Back to Journals » Patient Preference and Adherence » Volume 8
How to study determinants related to medication adherence in newly diagnosed polyarthritis patients for the development of a prediction instrument
Authors Pasma A, Hazes JM, Luime J, Busschbach JJ, van 't Spijker A
Received 28 April 2014
Accepted for publication 4 June 2014
Published 20 October 2014 Volume 2014:8 Pages 1437—1447
DOI https://doi.org/10.2147/PPA.S66922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Annelieke Pasma,1,2 Johanna MW Hazes,1 Jolanda J Luime,1 Jan JV Busschbach,2 Adriaan van ’t Spijker2
1Department of Rheumatology, Erasmus MC, University Medical Center Rotterdam, 2Department of Psychiatry, Section of Medical Psychology and Psychotherapy, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands
Introduction: For patients with a chronic disease, the appropriate use of medication is the key to manage their illness. Adherence to medication is therefore important. Adherence can be divided into three parts: the initiation part, the execution phase, and the discontinuation part. Little is known about the determinants of the initiation part. For this reason, we describe the conduct of a stepwise procedure to study determinants of medication initiation for patients with a chronic disease.
Methods/design: The stepwise procedure comprises of eliciting a list of all potential determinants via literature review, interviewing patients, and consulting an expert panel. This is followed by embedding the determinants in a theoretical framework, developing a questionnaire, and choosing adherence measurement methods. The consecutive steps that we conducted for the development of a tool for the prediction of adherence in our study sample of early arthritis patients are described.
Discussion: Although we used a thorough procedure, there are still some pitfalls to take into account, such as the choice of theoretical framework. A strength of this study is that we use multiple adherence measurement methods and that we also take clinical outcomes into account.
Keywords: adherence, chronic disease, methodology, arthritis, medication
Introduction
For patients with a chronic disease who respond well to drug treatment, appropriate use of medication is the key to managing their illness. However, over 30% of prescribed medication is not taken as directed.1 A meta-analysis from 2004 assessed over 50 years of research on chronic medication adherence and calculated an overall nonadherence rate of 24.8%.2 For rheumatoid arthritis (RA), the nonadherence rates range between 1.5% and 50.5%.3
Nonadherence wastes resources, and is related to preventable morbidity and mortality.4 For example, in the rheumatology practice, patients with RA are treated with disease-modifying antirheumatic drugs (DMARDs) to induce disease remission and prevent disability. When not sufficiently adhering to their treatments, patients may present as nonresponders to the treatment, resulting in a switch to a more expensive treatment with biologicals. When rheumatologists get better insight into patients who are potentially nonadherent, unnecessary switches to other therapies might be prevented.5
To prevent nonadherence, early recognition of potential nonadherence behavior is necessary. This requires patient profiling to distinguish those at risk for nonadherence from adherent patients. A frequently cited definition of adherence is “the extent to which a person’s behavior – taking medication, following a diet, executing lifestyle changes – follows medical advice.”6 It is a continuous process, which can be divided into three parts: acceptance, execution, and discontinuation.7 In the acceptance phase, the patient learns to accept the need for the medication and learns to fit the medication schedule into daily life.8 This phase initiates the execution of medication intake. For RA patients, this is the part where they start to experience the effect and side effects of the DMARDs. It takes approximately 3 months before the full effectiveness of the DMARDs can be felt and tested. Unfortunately, most studies on adherence focus on the execution phase and skip this important phase that precedes the execution phase: the initiation of medication.
The focus of studies on the execution phase means that, although we do have some insight into the prevalence of nonadherence in the execution phase, even in that phase we still do not have a clear view of what causes nonadherence. Nonadherence is a complicated phenomenon, and decades of research show unequivocal relationships with both modifiable factors and unmodifiable factors.6,9 Frequently studied factors are medication characteristics, perceptions and cognitions about illness and medication, socioeconomic and demographic factors, disease features, and the doctor-patient relationship.3,6 Although these factors are widely studied, the evidence for the association with adherence points in different directions.3 For example, some studies report higher age as a risk factor for nonadherence in RA patients, whereas other studies report lower age as a risk factor for nonadherence in RA.3 This can be due to a number of factors, such as the use of different adherence measurement instruments. Another problem is that most studies on medication adherence in chronic patients do not include a consistent behavioral model to explain nonadherence. A behavioral model directs research, indicates which factors are potentially relevant, and helps to gain insight into the relationship between the determinants that guide behavior. When a behavioral model is missing, it could be that relevant factors are missed.
