Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Hypoglycemia After Upper Gastrointestinal Surgery: Clinical Approach to Assessment, Diagnosis, and Treatment
Received 1 September 2020
Accepted for publication 28 October 2020
Published 19 November 2020 Volume 2020:13 Pages 4469—4482
DOI https://doi.org/10.2147/DMSO.S233078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Amanda Sheehan,1 Mary Elizabeth Patti1,2
1Research Division, Joslin Diabetes Center, Boston, MA, USA; 2Harvard Medical School, Boston, MA, USA
Correspondence: Mary Elizabeth Patti
Research Division, Joslin Diabetes Center, 1 Joslin Place, Boston, MA 02215, USA
Tel +1 617-309-1966
Fax +1 617-309-2593
Email [email protected]
Context: Post-bariatric hypoglycemia (PBH) is an increasingly encountered complication of upper gastrointestinal surgery; the prevalence of this condition is anticipated to rise given yearly increases in bariatric surgical procedures. While PBH is incompletely understood, there is a growing body of research describing the associated factors, mechanisms, and treatment approaches for this condition.
Evidence Acquisition: Data are integrated and summarized from studies of individuals affected by PBH and hypoglycemia following upper gastrointestinal surgery obtained from PubMed searches (1990– 2020).
Evidence Synthesis: Information addressing etiology, incidence/prevalence, clinical characteristics, assessment, and treatment were reviewed and synthesized for the practicing physician. Literature reports were supplemented by clinical experience as indicated, when published data were not available.
Conclusion: PBH can be life-altering and severe for a subset of individuals. Given the chronic nature of this condition, and sequelae of both acute and recurrent episodes, increasing provider awareness of both the condition and associated risk factors is critical for assessment, prompt diagnosis, treatment, and preoperative identification of individuals at risk.
Keywords: hypoglycemia, post-bariatric hypoglycemia, upper gastrointestinal surgery, bariatric surgery
Introduction
Increasing evidence indicates the importance of the gastrointestinal tract as a regulator of systemic metabolism. For example, metabolic surgery, such as Roux-en-Y gastric bypass (RYGB), produces profound improvements in glucose metabolism within days postoperatively, is more effective than medical therapies at improving control of type 2 diabetes (T2D), and reduces the need for medications.1–3 However, with this metabolic benefit comes an increased incidence of hypoglycemia, or post-bariatric hypoglycemia (PBH). While hypoglycemia most commonly occurs following Roux-en-Y gastric bypass (RYGB), it can also occur with other bariatric or upper gastrointestinal surgeries including sleeve gastrectomy (SG),4 duodenal switch (DS),5 Nissen fundoplication (NF),6 esophagectomy, gastrectomy,7 and others.
While hypoglycemia can be mild in some individuals, PBH can be life-altering and severe for a subset, with hypoglycemia-associated injuries, disability, job and income loss, as well as impairment in broader activities of daily living and social functioning. With ever-increasing rates of bariatric and other gastrointestinal surgical procedures, raising provider awareness to aid in assessment (including pre-operative assessment), diagnosis, treatment, and timely referral is important and a goal of this review.
Etiology
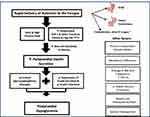
Following bariatric or upper gastrointestinal surgery there are profound alterations in postprandial glucose metabolism which are likely to contribute to the pathophysiology of PBH (summarized in Figure 1).8 Surgical alterations in gastrointestinal anatomy result in the rapid emptying of nutrients out of the pouch or sleeve and into the foregut.9 The ensuing rapid absorption of glucose leads to an early and high plasma glucose peak.9–11 In parallel, secretion of the hormone glucagon-like peptide-1 (GLP-1) from the small intestine is increased by as much as 10-fold.12 These high levels of GLP-1, together with high postprandial glucose levels, markedly stimulates insulin release.10,13,14 Surging postprandial insulin levels stimulate increased tissue glucose uptake, resulting in a rapid drop in plasma glucose levels 1–3 hours after meals.9,15–18 Decreased insulin clearance and reduced beta cell suppression with hypoglycemia may further contribute.13 Additionally, insulin-independent glucose uptake is increased in patients with PBH,17 potentially linked to a >3-fold increase in plasma levels of the intestinally-derived hormone fibroblast growth factor-19 (FGF-19).19
Increases in intestinally-derived GLP-1 and FGF-19 in PBH suggest that intestine-specific mechanisms may contribute to changes in glucose metabolism after bariatric surgery. These include changes in bile acid composition and metabolism, which can modulate both GLP-1 and FGF-19,20 and microbiome diversity and composition.21,22 Interestingly, a recent study reports that individuals post-bariatric surgery who have undergone cholecystectomy (vs those without cholecystectomy) have significantly increased risk for both PBH and dumping syndrome, further supporting the potential role of changes in the kinetics or composition of circulating or enteral bile acids in the pathogenesis of PBH.21,23 Likewise, differences in the microbiome may contribute to inter-individual differences in glycemic and hormonal response to meals. Fermentation products of the microbiome, such as short chain fatty acids (SCFA), which are increased after RYGB,24 can stimulate GLP1 release,25 and may modulate insulin secretion,6,27 potentially contributing to altered glucose metabolism.
Genetic factors may also be contributory.8 For example, mutations in voltage-gated potassium channels KCNQ1 and KCNH1, identified as pathogenic in some individuals with long QT syndrome, may also increase secretion of GLP-1, GIP, and insulin, and impair glucagon secretion.26 This would be predicted to increase the risk for an affected patient who chooses bariatric surgery, as in the case report by Mateo et al.27 Research is ongoing to elucidate additional possible genetic factors related to PBH.
Individuals who have had upper GI surgery also have blunting of counterregulatory mechanisms, such as cortisol, catecholamines, and sympathetic nerve responses,13,28 and these may be further impaired in patients with hypoglycemia.29 Additional mechanisms implicated in hypoglycemia are decreased alpha cell responsiveness, insufficient glucagon secretion, inadequate glycogen stores, or reductions in substrates for gluconeogenesis.12,30 Such impaired counterregulatory responses to hypoglycemia could result from recurrent hypoglycemia (sometimes unrecognized), but may also contribute to more prolonged duration of hypoglycemic episodes and reduced awareness of hypoglycemia. These patterns are similar to the impaired counterregulation and reduced awareness observed in patients with diabetes who experience frequent hypoglycemia.31
Recent studies have demonstrated elevated postprandial glucose levels induce macrophages to secrete interleukin-1β (IL-1β), which stimulates insulin secretion and also increases glucose uptake into macrophages.32 IL-1β also decreases the threshold for noradrenergic response in the hypothalamus (where IL-1B is expressed).33,34 Treatment of individuals with PBH with an Il-1B antagonist increased nadir glucose during meal testing.35 Whether aberrant inflammatory responses contribute more broadly to PBH will require additional study.
