Back to Journals » Cancer Management and Research » Volume 12
Hyperin Controls the Development and Therapy of Gastric Cancer via Regulating Wnt/β-Catenin Signaling
Authors Ping MH
Received 3 July 2020
Accepted for publication 1 October 2020
Published 17 November 2020 Volume 2020:12 Pages 11773—11782
DOI https://doi.org/10.2147/CMAR.S270544
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Mao-Hua Ping
Department of Rehabilitation Medicine, Hanyang Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei Province 430050, People ’s Republic of China
Correspondence: Mao-Hua Ping
Department of Rehabilitation Medicine, Hanyang Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei Province 430050, People ’s Republic of China
Email [email protected]
Background: Hyperin is an effective monomer extracted from Malvaceae plant Abelmoschus, which is a flavonol glycoside compound. Hyperin performs a variety of pharmacological activities, such as analgesia, antioxidation, anti-inflammation, avoiding microthrombosis, regulating immune function, inhibiting tumor cell growth. But the role of Hyperin on gastric cancer is unrevealed. Considering the essential role of Hyperin, Hyperin function in gastric cancer is necessary to explore.
Aim: To identify the function of Hyperin in gastric cancer.
Methods: The role of Hyperin on gastric cell progression was detected in our research. Proliferation, migration, and invasion ability were assessed by the CCK-8, colony formation, cell-cycle assay, wound healing, Transwell migration and invasion assays. TUNEL assay and flow cytometry showed the results of the apoptosis level. Further, caspase-3, -9 activity and apoptosis-associated protein were assessed by the Caspase activity kit and Western blot. Wnt/β-catenin signal pathway activity was appraised by TOP/FOP luciferase activity. Immunohistochemical staining was performed to detect the role of Hyperin on tumor growth in vivo.
Results: Functional experiments showed that Hyperin inhibited proliferation, migration, and invasion and induced apoptosis in gastric cancer cells. Meanwhile, Hyperin prevented tumor growth by suppressing Wnt/β-catenin signal pathway.
Conclusion: The present study revealed that Hyperin suppressed gastric cancer progression by controlling Wnt/β-catenin signal pathway, which provided a novel therapy in gastric cancer.
Keywords: Hyperin, gastric cancer, Wnt, β-catenin
Introduction
Gastric cancer is the most common malignant tumor in the world. There are now more than 900,000 new cases worldwide each year, accounting for about 9% of the new cancer cases and the second leading cause of cancer-related death worldwide.1 In recent years, the incidence of gastric cancer has continued to decline in western countries, but it still remains at a high level in Asia.2 It is reported that about 42% of the new cases in the world are in China, and most of them are in the advanced stage at the time of definite diagnosis, accounting for more than 80%. The five-year survival rate of gastric cancer in China is only 40%.3 The annual death toll of gastric cancer in China accounts for about 25% of the total deaths of the disease in the world.4 There is an urgent to explore novel methods for gastric cancer therapy.
As a kind of comprehensive therapy, traditional Chinese medicine has certain advantages of prevention and treatment.5 The research on the material basis and mechanism of its curative effect has been paid attention to by the majority of medical workers.6 In recent years, clinical research have identified that the treatment of traditional Chinese medicine could ameliorate the quality of life and prolong the survival time of patients with gastric cancer.7 Previous research have also shown that traditional Chinese medicine could inhibit gastric cancer progression.8
Hyperin belongs to flavonoids, also known as quercetin-3-O-β-D-galactoside, which widely exists in many plants such as Tengxanthaceae, Leguminosae, Azaleaceae and Celastraceae.9 At present, few reports on the anti-tumor effect and immunomodulatory function of Hyperin at home and abroad. Hyperin has the effect of anti-tumor cells such as lung adenocarcinoma cells,10–12 colon cancer cells13 and prostate cancer cells.14 Meanwhile, it can promote lymphocyte proliferation, enhance lymphocyte phagocytosis, antibody production, cytokine release and other immunomodulatory effects.15 However, there is no exact research on the function and mechanism of Hyperin on gastric cancer.
