Back to Journals » Lung Cancer: Targets and Therapy » Volume 9
Hypersensitivity in ALK-positive lung cancers exposed to ALK inhibitors: a case of successful switch to an alternative ALK inhibitor and systematic review of the literature
Authors Deng L , Sharma J, Ravera E, Halmos B, Cheng H
Received 12 May 2018
Accepted for publication 27 July 2018
Published 6 September 2018 Volume 2018:9 Pages 73—77
DOI https://doi.org/10.2147/LCTT.S173948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Lei Deng,1 Janaki Sharma,2 Elizabeth Ravera,2 Balazs Halmos,2 Haiying Cheng2
1Department of Medicine, Jacobi Medical Center/Albert Einstein College of Medicine, Bronx, NY, USA; 2Department of Oncology, Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, NY, USA
Abstract: Alectinib can cause rare, but severe hypersensitivity. The cross-reactivity between different ALK inhibitors is unknown and desensitization is the only reported management. We hereby report the first case of severe delayed hypersensitivity developed in a lung cancer patient treated by alectinib, who was successfully managed by switching to brigatinib, another ALK inhibitor. The patient achieved excellent anti-tumor response to brigatinib. Our case provides an alternative and safe strategy in patients with alectinib-related hypersensitivity.
Keywords: alectinib, brigatinib
Introduction
ALK rearrangement is a well-recognized oncogenic driver in non-small-cell lung cancer (NSCLC), accounting for about 8% of NSCLC population.1,2 Alectinib is currently the preferred first-line treatment for metastatic NSCLC harboring ALK gene rearrangement based on J-ALEX and ALEX studies.3,4 While in general, alectinib, a well-tolerated drug, may lead to adverse effects, including rare hypersensitivity reactions principally presenting with a skin rash. Grade 3 or 4 skin rash was reported in only 1% of patients in J-ALEX study. Because of the rarity, experience in managing such cases is very limited. Previously, only desensitization was reported although its role in delayed hypersensitivity remains controversial and usually requires close inpatient monitor.5,6 In the present study, we report a case of alectinib-induced type IV delayed hypersensitivity presenting with an extensive skin rash and high fever, after obtaining a written informed consent from the patient to publish the case details and accompanying images. This serious toxicity was successfully managed in this case by a brief course of prednisone and switching treatment to brigatinib which demonstrated excellent activity.
Case summary
A 49-year-old Hispanic never-smoking female, with a history of asthma and resected atrial myxoma, was found to have a left upper lobe perihilar lung nodule, mediastinal lymphadenopathy, and a large left pleural effusion. Cytology of the pleural effusion was consistent with TTF1-positive pulmonary adenocarcinoma. Further testing revealed EGFR wild type, no ALK rearrangement (by fluorescence in situ hybridization), and no ROS1 fusion. The PD-L1 TPS score was 40%. She was treated initially with four cycles of carboplatin and pemetrexed and subsequently eight cycles of pembrolizumab with the progression of disease. At the same time, EML4-ALK fusion was identified by NGS-based circulating tumor DNA analysis (FoundationACT). It is unclear why the ALK rearrangement was not detected by the FISH test at initial diagnosis. The quality, quantity, and process of tumor specimen may affect the FISH test and occasionally lead to false-negative results. In our case, the initial pleural effusion specimen might not be suitable for the FISH test.