Incidence/Prevalence
The precise incidence and prevalence of PBH is unknown, due to differences in diagnostic criteria, the challenges of self-reporting of symptoms which are often non-specific, and the high rates of asymptomatic low glucose values. Severe hypoglycemia requiring hospitalization occurs in less than 1% of individuals;36 however, estimates suggest that symptomatic hypoglycemia occurs in 10–30% of individuals,37,38 and occurs after both RYGB and vertical sleeve gastrectomy (SG), with similar presentation and spectrum of severity.39 There are no published data on the prevalence of hypoglycemia occurring after other upper gastrointestinal surgeries such as Nissen fundoplication or esophageal or gastric procedures, beyond case reports.6,7,40,41
Risk Factors for Hypoglycemia
Risk factors for PBH identified during retrospective epidemiologic studies include female sex, younger age, no diagnosis of diabetes pre-surgery, history of hypoglycemia pre-surgery (not associated with diabetes or diabetes medications), lower pre-surgery hemoglobin A1C, and greater excess weight loss postoperatively.42,43
Given that risk for PBH may be increased in those with pre-operative hypoglycemia, the preoperative history should include questions about symptoms suggestive of hypoglycemia (eg, sweating, palpitations, tremors, and lightheadedness). If these are present, additional evaluations should be considered, such as laboratory testing, meal testing, and/or blinded CGM, to identify patterns of glucose excursions.
Interestingly, prospective studies have demonstrated that individuals who have hypoglycemia postoperatively had lower glucose levels on preoperative oral glucose tolerance testing (OGTT)44,45
However, differences between those who develop hypoglycemia and those who do not are of relatively small magnitude, and there are no defined diagnostic thresholds at present. It will be important in future studies to identify diagnostic thresholds which could be used clinically to identify high-risk individuals during the preoperative evaluation and inform decision-making about the type of surgical procedure planned (or not).
EKG should be considered during the preoperative evaluation to rule out a long QT interval, given the association between long QT caused by mutations in genes KCNQ146 and KCNH147 and postprandial incretin and insulin hypersecretion. One would predict that this would potentially pose increased risk to an affected individual who chooses bariatric surgery as in the case report by Mateo et al.27 Moreover, as hypoglycemia can further prolong the QT interval, risk for arrhythmia may be increased.48 Thus, each clinician should use clinical judgment and carefully consider patient-specific factors before recommending surgery for an individual with long QT syndrome.
Assessment and Clinical Characteristics
Meticulous history-taking remains a critical first step in assessment of the patient with possible hypoglycemia. Information about episodes should include the severity (frequency, presence of neuroglycopenia, whether assistance required) and timing (relationship to fasting, meals, specific provocative foods, activity, and presence of nocturnal symptoms). Detailed records of symptoms, intake (both solid food and liquids), and activity can be helpful in identification of patterns linked to symptoms.
Symptoms of hypoglycemia can include sweating, tremor, profound hunger, palpitations, rapid heartbeat, changes in thinking, dizziness, difficulty concentrating, confusion, and alteration or loss of consciousness. Individuals may also report bizarre dreams or morning headaches, suggesting nocturnal hypoglycemia. Family or friends may report mood swings or changes in behavior during episodes of hypoglycemia, and that the patient has a distant stare or does not “look like themself”. With severe hypoglycemia, brain function can be impaired (neuroglycopenia), potentially causing altered cognition, seizures, falls, motor vehicle accidents, and loss of consciousness. Reduced memory, both acutely during a hypoglycemic event, as well as a more generalized change in memory, may be reported.
Standardized questionnaires can be useful tools for measuring and tracking hypoglycemia symptoms clinically as well as in research settings. The Edinburgh Hypoglycemia Symptom Scale (EHSS) assesses adrenergic, cholinergic, and neuroglycopenic symptoms, while Gold and Clarke scales measure the awareness of hypoglycemia.49,50
The key clinical characteristics suggesting that PBH may be the cause of hypoglycemia are detailed in Table 1. Symptoms typically begin at least 1 year after surgery, although diagnosis may be delayed even longer given the non-specific nature of symptoms. Symptoms typically do not occur in the fasting state, but occur most commonly 1–3 hours (or even longer) after eating, especially after ingestion of rapidly absorbed, high glycemic index carbohydrates.51 Other triggers for some patients may include activity, stress, prior alcohol ingestion, and caffeine. Ethanol inhibits hepatic gluconeogenesis, thereby increasing the risk of hypoglycemia,52,53 and potentially attenuating the counterregulatory effect of glucagon.54 Caffeine intake is associated with significant increases in cortisol, epinephrine, and norepinephrine, increased perception of symptoms of postprandial hypoglycemia at low-normal glucose levels, and greater symptoms at frankly hypoglycemic levels.55 By contrast, a meta-analysis reported caffeine acutely decreases insulin sensitivity in individuals without diabetes.56 Conflicting reports may explain the individual effects reported clinically to ingestion of caffeine. More studies will be required to fully assess relationships between caffeine intake and PBH. Consumption of liquids with meals can accelerate delivery of nutrients and stimulation of the intestine, further increasing the risk of hypoglycemia. Some patients with PBH have middle-of-the-night hypoglycemia; however, fasting hypoglycemia (upon arising) is not typical of PBH, and should prompt evaluation for other causes.