Here, we demonstrated that Hyperin inhibited tumor growth in vivo and in vitro via regulating the Wnt/β-catenin signal pathway.
Materials and Methods
Reagent
Hyperin (00180585) was purchased from Sigma. CP21R7 (CP21, S7954) was purchased from Selleck, which could effectively activate the classical Wnt signal pathway.
Cell Culture
Gastric cancer cell lines were purchased from CHI Scientific, Inc (Jiangsu, China). The cells were cultured with complete medium including 89% 1640 (Beit-Haemek, Israel), 10% FBS (Beit-Haemek, Israel) and 1% penicillin/streptomycin (Beyotime, China), and maintained in incubator with 37°C and 5% of CO2 saturated humidity.
Clone Formation Experiment
The drug-treated cell suspension was inoculated into a petri dish with 300 cells per dish. After the cells were evenly distributed, they were cultured in a cell incubator for 14 ~ 21 days. When a clone is visible to the naked eye, the culture is terminated. The medium was abandoned and 4% paraformaldehyde was incubated to fix the cells for 15 min. After abandoning the fixed solution, Giemsa was added to dye for 15 min. Rinse with running water and dry. The number of effective cloned cells was observed under microscope.
Western Blot
After RIPA cleavage, we extracted total protein and measured with BCA method. After quantitative denaturation, protein electrophoresis membrane transfer and blocked. The primary antibody anti-Bcl2 (12,789-1-AP, Proteintech), anti-Bax (50,599-2-Ig, Proteintech), anti-cleaved-caspases3 (ab2302, abcam), anti-DKK1 (21,112-1-AP, Proteintech), anti-p-GSK3β (#3548, Cell Signaling Technology), anti-GSK3β (#12,456, Cell Signaling Technology), anti-β-catenin (51,067-2-AP, Proteintech), Wnt1 (27,935-1-AP, proteintech), and anti-GAPDH (60,004-1-Ig, Proteintech) incubation and second incubation were carried out according to the operation steps. The expression of the protein was expressed by the gray value.
CCK8 Assay
The cells in logarithmic growth phase were collected by centrifugation after trypsin digestion and resuscitated, and the cell count was adjusted to 2 × 104/mL. The prepared cell suspension was gently mixed while adding a 96-well plate with 100 μ L per well, and the inoculated cell culture plate was put into the incubator overnight, while the blank control group only added medium without cells, and 6 multiple holes were set up in each group to take the average value. The experiment was repeated 3 times. The cell viability was measured by CCK8 method after 24 hrs, 48 hrs and 72 hrs, and 10 μ L CCK8 solution was added to each well in different time group and control group, and the incubator was incubated for about 2 hrs. The absorbance (A) value of wells was detected at 450 nm of enzyme labeling instrument.
Migration and Invasion Assay
Invasion of cells was determined with a transwell chamber (Corning, Corning, NY, USA) with Matrigel (Invitrogen, Carlsbad, CA, USA), and migration was determined in the same way but without Matrigel (Invitrogen, Carlsbad, CA, USA). The cells in the logarithmic growth phase in each group were digested and counted after treatment. Cells were seeded onto the membrane of the upper chamber of the transwell at a concentration of 5 × 105 mL in 2 mL of 1640. The medium in the upper chamber was serum-free. The medium in the lower chamber contained 5% FBS as a source of chemoattractant. After 24 h, migrated cells were fixed in 100% methanol for 30 mins. Those non-migrated cells were then removed by cotton swabs. Finally, the cells on the bottom surface of the membrane were stained for 20 mins with the 0.1% crystal violet. Eventually, pictures were taken in eight random views under a microscope for records.
In vivo Tumor Growth Assay
BALB/c nude mice (6–8 weeks) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (Beijing, China). For the experiment of Xenograft, MKN-45 cells (5 × 106) were suspended in 200 μ l normal saline and injected subcutaneously. Animals were given hyperin (50 mg/kg) by intraperitoneal injection once 5 days. Tumor volume (mm3): V (Mm3) = S2 (Mm2) × L (Mm)/2. After another 30 d of injection, mice were intraperitoneally injected with 3% pentobarbital sodium and were euthanatized by excessive anesthesia with a dose of 90 mL/kg, and the tumors were removed for follow-up study. The research protocol of this study was approved by the Animal Care and Use Committee of Hanyang Hospital Affiliated to Wuhan University of Science and Technology. This study was carried out the Guide for the Care and Use of Experimental Animals.