The patient started on alectinib 600 mg twice a day and had a rapid improvement of her left-sided chest pain. However, 10 days later, she developed a non-pruritic morbilliform rash spreading from trunk to the entire body, including palms, soles, and face with mild lip and eye swelling (Figure 1A). She had recurrent fevers with a maximum temperature of 105 degrees F and was hospitalized. A right arm lesion was punch biopsied, showing spongiotic and interface dermatitis with eosinophils, consistent with drug eruption (delayed type IV hypersensitivity, Figure 1B). She had no hematologic (eosinophilia), hepatic, renal, or pulmonary abnormality to suggest drug reaction with eosinophilia and systemic symptoms. Infectious disease workup was negative for streptococcal pharyngitis, HIV, parvovirus, herpes, syphilis, strongyloidiasis, and bacteremia. Alectinib was the only medication she was taking and was therefore withheld. Oral prednisone was started at 30 mg on day 1 and was tapered to 20 mg on the next day. She then continued at 10 mg daily for the next 5 days with a total course of 7 days. She also received one dose of diphenhydramine 50 mg intravenously prior to oral prednisone and topical triamcinolone cream. No antibiotics were administered. The rash improved rapidly, and the patient’s febrile episodes resolved following 1 day of prednisone administration. Desensitization was not instituted because of the perceived potentially serious nature of this delayed hypersensitivity reaction, in light of the presence of eosinophils in the skin biopsy, and high fevers. She was started with brigatinib 1 week after completion of prednisone taper. She tolerated the oral inhibitor well without rash and fever. Eight weeks later, rescanning showed markedly improved pleural effusion and tumor response (Figure 1C).
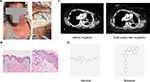  | Figure 1 (A) Morbilliform skin rash on the face, sole, palm, and legs. (B) Skin pathology of punch biopsy on right arm showing spongiotic and interface dermatitis with eosinophils, consistent with drug eruption. Left: 40× magnification; right: 200× magnification; (C) CT chest images before and 8 weeks after brigatinib treatment. (D) Chemical structures of alectinib (left) and brigatinib (right). Structure pictures of alectinib and brigatinib were downloaded from PubChem database.16 |
Systematic review
Medline database was searched (data cutoff as of May 1, 2018) with the following terms: (hypersensitivity or rash); cancer; (ALK or (anaplastic lymphoma kinase) or crizotinib or alectinib or brigatinib or ceritinib or lorlatinib or TSR-011 or ASP3026 or ensartinib)). Reports of hypersensitivity but lack of management details were not included. Reports identified through literature reading but not from Medline search were also included. Data are summarized in Table 1. The search generated 29 publications. After screening, we included three reports from Medline search. An additional case report was identified through literature reading (flowchart summarized in Figure S1).
  | Table 1 Summary of cases on hypersensitivity to ALK inhibitors with successful management Note: All patients are female. |
Discussion
Our unique case is the first report of the successful switch to an alternative ALK inhibitor, brigatinib, in a patient who developed alectinib-induced severe type IV hypersensitivity. To the best of our knowledge, two cases of delayed skin hypersensitivity to alectinib have been reported (Table 1).5,7 In both cases, patients were treated with oral desensitization for 9 and 14 days in the inpatient monitored setting, respectively.5 While desensitization seemed effective in the reported cases, we did not believe that this was an appropriate course for our patient. First, there has been no consensus of the utility and risk/benefit ratio of desensitization for delayed hypersensitivity. The European position recommends that desensitization is “restricted to mild, uncomplicated exanthems and fixed drug eruptions.”6 The extensive rash in our case was generalized to the entire body, associated with high fever. In addition, our patient was treated with standard dose of 600 mg twice daily in contrast to once daily in the previous case and desensitization to full dose may carry higher risk and requires longer time. Sustained exposure is also needed to maintain tolerance status, and interruption will require repeated desensitization.8 Our case is further unique given the type IV hypersensitivity with eosinophil infiltration and fever, which was lacking in the previous case. Of note, the patient’s history of asthma may suggest asthmatic involvement in eosinophilic findings in skin, but the patient had not been on any asthmatic medication (neither controller nor rescuer) and had no exacerbation for at least a year before the rash and fever onset. Eosinophilia was present in peripheral blood. At the onset of rash and fever, the patient had no shortness of breath and wheezing. We therefore believe that there is less chance that the skin eosinophils represent her asthma. So far, three more cases of desensitization to crizotinib, a first-generation ALK inhibitor, have been reported, but all these appeared more consistent with immediate and not delayed hypersensitivity, and the management was only desensitization (Table 1).9,10 Although reappereance of symptoms on readministration is very important to establish cause–effect relationship between a drug and hypersensitivity, the potential serious risk prevents us to test it in our case.