 |
Table 1 Clinical Characteristics of Hypoglycemia |
There is substantial overlap between symptoms of hypoglycemia and the so-called dumping syndrome. Typically, early dumping symptoms occur 1030– minutes after a meal, and may include diarrhea, palpitations, lightheadedness, severe fatigue, nausea, and vomiting. At the time of these symptoms, glucose values are typically not low. However, hypoglycemia can develop later after the meal, sometimes as a component of “late dumping”.57
Individuals can have multiple episodes of hypoglycemia per day in response to foods and drinks that cause a spike and then drop – a so-called “roller coaster” pattern.58 A vicious cycle can occur after self-treating severe symptoms of hypoglycemia with high glycemic index carbohydrates, sometimes in excess. While these raise glucose levels and improve symptoms in the short-term, they may cause another “spike” in glucose and subsequent recurrent hypoglycemia. This pattern may contribute to the observation that PBH is independently associated with significantly less weight loss at 2 years follow-up,43 and that symptoms of hypoglycemia are associated with increased risk of weight regain, and less self-reported adherence to nutritional recommendations.59
Diagnosis
If the history is typical for PBH (postprandial neuroglycopenia occurring >1 hour after meals in patients with a history of GI surgery at least 6–12 months before symptom onset, no hypoglycemia with fasting), laboratory testing is performed to confirm hypoglycemia, rule out other causes of hypoglycemia, and assess hormonal responses to hypoglycemia. The diagnosis can be established by documented hypoglycemia at the time of neuroglycopenic symptoms, with resolution of symptoms with treatment to raise glucose (Whipple’s triad).60 Glucose levels defining hypoglycemia vary widely. Recently published American Diabetes Association guidelines define glucose levels <70 mg/dL as level 1 hypoglycemia and <54 mg/dL as level 2 hypoglycemia,61 while Endocrine Society Guidelines define hypoglycemia as glucose <55 mg/dL in individuals without diabetes.62 Venous glucose levels are required to establish the diagnosis, as capillary glucose measurements can be falsely low in the setting of relative hypotension or Raynaud’s disease.63–65 This is an essential element of diagnosis of PBH, and is needed to help distinguish from symptoms of dumping syndrome.
Once hypoglycemia is established, differential diagnoses include malnutrition, side effects of medications or supplements, critical illness, hormone deficiencies (eg, adrenal), autoimmune hypoglycemia, insulinoma/proinsulinoma,66 or nonislet cell tumors.62,67 Laboratory analysis to assess these possibilities can include plasma glucose, insulin, proinsulin, C-peptide, beta-hydroxybutyrate, and cortisol. While it is preferable to analyze these hormonal responses at the time of spontaneous hypoglycemia, this is often challenging to accomplish. Alternative strategies include provocation of hypoglycemia using the triggers reported by the patient, with the same hormonal testing noted above. If this is not possible or cannot be accomplished safely, and suspicion is low for fasting hypoglycemia based on history, we often perform laboratory evaluation in the morning after an overnight fast to permit analysis of the adrenal axis and to assess the impact of fasting on blood glucose levels and beta cell peptides. If morning cortisol levels are low (<10 mcg/dL), an ACTH stimulation test should be considered to more fully define the adrenal axis.68
Other laboratory testing may be indicated depending upon individual or family history. A history of autoimmune or hematologic disease, postprandial hypoglycemia, or recent viral illness may raise suspicion for autoimmune hypoglycemia and prompt measurement of insulin auto-antibodies. Measurement of IGF-1 and IGF-2 should be considered in those with fasting hypoglycemia, suspicion for MEN syndromes, neuroendocrine tumors, or non-islet cell tumors.69,70 Additional symptoms such as flushing, hypotension, malnutrition, diarrhea, and weakness can be signs of carcinoid syndrome and should prompt measurement of urinary 5-HIAA.71
If history or laboratory testing suggest fasting hypoglycemia, a prolonged fast should be performed to determine whether beta-cell peptides are appropriately suppressed with hypoglycemia. This is typically performed in the hospital (traditional 72 hour fast); a shorter-term fast may be adequate for some patients and may be performed in the outpatient setting if appropriate monitoring to ensure safety is available.
Administering a provocative meal and then obtaining lab testing at the time of an episode is rarely required if history is typical. Most studies report the use of liquid meals (eg, commercial nutritional liquids) as these are less influenced by interindividual differences in gastric emptying rates, but these are not physiologic and there is no consensus for a standardized test meal. An alternative is to use a test meal identified by the patient as a provocative meal in daily life. Both of these possibilities are highlighted in the Endocrine Society guidelines on the evaluation of hypoglycemia.62 Some have advocated glucose tolerance testing, but this can yield false positive results, as 10% of the healthy asymptomatic population can have asymptomatic low glucose levels (nadirs of 47 mg/dL or below) during oral glucose tolerance testing.72 Moreover, this test can provoke severe “early” dumping syndrome in this population, with symptoms including abdominal pain, cramping, diarrhea, diaphoresis, dizziness, palpitations, nausea, and vomiting.62,73,74 Testing may also provoke severe hypoglycemia, and should only be done in the appropriate setting where rescue treatment is available to ensure safety. Moreover, there are no established diagnostic thresholds for testing, making interpretation of results challenging.
Continuous glucose monitoring (CGM) in the blinded (masked) mode is valuable – not for diagnosis, but for elucidating glycemic patterns and linking these patterns to symptoms. Individuals are asked to wear a CGM sensor for 7–10 days, while keeping a diary of food, activity, and symptoms. While CGM is less accurate in the hypoglycemic ranges, and should not be used for diagnostic purposes, this method allows pattern analysis and links to diary events can also help the patient identify their triggers for hypoglycemia.
Acute Treatment of Hypoglycemia
While there are some similarities in treatment of hypoglycemia between individuals with diabetes and PBH there are key differences as well. When symptomatic hypoglycemia develops, 10–15 g of oral carbohydrates (eg 3–4 glucose tabs or gel, 4 ounces of juice) are recommended to relieve symptoms and rapidly increase glucose to safe levels. However, if the individual is being treated with acarbose, treatment with glucose exclusively is recommended, as absorption of other forms of carbohydrate (eg, sucrose, juice) will be delayed. If severe neuroglycopenia develops, glucagon can be administered by family members via either injection or nasal delivery.
Since oral carbohydrates can produce a glucose “yo-yo” effect, initial treatment should be followed by a snack containing low glycemic index (LGI) carbohydrate mixed with fat/protein, such as one tablespoon of natural (unsweetened) peanut butter on a half-slice of whole-wheat bread, to reduce rebound hypoglycemia.8,75 While nut butters have high caloric density, they are convenient and generally effective for preventing rebound hypoglycemia. In the short-term, achieving safety is paramount. However, long-term nutritional guidance should incorporate the need to optimize overall caloric balance to reduce weight gain. Since some individuals are very sensitive to oral carbohydrate, the dose of treatment needed to increase glucose should be individualized after review of glycemic responses in order to balance efficacy of treatment vs avoidance of rebound hyperglycemia.
