Back to Journals » International Medical Case Reports Journal » Volume 13
Hypertension and Ischemic Stroke After Aflibercept for Retinopathy of Prematurity
Authors Bazvand F , Khalili pour E , Gharehbaghi G, Faghihi H, Khodabandeh A , Mehrabi Bahar M , Riazi-Esfahani H
Received 24 April 2020
Accepted for publication 23 June 2020
Published 3 July 2020 Volume 2020:13 Pages 243—247
DOI https://doi.org/10.2147/IMCRJ.S258881
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Fatemeh Bazvand,1 Elias Khalili pour,1 Golnaz Gharehbaghi,2 Hooshang Faghihi,1 Alireza Khodabandeh,1 Mohammadreza Mehrabi Bahar,1 Hamid Riazi-Esfahani1
1Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran; 2Ali Asghar Children’s Hospital, Iran University of Medical Sciences, Tehran, Iran
Correspondence: Mohammadreza Mehrabi Bahar
Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Farabi Eye Hospital, Qazvin Square, South Kargar Street, Tehran 1336616351, Iran
Tel +982155421002
Email [email protected]
Background: Retinopathy of prematurity is the leading cause of preterm infants’ blindness. The preferred method for the management of aggressive posterior ROP is the anti-vascular endothelial growth factor (anti-VEGF). However, systemic and ocular adverse effects of anti-VEGF drugs remain a concern.
Case Presentation: A case report of a preterm infant with a history of hypertension underwent intravitreal injection of aflibercept at the 50-week postmenstrual age because of aggressive posterior retinopathy of prematurity (ROP) in both eyes. Seven days after the intravitreal administration of aflibercept, he has a hypertension crisis and an ischemic stroke. Serial fundoscopies implied complete arrest of vascularization till seven months after receiving treatment.
Conclusion: We report a case of an infant, with a history of hypertension, had an ischemic stroke just one week after the intravitreal injection of aflibercept for aggressive posterior ROP. We can conclude that in cases of preterm infants with systemic comorbidities, like uncontrolled hypertension, that predispose patients to thromboembolic events, we should be cautious about the potential increase in the risk of thromboembolic events after administration of anti-vascular endothelial growth factor agents (anti-VEGF), especially those with a longer half-life, like aflibercept.
Keywords: retinopathy of prematurity, aflibercept, intravitreal injection, stroke, cerebrovascular accident
Introduction
Retinopathy of prematurity is a proliferative vasculopathy of immature retinal vessels in premature infants and the leading cause of preterm infants’ blindness. Although the preferred method for management of high risk-ROP was laser photoablation for many years, the BEAT-ROP study shows the efficacy and favorable results in managing of prethereshhold type 1 ROP with anti-vasoendothelial growth factor (anti-VEGF) especially in zone 1.1 Studies show a reduction in serum VEGF level after administration of Anti-VEGF intravitreally, and systemic adverse effects of these conditions remain a concern.2
Aflibercept is a potent recombinant fusion protein that blocks all isoforms of VEGF-A, VEGF-B, and placental growth factors. It has a higher binding capacity to VEGF and longer duration of action in the eye compared to previous anti-VEGF agents.3 The systemic administration of aflibercept in cancer patients is associated with a significantly increased risk of hypertension.4 But in a systematic review of ocular side effects of intravitreal aflibercept in adults shows that hypertension was similar to those of controls and similar across disease states in evaluated IAI trials. The Committee for Medicinal Products for Human Use pointed attention to the increase in cerebrovascular events with the use of aflibercept. However, systematic studies show no increase in cerebrovascular side effects in intravitreal use of aflibercept in adults’ retinal vasculopathy.5 However, there is no study to evaluate hypertension and cerebrovascular side effects of anti-VEGF used in ROP patients.
We report a case of cerebral stroke and hypertension crisis one week after administering intravitreal aflibercept and complete retinal vascular arrest until seven months after receiving treatment for aggressive posterior ROP.