Moreover, research mostly focused on unmodifiable determinants of adherence, such as disease features or demographic characteristics. An example is the work of Curtis et al who showed which osteoporosis patients are at risk for nonadherence for bisphosphonates, but only identified unmodifiable risk factors.10 Although these unmodifiable determinants give us some clues for the target of interventions, we also need to study modifiable determinants, so that interventions can be developed.
We know from the scarce literature on the initiation of medication that a) expecting health problems from not treating the disease; b) the ability to obtain information during treatment; c) negative attitudes toward medication; and d) a relative lack of insight, are associated.11,12 We do not know whether the results of the studies that were conducted on factors influencing medication adherence in the execution phase are also applicable to the starting phase.
We aim to study factors that are possibly associated to the initiation of medication therapy, with the final goal to develop a prediction instrument for the early recognition of patients at risk for nonadherence. In this article we describe the study protocol as a stepwise procedure with background information to examine possible determinants of medication initiation. We use the population of newly diagnosed inflammatory arthritis patients starting on DMARD therapy as an example. The aim of this paper is to describe how to study possible determinants of adherence by using a stepwise procedure.
Methods/design
We describe which steps are needed to develop a preliminary set of determinants. Thereafter, we describe the study setup and how to develop the prediction instrument. The process contains the following steps: systematic literature search, patient perspective, expert panel, application of a theoretical framework, selection of questions, and selection of adherence measurement instrument.
Systematic literature search
The first step to gain insight into relevant and modifiable determinants of adherence is systematically reviewing the literature. A large amount of research tried to assess determinants of adherence. Reviewing all the literature on this topic is therefore not advisable. Instead, it is useful to review the literature on the topic that is of interest: a particular disease or medication, or a particular adherence phase, such as the initiation or discontinuation of medication. It is important to remember that determinants may differ for various diseases. Patients with arthritis might be driven by feelings of pain to take their medication, whereas for hypertension, patients may have no symptoms, and their adherence behavior might be driven by adverse cardiovascular outcome.
In this example, we wanted to gain insight into factors affecting adherence in recent onset (rheumatoid) arthritis patients. When searching the literature, we discovered that there was no information on recent onset arthritis, so we broadened our search terms into established disease, because then we at least got insight into factors influencing adherence. The literature was systematically searched from inception to February 2012 to identify studies on factors affecting medication adherence in patients with (rheumatoid) arthritis. Studies were eligible if they addressed medication adherence in adult (rheumatoid) arthritis patients, evaluated factors related to adherence, used a reproducible definition or validated instrument to measure adherence, and provided a statistical measure to reflect the strength of the association between the determinant and adherence. Eighteen observational studies remained, and were assessed on their methodological quality. All studies were on established RA patients and focused on the execution phase of adherence. Adherence rates ranged from 49.5% to 98.5%.3 A level of evidence synthesis was conducted to find the strength of the evidence for every factor. The factor that was associated with adherence to biologicals was having had a prescription for DMARDs 6 months prior to biological treatment. The factor that was associated with adherence to DMARDs is the belief that the medication for RA is necessary to treat the illness. There is also some limited evidence that the communication between the health care provider and the patient is influencing medication adherence.