Chronic Treatment of PBH
Goals of treatment should include decreasing the frequency and severity of hypoglycemia, thereby helping to avoid injury, disability, and improving quality-of-life. Treatment approaches include medical nutrition therapy and medications (summarized below).
Patterns of Intake at Presentation
While there is a great degree of variability in how individuals attempt to manage hypoglycemia symptoms, two patterns are often observed clinically. Many individuals severely reduce overall food intake (or carbohydrates specifically) in an attempt to feel better and/or prevent hypoglycemia during critical periods of the day (eg, work hours). Modified ketogenic plans are often adopted. Unfortunately, we have observed clinically that this pattern can sometimes result in weight loss, malnutrition, fatigue, activity, or exercise-induced hypoglycemia, and in some cases emergence of more nocturnal hypoglycemia. Fear of eating, food intolerance, or aversion can be additional challenges to address.
A second pattern can be the near-continuous intake of either solid or liquid carbohydrates, in an effort to avoid or treat the symptoms. Consequences of this pattern can include weight gain, and repeated cycles of rebound hypoglycemia (initial treatment provoking hyperglycemia and then another episode of hypoglycemia) multiple times per day. Overnight hypoglycemia may follow bedtime or nocturnal eating. Individuals presenting with this pattern may be reluctant to decrease intake of foods or liquids which are perceived to help treat their hypoglycemia; however, in many cases, avoidance of high glycemic index carbohydrates will actually be effective in breaking this cycle and reducing hypoglycemia.
Medical Nutrition Therapy
Medical nutrition therapy (MNT) is foundational to the treatment of PBH, and ongoing follow-up with a registered dietician nutritionist who is experienced in this condition is essential. The key components of MNT are summarized in Table 2.75 It is recommended that individuals with PBH eat meals and snacks containing 20–30 grams and 10–15 grams of LGI carbohydrate, respectively, every 3–4 hours; these should be paired with proteins and healthy fats, while avoiding liquids for at least 30 minutes following intake of food. While this is an initial recommendation, it is important to review glycemic patterns and revise the meal plan (including carbohydrate content) as needed for the individual patient.
 |
Table 2 Key Components of MNT for Post-Bariatric Hypoglycemia |
Cornstarch (CS), in uncooked form, is another helpful adjunctive tool which has been used for years by individuals with glycogen storage disorders and in children with type 1 diabetes.76 CS can be combined with MNT in PBH.77 CS is not readily absorbed by the small intestine, but is slowly hydrolyzed by pancreatic amylase and intestinal glucoamylase to provide a steady supply of exogenous glucose. It can be purchased easily at the grocery store, mixed in unsweetened beverages, plain yogurt, or cottage cheese, and can be consumed during the day and/or before bed, depending upon the timing of hypoglycemia. Commercial products that contain uncooked CS (eg, nutritional bars, drinks) are also available.
The patient should be aware that MNT is unlikely to completely eliminate hypoglycemia, that MNT needs to be individualized based on initial response, and that even optimal MNT often requires the addition of one or more medications.
Micronutrient Status
Ensuring a lifetime intake of prescribed vitamins and monitoring of levels of vitamins D, A, B1, B12, and other B-complex vitamins, as well as iron, calcium, zinc, copper, and folic acid is recommended.78 Assessment of prior or current micronutrient deficiencies is important, as bariatric surgery may reduce both intake and absorption. While a direct link with PBH has not yet been formally established, the association of micronutrient deficiencies (eg, B1, B12, magnesium, D)79 and autonomic neuropathy has been reported in the literature.80,81 Compromised autonomic nervous system functioning could impair counterregulatory responses to hypoglycemia. For example, one case report of an individual with type 1 diabetes demonstrated that correction of B12 deficiency resulted in increased counter-regulatory hormone response and improved hypoglycemia awareness.82
CGM
Use of a personal CGM (not blinded) can be helpful in detection and treatment of dropping glucose levels before impairment in cognition (neuroglycopenia) develops, especially for those with unawareness or reduced awareness of hypoglycemia. Unfortunately obtaining insurance coverage for CGM devices can be difficult, as they are not uniformly approved for this indication at present, and the out-of-pocket cost is prohibitive for most.
Pharmacotherapy
Pharmacotherapy is utilized as an adjunctive therapy when MNT is insufficient (summarized in Table 3). Acarbose is a first step due to its effect of slowing carbohydrate absorption, thus attenuating the postprandial glucose and insulin spike.74,83–86 Additional strategies aimed at reducing incretin (eg, GLP1) and insulin responses to meals include diazoxide (oral medication which reduces insulin secretion), and somatostatin receptor analogs (inhibit secretion of insulin and GLP1, but also glucagon and other gastrointestinal peptides).74,87–90 Octreotide can be self-administered via subcutaneous injection 3–4 times per day depending upon individual patterns. While there is some evidence that this short-acting octreotide is more effective in terms of symptom control,74,91 long-acting somatostatin analogs (LAR)92 may be an easier option for some. These are administered once every 3–4 weeks, but require a nurse skilled in depot injections. Both short- and long-acting somatostatin analogs have been shown to also improve early dumping.74
 |
Table 3 Pharmacotherapy for Hypoglycemia After Upper GI Surgery |
The use of these medications is often limited by tolerability and side effects, and patients should be closely monitored. Cost and insurance approval can be an added barrier to care, and appeals may be needed to obtain coverage.
Additional therapies which have been reported to have efficacy in PBH include calcium channel blockers93 and liraglutide94 (Table 3), but caution must be used due to the potential for hypotension and worsening of hypoglycemia, respectively.
Additional Therapies
When MNT, CGM, and pharmacotherapy are not successful and quality-of-life is severely compromised, additional more invasive treatments may be considered. Placement of a feeding gastrostomy (G)-tube into the remnant stomach (bypassed portion) can be successful, as this delivery route does not stimulate incretin or insulin secretion.95–97 However, individuals will still develop hypoglycemia if they take carbohydrates by mouth, so oral carbohydrates should be avoided. Involvement of a RDN experienced in PBH is needed to help optimize the choice of formula and delivery rates.
