 28 days significantly reduce the fasting blood glucose level compared to the negative control. This finding was in line with other studies with related species Acanthus ilicifolius42 and Acanthus montanus.43 Besides this, the extract also reduces body weight loss associated with diabetes after 28 days of administration.
Lipid profile abnormality is a common occurrence in diabetes mellitus which is characterized by elevation of TC, TG, and LDL and decreases HDL. These abnormalities increase the risk of cardiovascular diseases.44 The elevation of TG is due to lack of lipoprotein lipase activation by insulin, whereas elevation of TC, LDL and decreasing of HDL is partly due to lack of insulin inhibitory action on key enzyme 3-hydroxy-3-methyl-glutaryl CoA reductase (HMG-CoA reductase), in de novo synthesis of cholesterol.45,46 In this study, the Acanthus polystachyus extract treated groups of rats had shown a significant effect on lipid abnormality compared with both negative and positive control, which is another rewarding effect of the extract, but the extract did not exhibit a significant effect on HDL-cholesterol.
The phytochemical screening of the plant revealed the presence of phenolic compounds, terpenoids, and flavonoids which is consistent with other studies previously done on the plant.17,38,47 In other similar studies, these secondary metabolites have shown to have antidiabetic activity.11,12,21,48 Thus, the antidiabetic activity of methanol root extract of Acanthus polystachyus may be due to the presence of these different secondary metabolites with possible synergistic effects.
The standard drug acts via selective blockage of ATP sensitive K+ channels (or KATP channel) in the plasma membrane of β-cells of the pancreas, thereby it leads to cytosolic depolarization and release of insulin, which cause hypoglycemia in normoglycemic rats.49 As the root extract of Acanthus polystachyus is devoid of hypoglycemic effect in normoglycemic rats, its mechanism of action is different from the standard drug, instead its mechanism may be similar with biguanides;50 it may act by increasing peripheral glucose uptake, enhancing glucose-dependent insulin secretion, promoting glucose-dependent glucose excretion and by decreasing glucose output in the liver and skeletal muscle. However, detailed molecular studies are required to identify the exact mechanism for the antihyperglycemic activity of Acanthus polystachyus observed in the study.
Experimental induction of diabetes using a chemical is characterized by body weight loss due to increased wasting of fat stores, muscle, and tissue proteins. Similarly, in this study, there is significant weight loss in negative control groups. However, the root extract of Acanthus polystachyus significantly reduces weight loss associated with diabetes mellitus which is comparable with the standard drug glibenclamide.51
After administration of the maximum OCED recommended dose 2000 mg/kg body weight, the crude extract of the plant does not show any death or behavioral and physical adverse effect at the end of 14 days, which is consistent with the previous studies.17,47 Thus, the median lethal dose of methanolic root extract of this plant is greater than 2000mg/kg, which agrees with the nontoxic World Health Organization classification of hazardous substances.52
Conclusion
This study revealed that the methanol extract of Acanthus polystachyus has significant antihyperglycemic activity in streptozotocin–nicotinamide induced diabetic rats compared with the negative control group, but lack hypoglycemic effect on normoglycemic. The extract had also a significant antihyperlipidemic effect, which is another rewarding effect of the plant to search for new antidiabetic drugs. Thus, the root extract of Acanthus Polystachyus may be a potential source of safe and effective antidiabetic drugs.
Abbreviations
ANOVA, Analysis of Variance; APRE, Acanthus Polystachyus root extract; DW, Distilled Water; GLC, Glibenclamide; HDL, High-Density Lipoproteins; IP, Intraperitoneal; OGTT, Oral Glucose Tolerance Test; LDL, Low-Density Lipoprotein; TC, Total Cholesterol; TG, Triglycerides.
Data Sharing Statement
All the data used to support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dipiro J, Talbert RL, Yee GC, et al. Pharmacotherapy a pathophysiologic approach. J Chem Inf Model. 2008;53:1689–1699.
2. Maithili V, Dhanabal SP, Mahendran S. Antidiabetic activity of ethanolic extract of tubers of Dioscorea alata in alloxan-induced diabetic rats. Indian J Pharmacol.2011;43(4):455.
3. Mellitus DIABETES. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Supplement 1):S37. doi:10.2337/diacare.28.suppl_1.S37
4. Ahmed MF, Kazim SM, Ghori SS, Mehjabeen SS, Ahmed SR. Antidiabetic activity of vinca rosea extracts in alloxan-induced diabetic rats. Int J Endocrinol. 2010;2010.