Brigatinib is a highly potent, CNS-penetrant third-generation ALK inhibitor that was recently approved by the US Food and Drug Administration for patients with metastatic ALK-positive NSCLC after progression on their initial treatment with crizotinib based on ALTA trial.11 Although there is no direct comparison of efficacy between brigatinib and alectinib, studies showed that in the second-line setting in crizotinib refractory patients, the progression-free survival was 8.3 and 9.2–12.9 months for alectinib and brigatinib, respectively.11,12 In addition to ALK rearrangement, the spectrum of brigatinib can also inhibit ROS-1. Preclinical research showed that brigatinib was effective in managing secondary ALK mutants, but clinical studies are needed to validate the findings in patients.13,14 Information on cross-reactivity between brigatinib and alectinib is not available, but certain aspects of the side effects are distinct between the two,15 with the former characterized by hypertension and the latter with mostly laboratory abnormalities for most common grade 3–5 toxicities.3,11 The different chemical structures of the two inhibitors (Figure 1D) may explain the lack of cross-hypersensitivity to brigatinib in our case. By switching to brigatinib instead of desensitization, we avoided the prolonged hospitalization requiring close monitoring and prolonged desensitization protocol.6
Conclusion
In summary, we describe the first case of successfully switching from alectinib to brigatinib in a patient with ALK-positive advanced lung adenocarcinoma with alectinib-induced type IV delayed hypersensitivity. Our experience thus provides an alternative and safe management strategy in patients with alectinib-related hypersensitivity.
Disclosure
BH received grants and consulting fees from Roche, Takeda, Pfizer, Eli Lilly, Boehringer Ingelheim, Astra-Zeneca, Novartis, Mirati, grants from Merck, and consulting fees from Foundation Medicine and Guardant Health 360. The other authors report no conflicts of interest in this work.
References
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. | ||
Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. | ||
Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. | ||
Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. | ||
Shirasawa M, Kubotaa M, Harada S, et al. Successful oral desensitization against skin rash induced by alectinib in a patient with anaplastic lymphoma kinase-positive lung adenocarcinoma: a case report. Lung Cancer. 2016;99:66–68. | ||
Scherer K, Brockow K, Aberer W, et al. Desensitization in delayed drug hypersensitivity reactions – an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013;68(7):844–852. | ||
Kimura T, Sowa-Osako J, Nakai T, et al. Alectinib-induced erythema multiforme and successful rechallenge with alectinib in a patient with anaplastic lymphoma kinase-rearranged lung cancer. Case Rep Oncol. 2016;9(3):826–832. | ||
Solomon B. Refining the toxicity profile of crizotinib. J Thorac Oncol. 2014;9(11):1596–1597. | ||
Awad MM, Lax TP, Slawski BR, Shaw AT. Successful desensitization of two patients with ALK-positive lung cancer and hypersensitivity to crizotinib. J Thorac Oncol. 2014;9(11):1726–1728. | ||
Sánchez-López J, Viñolas N, Muñoz-Cano R, et al. Successful Oral Desensitization in a Patient With Hypersensitivity Reaction to Crizotinib. J Investig Allergol Clin Immunol. 2015;25(4):307–308. | ||
Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–2498. | ||
Yang JC, Ou SI, de Petris L, et al. Pooled systemic efficacy and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small cell lung cancer. J Thorac Oncol. 2017;12(10):1552–1560. | ||
Huang WS, Liu S, Zou D, et al. Discovery of brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59(10):4948–4964. | ||
Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22(22):5527–5538. | ||
Rashdan S, Gerber DE. A crowded, but still varied, space: brigatinib in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Transl Cancer Res. 2017;6(Suppl 1):S78–S82. | ||
Kim S, Thiessen PA, Bolton EE, et al. PubChem substance and compound databases. Nucleic Acids Res. 2016;44(D1):D1202–D1213. |
Supplementary material
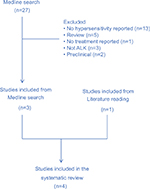  | Figure S1 Flowchart of literature search and study inclusion. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