Caspase‐3 and ‐9 Activity
Different group cells were harvested to assess caspase‐3 and caspase‐9 activity, 3 × 105 cells were placed in each tube and 1 μM of FITC‐DEVD‐FMK (cat no. 9499; BioVision, Milpitas, CA) was added to control and dose groups for determining caspase‐3 activity; then, cells were incubated for 1 h at 37°C incubator with 5% CO2 and centrifuged at 3000 rpm for 5 min. After that, the supernatant was immediately removed. The cells were resuspended in a 0.5 mL wash buffer and analyzed by flow cytometry. For the evaluation of caspase‐9 activity, the same manufacturer’s protocol was performed, but FITC‐LEHD‐FMK (cat no. 9534; Bio vision) was used as caspase‐9 in situ marker, instead of FITC‐DEVD‐FMK, which is a marker of caspase‐3.
Cell Cycle Assay
Cells were inoculated in a petri dish. When the cells adhered well and were in the logarithmic growth phase, the original culture medium was abandoned and different concentrations of Hyperin were added. The cells were first harvested after 72 hrs of transfection and the cell suspension was then digested, Afterwards, the cells were fixed with ethanol (75%) for 4 hrs at 4°C and the supernatant was then discarded, followed by incubation with an RNA enzyme containing iodide (PI, 40%, Sigma-Aldrich). After the cells were washed with PBS three times, the samples were detected by FC500 flow cytometry, and the proportion of each phase of cell cycle was analyzed and calculated by the software.
Apoptosis Analysis by Flow Cytometry
The percentage of apoptotic cells was quantified by flow cytometry (FACS-Calibur, Becton-Dickinson) using Annexin V-PE/7-AAD Kit (BD, USA). Cells were seeded into a 6-well plate for 24 hrs at 37°C and exposed to different drugs as described above. Collecting the adherent cells and washing twice with PBS, eventually, we resuspended the cells in binding buffer co-stained with 5 μL Annexin V-PE and 10 μL 7-AAD in the dark for 15 mins ahead of flow cytometric analysis.
Luciferase Reporter Assay
To analyze β-catenin transcriptional activity, TOP-flash luciferase (plasmid 16,558, Addgene) and FOP luciferase (plasmid 16,559, Addgene) were transfected into gastric cancer cells using Lipofectamine 2000. Luciferase activity was measured using Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol.
EdU Assay
The cells were placed in a laser confocal dish at 37°C and in a 5%CO2 incubator overnight. On the second day, the culture medium was replaced with the culture medium of the treatment group. Put it in the incubator for 48 hrs, change the culture medium to fresh medium, add EdU dye A solution, and continue to incubate for 6 hrs. After discarding the culture medium, 0.3%BSA washed the cells for 2 times and 5 min/times. Add 1 mL 3.7% formaldehyde and fix it at room temperature for 15 min. 0.3%BSA was cleaned twice and incubated at room temperature with 1 mL 0.5% Triton X-100 for 20 min, 0.3%BSA for 2 times. Add appropriate amount of 1 × Click-i T cocktail reaction solution. The horizontal shaker was incubated at room temperature and protected from light for 30 min. 0.3%BSA was cleaned twice, and Hoechst33342 shaker incubated at room temperature for 30 min,1 mL PBS for 2 times. Twenty visual fields of each group were randomly photographed under light-avoiding laser confocal microscope, and the total number of nuclei (blue) and the number of proliferating cells (red) were counted, respectively. Cell proliferation rate = the number of proliferating cells/total number of cells × 100%.