5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(SUPPL. 1):S62–S69. doi:10.2337/dc10-S062
6. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(SUPPL.1):81–90. doi:10.2337/dc14-S081
7. Bergman M, Buysschaert M, Schwarz PEH, Albright A, Venkat Narayan KM, Yach D. Diabetes prevention: global health policy and perspectives from the ground. Diabetes Manag (Lond). 2012;2(4):309–321. doi:10.2217/dmt.12.34
8. Melo da Cunha JDS, Alfredo TM, Dos Santos JM. Antioxidant, antihyperglycemic, and antidiabetic activity of Apis mellifera bee tea. PLoS One. 2018;13(6):e0197071. doi:10.1371/journal.pone.0197071
9. Williamson AR, Hunt AE, Pope JF, Tolman NM. Recommendations of dietitians for overcoming barriers to dietary adherence in individuals with diabetes. Diabetes Educ. 2000;26(2):272–279. doi:10.1177/014572170002600207
10. Rao MU, Sreenivasulu M, Chengaiah B, Reddy KJ, Chetty CM. Herbal medicines for diabetes mellitus: a review. Int J PharmTech Res. 2010;2(3):1883–1892.
11. Jain S, Pawan Gupta AK. Anti-diabetic activity of ethanol extract of praecitrullus fistulosus leaves on streptozotocin-induced diabetic rats. Int J Pharm Sci Res. 2017;8(2):740–745.
12. Kooti W, Farokhipour M, Asadzadeh Z, Damon Ashtary-Larky MA-S, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. 2016;8(1):1832–1842. doi:10.19082/1832
13. Hafidh RR. A comprehensive anticancer molecular study for genistein the promising anticancer drug. J Contemp Med Sci. 2017;3(11):264–269.
14. Tafesse TB, Hymete A, Mekonnen Y, Tadesse M. Antidiabetic activity and phytochemical screening of extracts of the leaves of ajuga remota benth on alloxan-induced diabetic mice. BMC Complement Altern Med. 2017;17(1):1–9. doi:10.1186/s12906-017-1757-5
15. Sagbo IJ, van de Venter M, Trevor Koekemoer GB, Bradley G. In vitro antidiabetic activity and mechanism of action of brachylaena elliptica (Thunb.) DC. Evid Based Complement Alternat Med. 2018;2018:1–13. doi:10.1155/2018/4170372
16. Derebe D, Abdulwuhab M, Wubetu M, Mohammed F. Investigation of the antidiarrheal and antimicrobial activities of 80% methanolic leaf extract of discopodium penninervum (hochst). Evid Based Complement Alternat Med. 2018;2018.
17. Demilew W, Adinew GM, Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of acanthus polystachyus delile (acanthaceae). Evid Based Complement Alternat Med. 2018;2018.
18. Venkataiah G, Ahmed MI, Reddy DS, Rejeena M. Anti-diabetic activity of Acanthus ilicifolius root extract in alloxan induced diabetic rats. Indo Am J Pharm Res. 2013;3(11):9007–9012.
19. National Research Council. Guide for the care and use of laboratory animals. Institute of laboratory animal resources, commission on life sciences. Natl Acad Sci. 1996.
20. Prashant Tiwari B, Kumar MK, Gurpreet Kaur HK. Phytochemical screening and extraction - a review. Int Pharm Sci. 2011;1(1):98–106.
21. Jones WP. Extraction of plant secondary metabolites. Methods Mol Biol. 2012;864:341–366.
22. Otsuka H. Purification by solvent extraction using partition coefficient. Nat Prod Isol. 2005;20:269–273. doi:10.1385/1-59259-955-9:269
23. OECD/OCDE (testing guidelines 407). OECD Guidelines for the Testing of Chemicals: Repeated Dose 28-Day Oral Toxicity Study in Rodents. 2008:1–13.
24. Masiello P, Broca C, Gross R, Roye M, Manteghetti M. Development of a new model of type II diabetes in adult rats administered with streptozotocin and nicotinamide. Diabetes. 1998;47(2):224–229. doi:10.2337/diab.47.2.224
25. Annie Shirwaikar K, Rajendran RB, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol. 2006;107(2):285–290. doi:10.1016/j.jep.2006.03.012
26. Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. J Appl Biomed. 2007;6(1):31–37. doi:10.32725/jab.2008.005
27. Belhekar SN, Chaudhari JS, Saryawanshi KK, Mali RBP. Antidiabetic and antihyperlipidemic effects of Thespesia populnea fruit pulp extracts on alloxan-induced diabetic rats. Indian J Pharm Sci. 2013;75(2):217–221.