Patient perspective
When searching for relevant determinants of health behavior, it is important to have an overview of possible determinants from all relevant viewpoints. The patient perspective is a very relevant viewpoint. To get to know this viewpoint, individual or group interviews with patients need to be conducted. When interviewing patients on possible determinants of adherence, it is of importance that the interviewees are representative of the group of patients the prediction instrument is targeted on. It is also advisable to keep on conducting interviews until saturation of the themes has been achieved. The main goal of the interviews has to be taken in mind when constructing an interview scheme. Furthermore, it is advisable to use a theoretical framework when analyzing the interview data.13,14
In the literature we reviewed, the viewpoint of the patient was not present. We also wanted to gain insight into factors affecting the initiation of therapy, because we did not gather information about these factors from the literature review. Therefore, we conducted a qualitative study to learn more about the initiation of medication from the patient perspective. This study was a combination of six focus groups and ten individual interviews, with a total of 33 patients. All patients gave informed consent for their participation and were aware that their data would be used for research purposes. The interviews were transcribed verbatim and imported into ATLAS.ti software. To ensure anonymity, all identifying information was removed from the transcripts. Responses that included reasons for adherence or nonadherence in the initiation part were extracted and coded by two coders separately. The same was done for the execution phase. Codes were classified into overarching themes. Seven factors that influenced medication intake behavior emerged: 1) severity of complaints, 2) experiences with medication, 3) perceptions about medication, 4) information about medication, 5) ability to adjust to the medication schedule, 6) need to make autonomous or shared decisions, and 7) communication with and trust in the rheumatologist.
Expert panel
The factors that were extracted from the literature review and the interviews were presented to an expert panel that consisted of one rheumatologist, one pharmacist, one psychologist, two specialized rheumatology nurses, and four researchers in the field of rheumatology. The expert panel ordered the factors according to theme and according to perceived importance. They also proposed other potentially relevant determinants of adherence behavior, which were integrated with the other factors in Table 1.
All potentially relevant determinants identified in the literature, during patient interviews, and by the expert panel were gathered, and ordered and clustered according to a theoretical framework in Table 1.
Theoretical framework
Adherence to medication requires behavior changes in the patient and could therefore benefit from the use of a theoretical framework to understand what facilitates and what inhibits medication intake.15
Numerous theoretical frameworks have been developed and tested, but how do we know which framework to use? Each model has its own advantages and disadvantages. A brief description of the social cognition models which are commonly used to predict health behavior can be found below.
The Health Belief Model (HBM)16 has the central assumption that behavior is determined by the perceived threat of the health problem and the evaluation of the health behavior. The benefits of the behavior have to be larger than the possible disadvantages. The protection motivation theory17 holds that behavior aimed at protecting one’s own health is called an “adaptive response”, where behavior that is regarded to be bad for one’s health is termed a “maladaptive response”. Two processes are distinguished: estimating the threat, and estimating the opportunities to cope with the threat. The person estimates the threats and, based on these estimations, the patient makes an adaptive or maladaptive response. The theory of reasoned action/theory of planned behavior18 depicts that the intention to follow a certain behavior is the best predictor of behavior. The intention is influenced by three determinants: attitude, subjective norms, and perceived behavioral control. The social cognitive theory19 describes how human behavior is directed through the expectations that one holds of a certain behavior. Behavior is seen as dynamic and the product of interactions and influences of environmental aspects, the person, and this person’s behavior.
There are also theories that focus on stepwise behavioral change; these are called “stage models”. The transtheoretical model of change20 describes five stages of behavioral change; precontemplation, contemplation, preparation, action, and maintenance. The precaution-adoption process21 is in some aspects comparable to the stages-of-change concept. In this process, the first step in the process from precontemplation to contemplation is to be aware of the risk behavior. The model distinguishes three stages of awareness.
What these models have in common is that they emphasize the rationality of human behavior. They assume that the health behaviors to be predicted, in our case adherence, are considered to be the end-product of a rational decision-making process based upon deliberate, systematic processing of the available information.
Which theoretical framework to apply should be based on the research question, the target health behavior, and the specific target group. The prerequisites for choosing a model are therefore to have adequate knowledge on the different theoretical frameworks and adequate knowledge on the specific empirical literature. The researcher can also decide to integrate several theoretical frameworks, because the goal is to get optimal insight into the determinants of behavior. One can add some concepts to an existing model or assemble two frameworks together. The drawback of this method is that connections or processes from specific theories can become disconnected or misinterpreted.
We choose to use the extended HBM (Figure 1) as a guide to explore and explain the numerous possible determinants of adherence, because it fits the possible determinants of adherence that we found the best (see Table 1, which is explained below). The HBM asserts that the decision to engage in preventive health behaviors, such as adherence, is influenced by four perceptions: the perceived severity of an illness, the perceived susceptibility of the individual to that illness, the perceived benefits associated with a health behavior to address the illness, and the perceived barriers to engage in the health behavior. The weighing of pros and cons of performing the health behavior was mentioned repeatedly in the focus group interviews as an important step toward taking the medication. This is also one of the key features of the HBM. The model focuses on severity, susceptibility, and perceived utility of the regimen (efficacy and the abundance of benefits over costs). These belief components have been found to affect intentions to adhere to various health-related behaviors.16 The model is thus in line with our findings from the literature review and the patient interviews.