Rare individuals with PBH continue to have episodic hypoglycemia despite continuous G-tube feeding. In this setting, reversal of the surgical procedure toward normal anatomy may be considered, when feasible; while this typically improves hypoglycemia, it does not always fully resolve hypoglycemia,97–100 and may result in additional (but typically modest) weight regain.101,102
While initial reports describing PBH utilized pancreatectomy to reduce hypoglycemia, pancreatectomy is no longer considered as an appropriate therapy for the majority of patients with severe PBH for several reasons. First, the pancreas is typically an innocent bystander, with altered nutrient flow and excessive incretin stimulation, rather than increased islet mass, driving insulin production. Consistent with this concept, pancreatectomy is associated with high rates of recurrent hypoglycemia.103
Investigational Treatments (Not FDA Approved)
Given that current therapeutic options are limited, clinicians often have questions regarding the status of investigational approaches described in the literature, and ongoing research aimed at identifying new therapeutic options for PBH. It is important to note that these are not FDA-approved.
To target the excessive secretion of GLP-1 in PBH, clinical trials of a GLP-1 receptor antagonist are underway. Twice-daily administration of SC avexitide increases the glucose nadir and prevents severe hypoglycemia.104 At the time of this publication, a Phase II clinical trial has been completed.
Another approach under investigation is the use of a closed-loop glucose-responsive automated glucagon delivery (AGD) system.105 Automation was achieved by development of a hypoglycemia algorithm,106 which was embedded in an artificial pancreas system, guided by CGM. The AGD system delivered a mini-dose of stable liquid glucagon via a patch pump when glucose levels were dropping rapidly, or when hypoglycemia developed. Compared with vehicle (placebo), the AGD closed-loop system resulted in significantly less hypoglycemia, with no rebound hypoglycemia.
Investigation of the same ready-to-use (stable) glucagon as a SQ injectable medication for use as rescue for hypoglycemia is also ongoing.
Challenges
Challenges in the treatment of PBH are many and multifactorial (summarized in Table 4). There is a spectrum of severity of PBH, and this can fluctuate over time. Even with strict adherence to recommendations and pharmacotherapies, individuals may still experience hypoglycemia that is unpredictable.
 |
Table 4 Potential Treatment and Self-Care Challenges Observed Clinically in Individuals with Hypoglycemia After Upper GI Surgery |
Some individuals respond to a combination of MNT and behavioral modifications (eg, avoiding meal-related triggers), and may not require pharmacologic therapy. However, many individuals will require medications, particularly in those with severe PBH. Individuals with severe PBH may experience frequent hypoglycemia requiring assistance, neuroglycopenia (seizures, loss of consciousness), falls, motor vehicle accidents, job, and income loss. Individuals may express feelings of social isolation and loneliness. Memory impairment or changes in cognition have been reported both acutely and chronically. The relationship between PBH, psychosocial impact, and cognitive functioning has not yet been reported in the literature.
Further, while clinical experience has demonstrated that PBH is usually a chronic condition, data are lacking on the long-term impact of PBH on health; thus providing guidance about the prognosis of PBH is challenging, and the uncertainty can be difficult for clinicians, patients, and their families alike.
Conclusion
Hypoglycemia is an increasingly encountered complication of upper gastrointestinal surgery and its prevalence is anticipated to rise in the setting of yearly increases in bariatric surgical procedures. Given the spectrum of severity, sequelae from both acute and recurrent episodes, and potential for life-altering disability, prompt diagnosis and treatment is essential for safety. Additionally, individuals with hypoglycemia who are considering bariatric surgery should be carefully evaluated for the presence of any signs and symptoms of hypoglycemia; if these are present, referral to an endocrinologist should be considered to help make informed decisions about the possible risk of PBH.
Future research should continue to investigate the mechanisms of PBH to increase understanding, identify risk factors, develop treatments, and possibly identify modifications to upper-gastrointestinal surgical procedures to reduce the risk of hypoglycemia while retaining the efficacy of surgery.
Acknowledgments
MEP gratefully acknowledges support from NIH R01 DK121995, R01 DK106193, U01 DK114156, and P30 DK036836 (DRC, Joslin Diabetes Center), and the Chan Zuckerberg Foundation.
Disclosure
MEP has been a coinvestigator on an NIH R44 grant together with Xeris Pharmaceuticals. MEP has consulted for Eiger Pharmaceuticals, has received investigator-initiated grant support from Janssen Pharmaceuticals, Dexcom, Medimmune, Sanofi, Astra-Zeneca, Jenesis, and Nuclea, has been a site investigator for XOMA and Xeris, acknowledges clinical trial research trial product support from Ethicon, Covidien, NovoNordisk, Nestle, and Dexcom within the past 5 years, and reports personal fees from Fractyl and grant support from Chan-Zuckerberg Initiative, and Helmsley Trust, during the conduct of the study. In addition, MEP has a patent hypoglycemia treatment issued for hypoglycemia markers and has submitted a patent application regarding plasma proteins contributing to hypoglycemia and pump therapies for hypoglycemia. The authors report no other potential conflicts of interest for this work.
References
1. Roth AE, Thornley CJ, Blackstone RP. Outcomes in bariatric and metabolic surgery: an updated 5-year review. Curr Obes Rep. 2020;9(3):380–389. doi:10.1007/s13679-020-00389-8
2. Yan Y, Sha Y, Yao G, et al. Roux-en-Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95(17):e3462. doi:10.1097/MD.0000000000003462
3. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England). 2015;386:9997.