Immunohistochemical Staining
Paraffin sections of carcinoma were dewaxing to water in xylene and descending series of ethanol. We penetrated sections using 0.5% Triton X-100. After washing three times, we blocked sections with 50% goat serum. Then, sections were incubated with ki-67, caspase3, wnt1, β-catenin antibody overnight. We incubated the sections using a secondary antibody followed by DAPI staining. The sections were photographed by light scope under an IX73 fluorescence microscope (Olympus, Valley, PA) and analyzed by Image J software.
TUNEL Staining
Tissues were embedded and incubated with proteinase K (40 µg/mL) for 1 hr at 37°C, then treated with 2% H2O2 in distilled water for 30 min at 37°C. After the enzymatic reaction, sections were washed with PBS, incubated with anti-digoxigenin peroxidase conjugate for 30 min at 37°C in a humified chamber. Sections were stained with diaminobenzidine and counterstained with hematoxylin, and observed under a light microscope.
The cells on the coverslip treated with Hyperin were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton-X 100. Next, the cells were stained using the In Situ Cell Death Detection kit (Roche, Basel, Switzerland). DNA fragmentation was visualized by TUNEL assay according to the manufacturer’s instructions. Finally, fluorescence images were obtained using a confocal microscope.
Statistical Analysis
Data were shown as mean ± SEM. Student’s t-test or one-way ANOVA was used to compare the groups. P<0.05 was considered significance.
Results
Hyperin Inhibits Cancer Progression in AGS and MKN-45 Cells
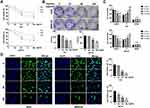
To detect the role of Hyperin in gastric cancer, we treated Hyperin (0, 25, 50, 100 µg/mL) in AGS and MKN-45 cells for 24hrs, 48hrs, 72hrs. CCK8 assay was carried out to assess the cell viability. The Hyperin-treated cells showed decreased cell viability in AGS and MKN-45 cells (Figure 1A). The clone formation assay evaluated the proliferation ability of cells following treatment with 0, 25, 50, 100 µg/mL concentration gradient of Hyperin in vitro. As Figure 1B shows, the Hyperin (50 and 100 µg/mL)-treated cells showed inhibition of clone formation in AGS and MKN-45 cells. Further, cell cycle analysis showed the cell distribution in each cell cycle. This indicated that Hyperin was able to block cell-cycle progression by inhibiting G1 to S phase transition of the cell cycle (Figure 1C). In addition, Edu assays confirmed that Hyperin treatment significantly suppressed the gastric cancer cell proliferation (Figure 1D).
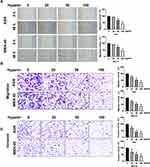
Subsequently, cells migration viability was detected. As Figure 2A and B shows, Hyperin prevented the migration of gastric cancer cells. Meanwhile, a Transwell invasion assay was conducted to explore cell invasion ability. The results revealed that Hyperin prevented invasion ability in gastric cancer cells (Figure 2C). Taken together, Hyperin could prevent tumor cells progression in AGS and MKN-45 cells (Figures 1 and 2).
Hyperin Induces Apoptosis in Gastric Cancer Cells
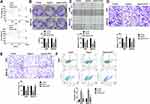
Further analysis of the data revealed that the apoptosis level of gastric cancer cells was increased after Hyperin treatment, which was verified by TUNEL assay (Figure 3A). TUNEL assay revealed that Hyperin induced apoptosis in gastric cancer cell. Then, flow cytometry also confirmed the pro-apoptosis effect of Hyperin in gastric cancer cells (Figure 3B). ELISA results indicated that Hyperin induced the up-regulation of caspase activity in AGS and MKN-45 cells (Figure 3C). The next section of the survey was concerned with apoptosis-associated protein. Western blot was executed to detect the protein level of Bcl2, Bax, cleaved-caspase3 in gastric cancer cells. Hyperin promoted the expression of cleaved-caspase 3 and Bax, while inhibited Bcl2 expression in gastric cancer cells (Figure 3D). (Figure 3)
Hyperin Prevents Wnt/β-Catenin Signal Pathway in Gastric Cancer Cells
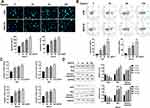
Here, we found that Hyperin could control the Wnt/β-catenin signal pathway. TOP/FOP luciferase assay was executed to explore the Wnt activity. As Figure 4A shows, Hyperin decreased the TOP/FOP luciferase activity in gastric cancer cells. GSK3β,16 DKK1,17 and NKD118 are suppressor genes of Wnt signal, which block the abnormal activation of classical Wnt/β-catenin signal pathway and regulate the polarity of epithelial cells, which indirectly inhibits tumorigenesis.19,20 Western blot results explored the protein level of GSK3β, DKK1, NKD1, Wnt1 and β-catenin in gastric cancer cells. The results performed that Hyperin induced the expression of DKK1 and NKD1, and phosphorylation of GSK3β, and prevented the level of Wnt1 and β-catenin in gastric cancer (Figure 4B). Taken together, Hyperin could regulate Wnt/β-catenin signal pathway (Figure 4).