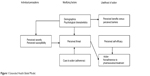  | Figure 1 Extended Health Belief Model. |
Another reason for choosing the HBM is that it has been used widely in the prediction of adherence behavior15 and has a larger level of evidence for the prediction of adherence than other theoretical frameworks.
Selection of questions
In deterministic research, one can distinguish between proximal, distal, and ultimate determinants. Proximal determinants are closely related to adherence and are usually modifiable; distal determinants influence adherence behavior indirectly; and ultimate determinants are even further away from the actual behavior but have an indirect influence on behavior through intermediate processes.22 Distal and ultimate determinants are mostly unmodifiable. In Table 1, factors that are thought to influence adherence behavior are clustered according to the HBM and ordered hierarchically from distal to proximal determinants. For example, the factor “race other than white”, which was found in the systematic literature review, is a demographic factor and distal. It is also unmodifiable.
It seems logical that only proximal factors will be included in our sample. However, we also chose to include some distal factors in our questionnaire, because we want to gain insight into the intermediate pathways in which distal determinants can influence adherence. Once we gain insight into all distal and proximal determinants, we can create tailored interventions for special target groups.
The underlined factors shown in Table 1 were the base of the constructs we used to develop our questionnaire. We searched in the scientific literature for validated questionnaires that measure the constructs from Table 1. The questionnaires that we extracted questions from were the short version Pain Anxiety Symptoms Scale,23 Arthritis Helplessness Index,24 the Revised Illness Perception Questionnaire,25 the Coping with Rheumatic Stressors Questionnaire,26 Pijn Coping Cognitie Lijst (Pain Coping and Cognition Scale),27 the Ways of Coping Questionnaire,28 the Multidimensional Health Locus of Control Scale,29 the Chronic Pain Acceptance Questionnaire,30 and the Nederlandse persoonlijkheidsvragenlijst (Dutch Personality Questionnaire).31 We studied the main articles on these questionnaires for the factor structure of the questionnaires and chose per factor the questions with the highest factor loading. We assumed that anxiety, depression, self-efficacy, and beliefs about medication might have a high impact on adherence behavior. Therefore, we used the complete scales to measure this construct. These scales are described in detail below.
Beliefs about Medicines Questionnaire
Patient beliefs about medicines were assessed using the Beliefs about Medicines Questionnaire (BMQ), which has been validated for use in patients with somatic chronic illnesses.32 The BMQ measures patient beliefs about the necessity of a prescribed medication to control their illness, and their concerns about the potential adverse consequences of taking the medication. Beliefs about necessity and concerns are both measured with five items rated on a five-point Likert scale. The total scores of the Necessity and Concerns scales range from 5 to 25, with higher scores indicating stronger beliefs. Among general medical patients, the subscales have reported Cronbach’s alpha of 0.86 for the Necessity scale to 0.51 for the Concerns scale.
Hospital Anxiety and Depression Scale
Anxiety and depression were measured with the Dutch version of the Hospital Anxiety and Depression Scale (HADS),33 screening anxiety and depression symptoms in a hospital setting on two subscales. The HADS consists of 14 items; seven items measuring anxiety and seven items measuring depression. Each item presents a statement, and patients are asked to respond to these items on a four-point Likert scale ranging from 1 (do not agree at all) to 4 (agree very much).
Medication Understanding and Use Self-efficacy Scale
The Medication Understanding and Use Self-efficacy (MUSE) questionnaire is a research tool that can be used in clinical and research settings to assess patients’ understanding and use of prescription medication. The MUSE questionnaire consists of two scales: “learning about medication,” with Cronbach’s alpha of 0.68, and “taking medication” with Cronbach’s alpha of 0.77.34 Taken together, the two factors account for 55% of the total variance of understanding medication instructions. MUSE scores are continuous and can range from 0 to 10, with low scores indicating patients’ low understanding of prescription medication use.