4. Lazar LO, Sapojnikov S, Pines G, et al. Symptomatic and asymptomatic hypoglycemia post three different bariatric procedures: a common and severe complication. Endocrine Cancer. 2019. doi:10.4158/EP-2019-0185
5. Abrahamsson N, Edén Engström B, Sundbom M, Karlsson FA. Hypoglycemia in everyday life after gastric bypass and duodenal switch. Eur J Endocrinol. 2015;173(1):91–100. doi:10.1530/EJE-14-0821
6. Zaloga GP, Chernow B. Postprandial hypoglycemia after nissen fundoplication for reflux esophagitis. Gastroenterology. 1983;84(4):840–842. doi:10.1016/0016-5085(83)90155-5
7. Kubota T, Shoda K, Ushigome E, et al. Utility of continuous glucose monitoring following gastrectomy. Gastric Cancer. 2020;23(4):699–706. doi:10.1007/s10120-019-01036-5
8. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815–2826. doi:10.1210/jc.2018-00528
9. Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–2009. doi:10.1002/oby.20791
10. Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–680.e662. doi:10.1053/j.gastro.2013.11.044
11. Dirksen C, Eiken A, Bojsen-Moller KN, et al. No islet cell hyperfunction, but altered gut-islet regulation and postprandial hypoglycemia in glucose-tolerant patients 3 years after gastric bypass surgery. Obes Surg. 2016;26(9):2263–2267. doi:10.1007/s11695-016-2197-x
12. Patti ME, Goldfine AB. Hypoglycemia after gastric bypass: the dark side of GLP-1. Gastroenterology. 2014;146(3):605–608. doi:10.1053/j.gastro.2014.01.038
13. Salehi M, Woods SC, D’Alessio DA. Gastric bypass alters both glucose-dependent and glucose-independent regulation of islet hormone secretion. Obesity (Silver Spring). 2015;23(10):2046–2052. doi:10.1002/oby.21186
14. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017. doi:10.1210/jc.2013-2686
15. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. doi:10.1056/NEJMoa043690
16. Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685. doi:10.1210/jc.2007-0918
17. Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver Spring). 2015;23(4):798–807. doi:10.1002/oby.21043
18. Jacobsen SH, Bojsen-Møller KN, Dirksen C, et al. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56(10):2250–2254. doi:10.1007/s00125-013-3003-0
19. Mulla CM, Goldfine AB, Dreyfuss JM, et al. Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes Surg. 2019;29(7):2092–2099. doi:10.1007/s11695-019-03845-0
20. Nielsen S, Svane MS, Kuhre RE, et al. Chenodeoxycholic acid stimulates glucagon-like peptide-1 secretion in patients after Roux-en-Y gastric bypass. Physiol Rep. 2017;5(3):e13140. doi:10.14814/phy2.13140
21. Zhou LY, Deng MQ, Xiao XH. Potential contribution of the gut microbiota to hypoglycemia after gastric bypass surgery. Chin Med J. 2020;133(15):1834–1843. doi:10.1097/CM9.0000000000000932
22. Mulla CM, Middelbeek RJW, Patti ME. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann N Y Acad Sci. 2018;1411(1):53–64. doi:10.1111/nyas.13409
23. van Furth AM, van den Broek M, Emous M, de Heide LJM, Kuipers F, van Beek AP. Cholecystectomy increases the risk of dumping syndrome and postbariatric hypoglycemia after bariatric surgery. Surg Obes Related Dis. 2020. doi:10.1016/j.soard.2020.08.009
24. Ilhan ZE, DiBaise JK, Isern NG, et al. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017;11(9):2047–2058. doi:10.1038/ismej.2017.71
25. Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088–25097. doi:10.1074/jbc.M113.452516
26. Hyltén-Cavallius L, Iepsen EW, Wewer Albrechtsen NJ, et al. Patients with long-QT syndrome caused by impaired hERG-encoded K v 11.1 potassium channel have exaggerated endocrine pancreatic and incretin function associated with reactive hypoglycemia. Circulation. 2017;135(18):1705–1719. doi:10.1161/CIRCULATIONAHA.116.024279
27. Mateo CR, Patti ME, Long QT. Syndrome and risk for hypoglycemia in a postbariatric surgery patient: bariatric times; 2019. Available from: https://bariatrictimes.com/long-qt-syndrome-postbariatric-surgery/.
28. Abrahamsson N, Borjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667–2675. doi:10.2337/db16-0341
29. Øhrstrøm CC, Hansen DL, Kielgast UL, et al. Counterregulatory responses to postprandial hypoglycemia after Roux-en-Y gastric bypass. Surg Obes Related Dis. 2020.
30. Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes. 2011;35(Suppl S3):S22–S25. doi:10.1038/ijo.2011.143
31. Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6(7):912–926. doi:10.4239/wjd.v6.i7.912
32. Dror E, Dalmas E, Meier DT, et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol. 2017;18(3):283–292. doi:10.1038/ni.3659
33. Ota K, Wildmann J, Ota T, Besedovsky HO, Del Rey A. Interleukin-1beta and insulin elicit different neuroendocrine responses to hypoglycemia. Ann N Y Acad Sci. 2009;1153.
34. Del Rey A, Roggero E, Randolf A, et al. IL-1 resets glucose homeostasis at central levels. Proc Natl Acad Sci U S A. 2006;103(43).
35. Hepprich M, Wiedemann SJ, Schelker BL, et al. Postprandial hypoglycemia in patients after gastric bypass surgery is mediated by glucose-induced IL-1β. Cell Metab. 2020;31(4):699–709.e5. doi:10.1016/j.cmet.2020.02.013
36. Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. doi:10.1007/s00125-010-1798-5
37. Goldfine AB, Patti ME. How common is hypoglycemia after gastric bypass? Obesity (Silver Spring). 2016;24(6):1210–1211. doi:10.1002/oby.21520
38. Belligoli A, Sanna M, Serra R, et al. Incidence and predictors of hypoglycemia 1 year after laparoscopic sleeve gastrectomy. Obes Surg. 2017;27(12):3179–3186. doi:10.1007/s11695-017-2742-2
39. Capristo E, Panunzi S, De Gaetano A, et al. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. J Clin Endocrinol Metab. 2018;103(6):2136–2146. doi:10.1210/jc.2017-01695
40. Palladino AA. Increased glucagon-like peptide-1 secretion and postprandial hypoglycemia in children after nissen fundoplication. J Clin Endocrinol Metab. 2020;94(1):39–44.