Case Report
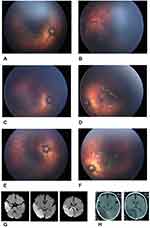
The case concerned a preterm infant with a gestational age of 34 weeks (36 weeks postmenstrual age (PMA)) and birth weight of 1970 mg. He was admitted to the neonatal intensive care unit (NICU) because of respiratory distress syndrome and treated with a single dose of intratracheal surfactant. He had neonatal jaundice that responded well to phototherapy alone. He developed hypertension and was treated with propranolol. His first visit for ROP was in his 41 weeks PMA and showed ROP in zone 1–2. The ophthalmologist recommended revisiting in 1 week to decide any necessary treatment. However, he was non-adherent to eye exams due to systemic comorbidities and needed hospitalization for necessary workup. He was admitted in 42 weeks PMA due to uncontrolled hypertension, and his cardiac workup revealed no abnormality, and they referred him to a pediatric nephrologist. In 43 weeks PMA, he was admitted for subsequent nephrology workup, they found renal artery stenosis confirmed with computed tomography angiography (CT-Angiography) and Color Doppler sonography. He was treated with amlodipine after consultation with a pediatric nephrologist. He had one episode of tonic-clonic seizure in his 48 weeks PMA, and in obtained electroencephalography, no abnormalities were detected. He was treated with phenytoin and phenobarbital.
After serial systemic workups, he was discharged from the pediatric hospital with controlled blood pressure with enalapril and amlodipine medication. One day after discharge, he visited in our hospital at his 50 weeks PMA. He underwent bilateral intravitreal aflibercept (1mg/0.025mL) (Eylea®, Regeneron) injection under topical anesthesia due to aggressive posterior ROP (zone 1 stage 3 flat neovascularization with plus) as shown in Figure 1A and B. In our center, routine anti-VGEF is bevacizumab. However, at that time, due to the unavailability of bevacizumab, aflibercept was prescribed. He was treated with a topical antibiotic (chloramphenicol 0.5% four times a day) to prevent infection. One week after the administration of aflibercept, he presented in the emergency ward for a hypertension crisis (BP 110/87) and a decrease in left upper and lower extremities’ motion and agitation. Based on neurologic examination, computed tomography scan (CT scan), and magnetic resonance imaging (MRI), they confirmed the acute ischemic cerebrovascular stroke at the occipital lobe, parietal lobe, and also splenium of the corpus callosum at territories of posterior cerebral and middle cerebral artery (Figure 1G and H). His systemic workup includes hypercoagulable state like antiphospholipid and anticardiolipin antibody was done, and all of them were normal. He was discharged with aspirin, phenytoin, phenobarbital, amlodipine, and enalapril.
Because of uncontrolled hypertension, and suspicious to the vascular anomaly, he underwent comprehensive examinations and imagings including thoracic and abdominal CT-Angiography, cervical color Doppler sonography, and transesophageal echocardiography. He revealed no apparent vascular involvement except for the right renal artery stenosis. Serial cardiac and nephrology workups and visits, till 75 weeks PMA, documented no further abnormality and controlled Blood Pressure with amlodipine and enalapril.
In eye exams, after intravitreal aflibercept injection in serial fundoscopies, we found a complete stop in vascularization progression until 75 weeks PMA (Figure 1C and D. fundus photographs of the right and left eye at the 65 weeks PMA and Figure1E and F at the 75 weeks PMA). In this time, due to non-adherent to eye exams, we have done laser photoablation in the retinal avascular zone.
Discussion
In cases of treatment-requiring ROP, the standard of care is laser photoablation of the avascular retina. BEAT-ROP study revealed favorable positive results and low ocular morbidity compared to conventional laser in the treatment of ROP in posterior zones.1 More recently, with the increased availability and easy administration of Anti-VEGF drugs in ROP, interests in the administration of all kinds of Anti-VEGF has been increased. But still, concerns and questions remain regarding timing, dosage, and ocular and systemic adverse events of using anti-VEGF agents for ROP.6 An interestingly reviewed data from the Canadian Neonatal Network showed higher odds of severe neurodevelopmental disabilities in preterm infants born before 29 weeks’ gestation and treated with bevacizumab, after adjusting for all key confounders like gestation, gender, maternal education, Score for Neonatal Acute PhysiologyII (SNAP-II) score, bronchopulmonary dysplasia, sepsis, and severe brain injury.7
Aflibercept has several advantages in comparison to other Anti-VEGF drugs. It has a high binding affinity to VEGF receptors, and it binds to VEGF-A, VEGF-B, and PLGF receptors. The high efficacy and the long duration of action of Aflibercept, possibly will help in inducing regression of ROP in very severe cases and benefit from preserving the regression. Salman et al were the first to report that a single injection of aflibercept was an effective therapy for high-risk prethreshold type 1 ROP, with favorable structural, visual, and refractive outcomes.3 Furthermore, several studies show the efficacy of these agents in the management of ROP.6