Hyperin Prevents Tumor Progression by Inhibiting Wnt/β-Catenin Signal Pathway
Next, we would explore the relationship between Hyperin and Wnt/β-catenin signal pathway. We treated the cells with Hyperin and Wnt/β-catenin signal pathway activating agent CP21R7 (CP21). Then, we evaluated the cell viability by performing CCK8. We found that CP21 blocked the function of Hyperin on cell viability (Figure 5A). Meanwhile, the inhibition function of Hyperin on cell clone formation was hindered by CP21 treatment (Figure 5B). Wound healing assay and Transwell migration assay were performed to explore migration ability. CP21 inhibited the function of Hyperin on cell migration (Figure 5C). Further, Transwell invasion ability was inhibited by Hyperin, which was recovered by CP21 (Figure 5D and E). Next, we found that CP21 blocked the pro-apoptosis effect of Hyperin in AGS cells, which was provided by flow cytometry (Figure 5F). In summary, Hyperin prevented gastric cancer progression by inhibiting Wnt/β-catenin signal pathway (Figure 5).
Hyperin Impedes Xenograft Tumor Growth
A xenograft tumor model was constructed to explore the tumor growth after subcutaneous injection with MKN-45 cells. We observed that the tumor size was decreased in Hyperin group (Figure 6A). The tumor weight and volumes were calculated and reduced parameters were shown in Hyperin group (Figure 6B and C). H&E staining showed the morphological changes for the tumor cell growth between control and Hyperin groups, TUNEL assay showed increased apoptosis level in Hyperin (Figure 6D). IHC analysis indicated the decreased expression levels of Ki-67, wnt1 and β-catenin and increased expression of caspase3 in Hyperin group (Figure 6E). Western blot was executed to detect the protein level of Bcl2, Bax, cleaved-caspase3 in tumor tissues. Hyperin promoted the expression of cleaved-caspase 3 and Bax, while inhibited Bcl2 expression in gastric cancer cells (Figure 6F). Western blot results explored the protein level of GSK3β, DKK1, NKD1, Wnt1 and β-catenin in tumor tissues. The results performed that Hyperin induced the expression of DKK1 and NKD1, and phosphorylation of GSK3β, and prevented the level of Wnt1 and β-catenin in gastric cancer (Figure 6G). (Figure 6)
Discussion
In recent years, traditional Chinese medicine plays an important role in the treatment of malignant tumors. Natural plant drugs have been paid more and more attention because of their low toxicity and good curative effect.21,22 Here, we explore the anti-tumor activity of Hyperin. The experimental results showed that Hyperin could significantly inhibit the growth of tumor cells in a certain concentration range, and play an important role as a natural, low toxic and effective anticancer substance in the treatment and prevention of gastric cancer.
Previous research performed that Hyperin showed the anti-tumor function in different cancer. Qiu et al found that hyperoside acted as an anticancer drug through ROS-related apoptosis and its mechanism included activation of the Bax-caspase-3 axis and the inhibition of the NF-κB signaling pathway.23 Li et al showed that hyperoside and microRNA-let7a-5p might provide a synergistic effect on anti-cancer, which may provide a new idea for lung cancer treatment.10 Liu et al indicated that hyperoside completely impeded tumor growth through angiogenesis inhibition. Other study illustrated that hyperoside might provide a synergistic anticancer effects that warrant further study and investigation due to its potential role in clinical applications.24 Here, we found that Hyperin performed the inhibition effect on gastric cancer cells proliferation and growth, meanwhile, Hyperin promoted apoptosis of gastric cancer cells, which provided a new therapy on gastric cancer.