The final item pool consists of 217 items. Besides the constructs described before, the questionnaire also covers demographic questions such as sex, age, education, work, and social situation. The final item pool was tested by ten individual established RA patients. These patients were asked if they found some questions difficult to understand or difficult to answer. They were also asked what they thought of the length of the questionnaire. Following their responses, some questions were adapted.
Methods to measure adherence
Methods to measure adherence are described by de Klerk (2001).35 There are many methods, with their own advantages and disadvantages. Measurement instruments can be divided in direct measurement methods, which prove that the drug reached the site of action, and indirect measurement methods, where there is no proof of ingestion. An ideal measurement instrument should be: 1) valid, 2) reliable and sensitive to change, and 3) feasible: the patient should not be aware of compliance measurement, the method should not be invasive, and the researcher/physician should always have access to the data. However, an adherence measurement instrument that possesses all these features does not exist. Therefore, when choosing a measurement method, de Klerk suggests to pay attention to: 1) the objective of measuring adherence, 2) the desired level of precision of the instrument, 3) the need to prove ingestion of the medication, and 4) whether or not the patient has to be unaware of the adherence measurement.
The objective of measuring adherence in our study is to ensure that the drug is approximately ingested as prescribed. The desired level of precision of the instrument needs to be high. Although timing adherence is not relevant when using DMARDs, patients in this study will use different DMARDs with different regimens. Since we also need to look for variations in adherence between different DMARDs, we need a precise measurement instrument. Ideally, we want the patients to be unaware of the adherence measurement, but since we are ethically obliged to inform patients about the scope of the study, this is not entirely possible. From daily practice, it is however known that patients easily forget that they are being monitored and that electronic monitoring does not interfere with adherence behavior.36
We therefore chose as a primary measurement instrument the electronic monitoring with medication event monitoring systems (MEMS). It is noninvasive and gives stable results. It is also one of the best indirect methods.3 The MEMS uses a microprocessor in the medication container cap to record the day and time of each vial opening. Electronic monitoring offers the advantage of assessing adherence over a continuum. This method has proven to be superior to patient self-reports and pill counts in the measurement of adherence in studies of adults requiring chronic medication.37,38 The only drawbacks of using MEMS is that it is relatively expensive and that it does not prove ingestion of the medication. The primary outcome measure of our study is the adherence rate to oral DMARDs in the first 3 months of disease measured with MEMS. The MEMS adherence rate will be calculated by dividing medication events or bottle openings by doses prescribed for the interval. The adherence rate varies between 0 (complete nonadherence) and 100 (complete adherence).
Because it is recommended to use multiple measurement methods of adherence to increase reliability,3 we also chose to use questionnaires for the measurement of adherence. Patient questionnaires do not give detailed overviews on the time of ingestion, but they are a cheap way to measure adherence. When a patient needs to start using DMARDs subcutaneously, we cannot use MEMS anymore, but we can still use the questionnaires for adherence measurement.
The Compliance Questionnaire Rheumatology (CQR) is a self-report measure consisting of 19 statements related to compliance. Patients are asked to respond to these items on a four-point Likert scale ranging from 1 (do not agree at all) to 4 (agree very much). The total score is a continuous variable ranging from 0 (complete noncompliance) to 100 (perfect compliance). The CQR has been validated in patients with inflammatory rheumatic diseases, against an MEMS. The 19-item CQR compared well with electronic monitoring over 6 months with a sensitivity of 98%, specificity of 67%, and an estimated kappa of 0.78, to detect nonadherence.39 As the CQR does not measure adherence directly, but relies partly on behavioral items, the use of the CQR could teach us more about the correlation between specific cognitions and adherence behavior.
We also chose a direct physical method to measure adherence: concentration measurement. The advantage of this method is that it proves ingestion of the medication. The drawback of concentration measurements is that at the time of measurement, you only measure drug concentrations from medication which has been ingested the previous day. So if a patient has taken their medicine the day before, the method will report a perfectly adherent patient. This method is mostly used intermittently, but if you want to retrieve full adherence data, ideally you will have to do concentration measurements every day, which is, of course, invasive and therefore difficult to employ.