Aflibercept (Eylea®, Regeneron) has the FDA-approval for intravitreal injections in adults, but its use in ROP remains off-label.3 There is some concern about thromboembolic events in patients using intravitreal aflibercept.5 Anti-VEGF agents act on endothelium directly and considering the imperative role of the endothelium to sustain hemostatic balance, anti-VEGF agents may have the potential to increase the risk of thromboembolic events. However, several clinical trials show no difference in hypertension and thromboembolic events between intravitreal ranibizumab and placebo, nor between ranibizumab and aflibercept. On the other hand, patients receiving anti-VEGF have several risk factors for thromboembolic events.5
There are several case reports on Ischemic stroke after administration of aflibercept in adults, and all of them mentioned that potential risk factors like hypertension might play a role in thromboembolic adverse events but, to the best of our knowledge, there is no case report about ischemic stroke after anti-VEGF use in ROP patients even with systemic comorbidities like uncontrolled hypertension.5
Recently an interesting case report of a premature infant treated for stage 3 ROP with intravitreal bevacizumab developed new-onset systemic hypertension and neuroimaging changes. Neuroimaging showed new areas of vasogenic edema, which improved over time.8
We report a preterm infant with an ischemic stroke just one week after the intravitreal injection of aflibercept for ROP. Still, we cannot directly address that because of the anti-VEGF agent’s pharmacodynamics effect. Our patient was suffered from poorly controlled hypertension. Importantly, our patient also showed a delayed retinal vascularization. Although his birth age was 36 weeks PMA, in the funduscopic exam in 41 weeks PMA, he had ROP in zone 1–2. Additionally, in 50 weeks PMA, he had aggressive posterior ROP, which is atypical for that age. With all together and systemic comorbidities like renal artery stenosis, he probably had a disorder that delayed retinal vascularization. Moreover, in serial fundus exams following administration of intravitreal aflibercept, complete arrest of retinal vascular growth was noted; it might be due to the long duration of aflibercept’s action, even though he probably had the disorder to slow down retinal vascularization. We do not know these both complications were due to the side effects of aflibercept or due to the systemic condition of our patient.
In conclusion, we mentioned, in similar cases of preterm infants with systemic comorbidities, especially uncontrolled hypertension, we should be vigilant about the potential increase in the risk of thromboembolic events after the administration of anti-VEGF agents, particularly agents with a longer half-life, like aflibercept. Although we should be cautious about infants with delayed retinal vascularization like our patients, and should not use such long-acting agents.
Abbreviations
ROP, retinopathy of prematurity; VEGF, vascular endothelial growth factor; CT-Angiography, computed tomography angiography; PMA, postmenstrual age.
Data Sharing Statement
Not applicable.
Ethics Approval and Consent to Participate
The Health Research Authority decision tool (http://www.hra-decisiontools.org.uk/research) confirmed this project was not research and so ethical approval was not sought.
Consent for Publication
Written informed consent was obtained from his parents for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi:10.1056/NEJMoa1007374
2. Pertl L, Steinwender G, Mayer C, et al. A systematic review and meta-analysis on the safety of Vascular Endothelial Growth Factor (VEGF) inhibitors for the treatment of retinopathy of prematurity. PLoS One. 2015;10(6):e0129383. doi:10.1371/journal.pone.0129383
3. Salman AG, Said AM. Structural, visual and refractive outcomes of intravitreal aflibercept injection in high-risk prethreshold type 1 retinopathy of prematurity. Ophthalmic Res. 2015;53(1):15–20. doi:10.1159/000364809
4. Qi W-X, Shen Z, Tang L-N YY. Risk of hypertension in cancer patients treated with aflibercept: a systematic review and meta-analysis. Clin Drug Investig. 2014;34(4):231–240. doi:10.1007/s40261-014-0174-5
5. Beaumont PE, Petocz P, Kang HK. Is there risk of stroke with aflibercept? Ophthalmology. 2014;121(1):e4. doi:10.1016/j.ophtha.2013.09.020
6. Klufas MA, Chan RV. Intravitreal anti-VEGF therapy as a treatment for retinopathy of prematurity: what we know after 7 years. J Pediatr Ophthalmol Strabismus. 2015;52(2):77–84. doi:10.3928/01913913-20150216-01
7. Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137:4. doi:10.1542/peds.2015-3218
8. Twitty G, Weiss M, Albayram MS, O’Mara K, Mowitz ME. Hypertension and neuroimaging changes after bevacizumab for retinopathy of prematurity. Pediatrics. 2020;145(1):e20191814. doi:10.1542/peds.2019-1814
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.