Wnt signaling pathway plays a fundamental role in cell growth and development.20,25 Wnt signaling pathway affects cell growth, embryonic development, regulation of tumor cell division, proliferation, tumor development, metastasis and prognosis.26,27 Wang et al demonstrated through experiments that Wnt/β-catenin signal pathway plays an important role in the renewal process of gastric cancer stem cells, and the interruption of Wnt signal pathway may lead to the interruption of the functional pathway in the pathogenesis of gastric cancer.28 Yu et al also found that DACT2 gene is involved in the regulation of the Wnt/β-catenin signal pathway in gastric cancer, and its down-regulation or deletion will lead to an abnormal Wnt signal pathway.29 It can be seen that some proteins in the Wnt signal pathway are closely related to the occurrence, development and metastasis of gastric cancer. In our research, we found that Hyperin inhibited the protein level of Wnt and β-catenin, and decreased the TOP/FOP luciferase activity. Taken together, Hyperin regulated gastric cancer growth by inactivating the Wnt/β-catenin signal pathway.
Traditional Chinese medicine is an excellent traditional cultural heritage with a long history in China, and our country is rich in traditional Chinese medicine resources. How to make effective use of it to benefit the majority of gastric cancer patients and even all cancer patients is an urgent problem for our medical workers. It is believed that with the rapid development and progress of science and technology and the in-depth study of traditional Chinese medicine, more and more traditional Chinese medicine will play a greater role in the treatment of gastric cancer and go further in the human struggle to overcome malignant tumors.
Conclusion
Hyperin prevented proliferation, migration and invasion, and induced apoptosis in gastric cancer by inhibiting the wnt/β-catenin signal pathway, which would a new therapy for gastric cancer.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (London, England). 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
2. Cai H, Ye F, Michel A, et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia. Int J Epidemiol. 2016;45(3):774–781. doi:10.1093/ije/dyw078
3. Wang SM, Zheng RS, Zhang SW, et al. [Epidemiological characteristics of gastric cancer in China, 2015]. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2019;40(12):1517–1521. Chinese.
4. Zhu Z, Wu P, Du N, et al. Surgical choice of proximal gastric cancer in China: a retrospective study of a 30-year experience from a single center in China. Expert Rev Gastroenterol Hepatol. 2019;13(11):1123–1128. doi:10.1080/17474124.2019.1689816
5. Oncology The Lancet. Rethinking traditional Chinese medicines for cancer. Lancet Oncol. 2015;16(15):1439. doi:10.1016/S1470-2045(15)00406-4
6. Xue T, Roy R. Studying traditional Chinese medicine. Science (New York, NY). 2003;300(5620):740–741. doi:10.1126/science.300.5620.740
7. Yang J, Zhu L, Wu Z, Wang Y. Chinese herbal medicines for induction of remission in advanced or late gastric cancer. Cochrane Database System Rev. 2013;(4):CD005096.
8. Zhou Y, Xu Q, Shang J, Lu L, Chen G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol. 2019;234(10):17876–17885. doi:10.1002/jcp.28418
9. Wang LM, Zhang MY, Zhu QS, Lu CF, Bai X. Hyperin enhances the sensitivity of HCT8/VCR colon cancer cell line to vincristine by down-regulating P-glycoprotein. Clin Lab. 2018;64(3):269–275. doi:10.7754/Clin.Lab.2017.170923
10. Li JP, Liao XH, Xiang Y, et al. Hyperoside and let-7a-5p synergistically inhibits lung cancer cell proliferation via inducing G1/S phase arrest. Gene. 2018;679:232–240. doi:10.1016/j.gene.2018.09.011
11. Lü P. Inhibitory effects of hyperoside on lung cancer by inducing apoptosis and suppressing inflammatory response via caspase-3 and NF-κB signaling pathway. Biomed Pharmacother. 2016;82:216–225. doi:10.1016/j.biopha.2016.05.006
12. Fu T, Wang L, Jin XN, Sui HJ, Liu Z, Jin Y. Hyperoside induces both autophagy and apoptosis in non-small cell lung cancer cells in vitro. Acta Pharmacol Sin. 2016;37(4):505–518. doi:10.1038/aps.2015.148
13. Hu Z, Zhao P, Xu H. Hyperoside exhibits anticancer activity in non‑small cell lung cancer cells with T790M mutations by upregulating FoxO1 via CCAT1. Oncol Rep. 2020;43(2):617–624.