41. Casden CE. A case of hypoglycemia in an adult after Nissen fundoplication.
42. Lee CJ, Craig Wood G, Lazo M, et al. Risk of post-gastric bypass surgery hypoglycemia in nondiabetic individuals: a single center experience. Obesity (Silver Spring). 2016;24(6):1342–1348. doi:10.1002/oby.21479
43. Rebelos E, Moriconi D, Scalese M, et al. Impact of postprandial hypoglycemia on weight loss after bariatric surgery. Obes Surg. 2020;30(6):2266–2273. doi:10.1007/s11695-020-04465-9
44. Raverdy V, Baud G, Pigeyre M, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: a five year longitudinal study. Ann Surg. 2016;264(5):878–885. doi:10.1097/SLA.0000000000001915
45. Guarino D, Moriconi D, Mari A, et al. Postprandial hypoglycaemia after Roux-en-Y gastric bypass in individuals with type 2 diabetes. Diabetologia. 2019;62(1):178–186. doi:10.1007/s00125-018-4737-5
46. Torekov SS, Iepsen E, Christiansen M, et al. KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes Care. 2014;6.
47. Engelbrechtsen L, Mahendran Y, Jonsson A, et al. Common variants in the hERG (KCNH2) voltage-gated potassium channel are associated with altered fasting and glucose-stimulated plasma incretin and glucagon responses. BMC Genet. 2018;19(1).
48. Marques JL, George E, Peacey SR, et al. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabetic Med. 1997;14(8):648–654. doi:10.1002/(SICI)1096-9136(199708)14:8<648::AID-DIA418>3.0.CO;2-1
49. Gold AE, Macleod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697–703. doi:10.2337/diacare.17.7.697
50. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517–522. doi:10.2337/diacare.18.4.517
51. Marques AR, Lobato CB, Pereira SS, et al. Insights from the impact of meal composition on glucose profile towards post-bariatric hypoglycemia management. Obes Surg. 2020;30(1):249–255. doi:10.1007/s11695-019-04147-1
52. Krebs HA. Effects of ethanol on gluconeogenesis. In: Martini GA, editor. Metabolic Changes Induced by Alcohol. Berlin, Heidelberg: Springer; 1971:152–156.
53. Siler SQ, Neese RA, Christiansen MP, Hellerstein MK. The inhibition of gluconeogenesis following alcohol in humans. Am J Physiol. 1998;275(5).
54. Ranjan A, Nørgaard K, Tetzschner R, et al. Effects of preceding ethanol intake on glucose response to low-dose glucagon in individuals with type 1 diabetes: a randomized, placebo-controlled, crossover study. Diabetes Care. 2018;41(4):797–806. doi:10.2337/dc17-1458
55. Kerr D, Sherwin RS, Pavalkis F, et al. Effect of caffeine on the recognition of and responses to hypoglycemia in humans. Ann Intern Med. 1993;119(8):799. doi:10.7326/0003-4819-119-8-199310150-00005
56. Shi X, Xue W, Liang S, Zhao J, Zhang X. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: a systematic review and meta-analysis. Nutr J. 2016;15(1). doi:10.1186/s12937-016-0220-7
57. Øhrstrøm CC, Worm D, Kielgast UL, Holst JJ, Hansen DL. Evidence for relationship between early dumping and postprandial hypoglycemia after Roux-en-Y gastric bypass. Obes Surg. 2020;30(3).
58. Patti ME, Goldfine AB. The rollercoaster of post-bariatric hypoglycaemia. Lancet Diabetes Endocrinol. 2016;4(2):94–96. doi:10.1016/S2213-8587(15)00460-X
59. Varma S, Clark JM, Schweitzer M, Magnuson T, Brown TT, Lee CJ. Weight regain in patients with symptoms of post-bariatric surgery hypoglycemia. Surg Obes Related Dis. 2017;13(10):1728–1734. doi:10.1016/j.soard.2017.06.004
60. Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg. 1935;101(6):1299–1335. doi:10.1097/00000658-193506000-00001
61. Association AD. 6. Glycemic targets: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S61–S70.
62. Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi:10.1210/jc.2008-1410
63. El Khoury M, Yousuf F, Martin V, Cohen RM. Pseudohypoglycemia: a cause for unreliable finger-stick glucose measurements. Endocrine Cancer. 2008;14(3):337–339. doi:10.4158/EP.14.3.337
64. Bishay RH, Suryawanshi A. Artifactual hypoglycaemia in systemic sclerosis and raynaud’s phenomenon: a clinical case report and short review. Case Rep Endocrinol. 2016;2016.
65. Tarasova VD, Zena M, Rendell M. Artifactual hypoglycemia: an old term for a new classification. Diabetes Care. 2014;37(5):e85–e86. doi:10.2337/dc13-2891
66. Celli R, Tang LH, Cai G, et al. Proinsulin expressing neuroendocrine tumors of the pancreas: an underrecognized entity. Pancreas. 2019;48(1):55–59. doi:10.1097/MPA.0000000000001196
67. Mulla CM, Storino A, Yee EU, et al. Insulinoma after bariatric surgery: diagnostic dilemma and therapeutic approaches. Obes Surg. 2016;26(4):874–881. doi:10.1007/s11695-016-2092-5
68. Nieman LK. Dynamic evaluation of adrenal hypofunction. J Endocrinol Invest. 2003;26(7Suppl).
69. Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med. 2018;6(17):335. doi:10.21037/atm.2018.07.32
70. Dynkevich Y, Rother KI, Whitford I, et al. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev. 2013;34(6).
71. Furrer J, Hättenschwiler A, Komminoth P, Pfammatter T, Wiesli P. Carcinoid syndrome, acromegaly, and hypoglycemia due to an insulin-secreting neuroendocrine tumor of the liver. J Clin Endocrinol Metab. 2001;86(5):2227–2230. doi:10.1210/jcem.86.5.7461
72. Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycemia. Diabetes. 1981;30(12):996–999. doi:10.2337/diab.30.12.996
73. Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6(10):583–590.