28. Ghasemi A, Khalifi S, Jeddy S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiol Hung. 2014;101(4):408–420. doi:10.1556/APhysiol.101.2014.4.2
29. Kumar GPS, Arulselvan P, Kumar DS, Subramanian SP. Anti-diabetic activity of fruits of terminalia chebula on streptozotocin induced diabetic rats. J Health Sci. 2006;52(3):283–291. doi:10.1248/jhs.52.283
30. Ledoux SP, Hall CR, Forbes PAMM, Patton NJ, Wilson GL. Mechanisms of nicotinamide and thymidine protection from alloxan and streptozocin toxicity. Diabetes. 1988;37(8):1015–1019. doi:10.2337/diab.37.8.1015
31. Kumar R, Pate DK, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic leaves extract of alangium lamarckii thwaites on streptozotocin-nicotinamide induced type 2 diabetic rats. Asian Pac J Trop Med. 2011;4(11):904–909. doi:10.1016/S1995-7645(11)60216-2
32. Chandran R, Primelazhagan T, Shanmugam S, Thankarajan S. Antidiabetic activity of syzygium calophyllifolium in streptozotocin-nicotinamide induced type-2 diabetic rats. Biomed Pharmacother. 2016;82:547–554. doi:10.1016/j.biopha.2016.05.036
33. Shirwaikar A, Rajendran K, Kumar CD, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin – nicotinamide type 2 diabetic rats. J Ethnopharmacol. 2004;91(1):171–175. doi:10.1016/j.jep.2003.12.017
34. Jadhav R, Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int J Pharm Pharm Sci. 2012;4(2):2–7.
35. Sam I, Punitha R, Shirwaikar A, Shirwaikar A. Antidiabetic activity of benzyl tetra isoquinoline alkaloid berberine in streptozotocin-nicotinamide induced type 2 diabetic rats. Diabetol Croat. 2006;117–128.
36. Jadhav R, Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoid on streptozotocin-nicotinamide induced type 2 diabetic rats. Eur J Biol Sci. 2011;3(4):126–130.
37. Prabhu KS, Lobo R, Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide diabetic rats. J Pharm Pharmacol. 2008;60(7):909–916. doi:10.1211/jpp.60.7.0013
38. Asplund K, Wiholm B-E, Lithner F. Glibenclamide-associated hypoglycaemia: a report on 57 cases. Diabetologia. 1983;24(6):412–417. doi:10.1007/BF00257338
39. Kumar R, Patel DK, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic root extract of Caesalpinia digyna in streptozotocin-nicotinamide induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(2):S934–40. doi:10.1016/S2221-1691(12)60340-2
40. Subramani Srinivasan UM, Muruganathan U. Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chem Biol Interact. 2016;250:38–46. doi:10.1016/j.cbi.2016.02.020
41. Porchezhian E, Ansari SH, Shreedharan NKK. Antihyperglycemic activity of Euphrasia officinale leaves. Fitoterapia. 2000;71(5):522–526. doi:10.1016/S0367-326X(00)00204-5
42. Ahmed MN, Sultanaa T. A preliminary antihyperglycemic and antinociceptive activity evaluation of a mangrove species Acanthus ilicifolius L. leaves in mice. Asian J Tradit Med. 2014;(April 2015).
43. Chinwe Victoria Ukwe CMU. Hypoglycemic activity of leaves of acanthus montanus T.anderson (acanthaceae) in rats. Planta Med. 2012;78(11):CL70.
44. Rodrigues B, Goyal RK. Effects of hydralazine on streptozotocin-induced diabetic rats: prevention of hyperlipidemia and improvement in cardiac function. J Pharmacol Exp Ther. 1986;237(1):292–299.
45. Parmar GR, Pundarikakshudu K, Balaraman R. Antidiabetic and antihyperlipidemic activity of Euphorbia thymifolia L. extracts on streptozotocin-nicotinamide induced type 2 diabetic rats. J Appl Pharm Sci. 2017;7(8):078–84.
46. Edwin Jarald E, Joshi SB, Jain DC. Antidiabetic activity of flower buds of Michelia champaca linn. Indian J Pharmacol. 2008;40(6):256–260. doi:10.4103/0253-7613.45151
47. Derebe D, Wubetu M. Antimalarial activity of hydroalcoholic root extract of acanthus polystachyus delile (acanthaceae) against plasmodium berghei–infected mice. J Evid Based Integr Med. 2019;24.
48. Otsuka H. Purification by solvent extraction using partition coefficient. In: Natural Products Isolation. Humana Press; 2006:269–273.
49. Shiao-Wei Shen RB. Clinical pharmacology of oral antidiabetic agents. N Engl J Med. 1997;296(9):493–497.
50. Jangaard NO, Pereira JN, Pinson R. Metabolic effects of the biguanides and possible mechanism of action. Diabetes. 1968;17(2):96–104. doi:10.2337/diab.17.2.96
51. Pillai KK, Chidambaranathan N, Halith MM, Jayaprakash S, Narayanan N. Antihyperglycemic effect of alcoholic extracts of cnidoscolus chayamansa in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. Int J Res Pharm Chem. 2012;2(1):179–187.
52. World Health Organization. The guidebook to the registration of public health pesticides and repellents against vectors: hazard classification-acute LD50 values of formulated products. Available from: https://www.nea.gov.sg/docs/default-source/resource/guidebook-for-the-. https://www.nea.gov.sg/docs/default-source/resource/guidebook-for-the-registration-of-public-health-pesticides-and-repellents.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