14. Yang FQ, Liu M, Li W, Che JP, Wang GC, Zheng JH. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA‑21. Mol Med Rep. 2015;11(2):1085–1092. doi:10.3892/mmr.2014.2813
15. Geng M, Wang JH, Chen HY, Yang XB, Huang ZM. [Effects of hyperin on the cccDNA of duck hepatitis B virus and its immunological regulation]. Yao Xue Xue Bao = Acta Pharmaceutica Sinica. 2009;44(12):1440–1444. Chinese.
16. Yang Y, Li Z, Chen G, et al. GSK3β regulates ameloblast differentiation via Wnt and TGF-β pathways. J Cell Physiol. 2018;233(7):5322–5333. doi:10.1002/jcp.26344
17. Lyros O, Lamprecht AK, Nie L, et al. Dickkopf-1 (DKK1) promotes tumor growth via Akt-phosphorylation and independently of Wnt-axis in Barrett’s associated esophageal adenocarcinoma. Am J Cancer Res. 2019;9(2):330–346.
18. Twaroski K, Mallanna SK, Jing R, DiFurio F, Urick A, Duncan SA. FGF2 mediates hepatic progenitor cell formation during human pluripotent stem cell differentiation by inducing the WNT antagonist NKD1. Genes Dev. 2015;29(23):2463–2474. doi:10.1101/gad.268961.115
19. Onyido EK, Sweeney E, Nateri AS. Wnt-signalling pathways and microRNAs network in carcinogenesis: experimental and bioinformatics approaches. Mol Cancer. 2016;15(1):56.
20. Masuda M, Sawa M, Yamada T. Therapeutic targets in the Wnt signaling pathway: feasibility of targeting TNIK in colorectal cancer. Pharmacol Ther. 2015;156:1–9. doi:10.1016/j.pharmthera.2015.10.009
21. Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8:21. doi:10.1186/1476-4598-8-21
22. Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13(8):353–361. doi:10.1016/j.molmed.2007.07.001
23. Qiu J, Zhang T, Zhu X, et al. Hyperoside induces breast cancer cells apoptosis via ROS-mediated NF-κB signaling pathway. Int J Mol Sci. 2019;21(1):131. doi:10.3390/ijms21010131
24. Liu YH, Liu GH, Mei JJ, Wang J. The preventive effects of hyperoside on lung cancer in vitro by inducing apoptosis and inhibiting proliferation through Caspase-3 and P53 signaling pathway. Biomed Pharmacother. 2016;83:381–391. doi:10.1016/j.biopha.2016.06.035
25. Wang B, Tian T, Kalland KH, Ke X, Qu Y. Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39(7):648–658. doi:10.1016/j.tips.2018.03.008
26. Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106(1):djt356. doi:10.1093/jnci/djt356
27. Galluzzi L, Spranger S, Fuchs E, López-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2019;29(1):44–65. doi:10.1016/j.tcb.2018.08.005
28. Wang T, Wu H, Liu S, et al. SMYD3 controls a Wnt-responsive epigenetic switch for ASCL2 activation and cancer stem cell maintenance. Cancer Lett. 2018;430:11–24. doi:10.1016/j.canlet.2018.05.003
29. Yu Y, Yan W, Liu X, et al. DACT2 is frequently methylated in human gastric cancer and methylation of DACT2 activated Wnt signaling. Am J Cancer Res. 2014;4(6):710–724.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.