74. Scarpellini E, Arts J, Karamanolis G, et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16(8):448–466. doi:10.1038/s41574-020-0357-5
75. Suhl E, Anderson-Haynes SE, Mulla C, Patti ME. Medical nutrition therapy for post-bariatric hypoglycemia: practical insights. Surg Obes Relat Dis. 2017;13(5):888–896. doi:10.1016/j.soard.2017.01.025
76. Gremse DA, Bucuvalas JC, Balistreri WF. Efficacy of cornstarch therapy in type III glycogen-storage disease. Am J Clin Nutr. 1990;52(4):671–674. doi:10.1093/ajcn/52.4.671
77. Lembo E, Lupoli R, Ciciola P, et al. Implementation of low glycemic index diet together with cornstarch in post-gastric bypass hypoglycemia: two case reports. Nutrients. 2018;10(6):670. doi:10.3390/nu10060670
78. Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–474. doi:10.4239/wjd.v8.i11.464
79. Wadhwania R. Is vitamin D deficiency implicated in autonomic dysfunction? J Pediatr Neurosci. 2017;12:119–123.
80. Lonsdale D. Dysautonomia, a heuristic approach to a revised model for etiology of disease. Evid Based Complement Alternat Med. 2009;6:3–10. doi:10.1093/ecam/nem064
81. Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521–4539. doi:10.3390/nu5114521
82. Fujita S, Kozawa J, Ishibashi C, et al. An impaired awareness of hypoglycemia improved after vitamin B12 treatment in a type 1 diabetic patient. Inter Med (Tokyo, Japan). 2017;56(11):1383–1385. doi:10.2169/internalmedicine.56.8489
83. Frankhouser SY, Ahmad AN, Perilli GA, Quintana BJ, Vengrove MA. Post-gastric-bypass hypoglycemia successfully treated with alpha-glucosidase inhibitor therapy. Endocr Pract. 2013;19(3):511–514. doi:10.4158/EP12281.RA
84. Moreira RO, Moreira RB, Machado NA, Gonçalves TB, Coutinho WF. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. 2008;18(12):1618–1621. doi:10.1007/s11695-008-9569-9
85. Valderas JP, Ahuad J, Rubio L, Escalona M, Pollak F, Maiz A. Acarbose improves hypoglycaemia following gastric bypass surgery without increasing glucagon-like peptide 1 levels. Obes Surg. 2012;22(4):582–586. doi:10.1007/s11695-011-0581-0
86. Imhof A, Schneemann M, Schaffner A, Brändle M. Reactive hypoglycaemia due to late dumping syndrome: successful treatment with acarbose. Swiss Med Wkly. 2001;131(5–6):81–83.
87. Spanakis E, Gragnoli C. Successful medical management of status post-Roux-en-Y-gastric-bypass hyperinsulinemic hypoglycemia. Obes Surg. 2009;19(9):1333–1334. doi:10.1007/s11695-009-9888-5
88. Gonzalez-Gonzalez A, Delgado M, Fraga-Fuentes MD. Use of diazoxide in management of severe postprandial hypoglycemia in patient after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(1):e18–19. doi:10.1016/j.soard.2011.05.010
89. Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol. 2012;166(5):951–955. doi:10.1530/EJE-11-1065
90. Mohammadi A, Sulaiman RA, Grossman AB. Pasireotide and octreotide in the treatment of severe late dumping syndrome. Clin Case Rep. 2017;5:1608–1611. doi:10.1002/ccr3.1025
91. van Beek AP, Laville M, Tack J, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18(1):68–85. doi:10.1111/obr.12467
92. Øhrstrøm CC, Hansen DL, Kielgast UL, et al. Dose of pasireotide prevents hypoglycemia in Roux-en-Y gastric bypass-operated individuals. Obes Surg. 2020;30(4).
93. Ames A, Lago-Hernandez CA, Grunvald E. Hypoglycemia after gastric bypass successfully treated with calcium channel blockers: two case reports. J Endocr Soc. 2019;3:1417–1422. doi:10.1210/js.2019-00097
94. Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. 2013;169(6):885–889. doi:10.1530/EJE-13-0504
95. McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95(4):1851–1855. doi:10.1210/jc.2009-1628
96. Craig CM, Lamendola C, Holst JJ, Deacon CF, McLaughlin TL. The use of gastrostomy tube for the long-term remission of hyperinsulinemic hypoglycemia after Roux-en-y gastric bypass: a case report. AACE Clin Case Rep. 2015;1(2):e84–e87. doi:10.4158/EP14218.CR
97. Davis DB, Khoraki J, Ziemelis M, Sirinvaravong S, Han JY, Campos GM. Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: confirming a non-pancreatic etiology. Mol Metab. 2018;9:15–27. doi:10.1016/j.molmet.2017.12.011
98. Arora I, Patti ME. Can reversal of RYGB also reverse hypoglycemia? Mol Metab. 2018;9:1–3. doi:10.1016/j.molmet.2018.01.004
99. Lee CJ, Brown T, Magnuson TH, Egan JM, Carlson O, Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J Clin Endocrinol Metab. 2013;98(7):E1208–1212. doi:10.1210/jc.2013-1151
100. Qvigstad E, Gulseth HL, Risstad H, et al. A novel technique of Roux-en-Y gastric bypass reversal for postprandial hyperinsulinemic hypoglycaemia: a case report. Int J Surg Case Rep. 2016;21:91–94. doi:10.1016/j.ijscr.2016.02.033
101. Ma P, Ghiassi S, Lloyd A, et al. Reversal of Roux en Y gastric bypass: largest single institution experience. Surg Obes Related Dis. 2019;15(8):1311–1316. doi:10.1016/j.soard.2019.05.005
102. Shoar S, Nguyen T, Ona MA, et al. Roux-en-Y gastric bypass reversal: a systematic review. Surg Obes Relat Dis. 2016;12(7):1366–1372. doi:10.1016/j.soard.2016.02.023
103. Vanderveen KA, Grant CS, Thompson GB, et al. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery. 2010;148(6):1237–1246. doi:10.1016/j.surg.2010.09.027
104. Tan M, Lamendola C, Luong R, McLaughlin T, Craig C. Safety, efficacy and pharmacokinetics of repeat subcutaneous dosing of avexitide (exendin 9-39) for treatment of post-bariatric hypoglycaemia. Diabetes Obes Metab. 2020;22(8):1406–1416. doi:10.1111/dom.14048
105. Mulla CM, Zavitsanou S, Laguna Sanz AJ, et al. A randomized, placebo-controlled double-blind trial of a closed-loop glucagon system for postbariatric hypoglycemia. J Clin Endocrinol Metab. 2020;105(4):e1260–e1271. doi:10.1210/clinem/dgz197
106. Laguna Sanz AJ, Mulla CM, Fowler KM, et al. Design and clinical evaluation of a novel low-glucose prediction algorithm with mini-dose stable glucagon delivery in post-bariatric hypoglycemia. Diabetes Technol Ther. 2018;20(2):127–139. doi:10.1089/dia.2017.0298
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.