For RA patients, it would be interesting to measure levels of DMARDs. RA is commonly treated with one or more DMARD, of which methotrexate (MTX) is the first drug of choice. MTX needs to be ingested weekly, and it takes 6 to 8 weeks to have an effect on the arthritis symptoms.40 We expect that this drug will be prescribed to approximately 95% of our study sample. Measurement of MTX serum concentration is not possible, since this is cleared rapidly.41 However, MTX accumulates intracellularly into polyglutamates (PGs) when ingested. This process takes approximately 6 months. Longer retained intracellular red blood cell MTX concentrations might be a good indicator for adherence. Incorporating these PG measurements in normal clinical practice is relatively easy, as it is normal practice to draw blood from rheumatology patients on a regular basis, and that blood can also be used for the MTX PG measurement. Blood samples will be drawn, and intracellular MTX will be measured and compared to MEMS adherence data. Patients will be classified as nonadherent, partially adherent, and adherent. Nonadherence will be defined as MTX PG levels below the analytical detection limit.
Clinical outcome
In adherence studies, it is recommended to take clinical outcomes into account. While clinical outcomes cannot stand alone as an adherence measure, they can tell us something about the relationship between the adherence percentage and clinical outcome. Especially when you want to dichotomize an adherence percentage into “adherent” and “nonadherent”, measures of clinical outcome can be helpful to find a clinically relevant cut-off point. We use the Disease Activity Score 28 (DAS28)42 and the Health Assessment Questionnaire (HAQ)43 to measure clinical outcome of disease. These measures are described below.
DAS28
To evaluate disease activity, the DAS28 is calculated every 3 months. The DAS28 is a composite score of erythrocyte sedimentation rate and the number of tender and swollen joints as per the 28-joint count as well as a patient global assessment.
HAQ
Physical functioning was measured using the validated Dutch version of the consensus HAQ.43 This self-administered questionnaire is a validated measure of disability which includes 20 specific functions that are grouped into categories: dressing and grooming, arising, eating, walking, personal hygiene, reaching, gripping, and other activities. The average of these scores represents a physical functioning score. HAQ scores range from 0 (no difficulty) to 3 (unable to do). The HAQ has been found to have good criterion validity (correlations between questionnaire or interview scores and task performance 0.71–0.95) as well as test-retest reliability (correlations 0.87–0.99).
Study design
Ethics statement
This study was approved by the Medical Ethics Committee. Written informed consent will be obtained before the start of the study. When giving informed consent, patients state that they are aware that their data will be used for research purposes and that the data will be anonymized.
Determinants of adherence in the first 3 months of DMARD use are studied in a cohort study with 1 year follow-up, which will take place in the rheumatology outpatient clinic of the hospital where the patient is treated. The study will be performed in eleven different rheumatology outpatient clinics of eleven participating hospitals. Adult patients who are diagnosed with RA (according to the new or old American College of Rheumatology criteria for RA), psoriatic arthritis, or undifferentiated arthritis and are prescribed oral DMARDs will be invited by their rheumatologist to participate in the study. Data will be collected at five time points by one of the research nurses or specialized rheumatology nurses in the outpatient clinic where the patient is treated. T0 is the baseline measurement, performed within 2 weeks after the prescription for DMARDs. T1 will be performed 3 months after T0, T2 at 6 months, T3 at 9 months, and T4 at 12 months after start of medication. This study has been approved by the Medical Ethics Committee and all medical boards of the participating hospitals gave their consent for participation in the study.
Participants are asked to fill in the item pool at baseline (T0) and at the 6 month follow-up (T2). Every 3 months, physical functioning is measured, and patients are asked to fill in an adherence self-report measure. In Table 2, a schematic overview of measures is presented.
Study population
Inclusion criteria for participation in the study are: being newly diagnosed with RA, psoriatic arthritis, or undifferentiated arthritis; being prescribed a DMARD or prednisone for the first time; aged above 18 years; and being able to take their medication without the assistance of others. Reasons for exclusion are illiteracy and the inability to use the MEMS. Patients with cognitive impairments, visual impairments, and serious addictions were excluded from participation in the study, because they are mostly not able to take their medication without the assistance of others. The inclusion of patients started January 2012 and ended in December 2013. The 1-year follow-up of all patients will be finished by January 2015.
Construction of prediction rule
We strive to include 300 early polyarthritis patients, so that we have enough cases to include approximately nine predictive factors for adherence in the prediction rule.39,44
The amount of items will be reduced stepwise. First, items are reduced based on the frequency distribution of answer categories. Second, an exploratory factor analysis will be performed. The minimum amount of questions allowed per factor is five. Third, per factor, items will be reduced with item response theory. Only those questions will be included that distinguish the most within the latent factor.
Having reduced the items, a multivariate logistic regression with backward selection will be performed. After having fitted the significant factors in the model, we prevent the model from overfitting using a shrinkage method.45,46
Discussion
This article describes how to develop a tool for the prediction of adherence for patients with a chronic disease. It describes the various steps to be taken for the development of our tool for the prediction of adherence to DMARDs for early arthritis patients.
The stepwise procedure that we describe is thorough, but there are some pitfalls to take into account. First, the choice for a theoretical framework can be arbitrary. Although we do feel that the HBM fits our determinants the best, a different framework could have been applied as well. We focused on factors such as social norm and communication with the health care provider, and thus chose the HBM. If we had chosen a different framework, for example the Transtheoretical Stages of Change model, the focus of our study would have been on intrinsic factors. Using a theoretical framework for the prediction of adherence has many advantages, but it has one major drawback. A social cognition model can only be used to predict conscious and motivated behavior. Unconscious nonadherence, for example through simply forgetting to take the medication, cannot be predicted with a social cognition model.
Second, the selection of questions from validated questionnaires depended on what was available in the literature. This set of questions might therefore not be complete. We also had to make some choices concerning the questionnaires. There are for example many questionnaires available on coping, all based on slightly different theories. We chose to use questions from a general coping questionnaire as well as items from specific coping with chronic pain, and coping with arthritis questionnaires. We chose these questionnaires because these were most often used in research.
The outcome measure of our study is adherence. Previous literature has shown that adherence outcomes strongly depend on the measurement instrument chosen. We gave an overview of the different methods to measure adherence and pointed out the issues around choosing the right method. Because there is no “gold standard” to measure adherence, we will use in our example three different methods, both direct and indirect, to assess adherence behavior. Within the measurement of MTX PGs we can differentiate between nonresponse to MTX treatment and nonadherence or partial adherence to MTX treatment. We can correlate these measurements with the indirect adherence measurements with the MEMS, and by that means validate the MTX polyglutamate measure. When validated, this is the first direct measure for adherence to MTX treatment. It is also a relatively cheap method which is easy to implement in daily practice.
The item pool that we constructed is ready to be used in the study. In this phase, we can encounter some pitfalls. First, the patient may find the item pool too long and may find that items are similar. This may cause patients to become unmotivated to be followed up in the study. It is therefore important to try to reduce the number of questions in the item pool and to explain to patients that they are not filling out an end-stage questionnaire, but an item pool that has to become a questionnaire. We also expect that our cohort is vulnerable to selection bias. Those patients who do not adhere to their therapy are less likely to participate in the study, or are likely to become lost to follow-up. When setting up a cohort study for the measurement of medication adherence, researchers should be aware of these pitfalls and also try to gather information about patients who are not willing to participate or who are lost to follow-up.
In this article we described the steps to be taken for the development of a questionnaire for the prediction of adherence in patients with a chronic disease. We discussed for every step the different options and pitfalls. We concluded each section with the choices that we made regarding these steps.
Disclosure
The authors declare no conflicts of interest in this work.
References
Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In: Haynes NR, Taylor DW, Sackett DL, eds. Compliance in health care. Baltimore: John Hopkins University Press; 1979:11–22. | ||
DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. | ||
Pasma A, van’t Spijker A, Hazes JM, Busschbach JJ, Luime JJ. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum. 2013; 43(1):18–28. | ||
Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. | ||
Waimann CA, Marengo MF, de Achaval S, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum. 2013;65(6):1421–1429. | ||
World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland, 2003. | ||
Urquhart J, Vrijens B. New findings about patient adherence to prescribed drug dosing regimens: an introduction to pharmionics. Eur J Hosp Pharm Sci. 2005;11(5):103–106. | ||
van Geffen EC, Gardansdottir H, van Hulten R, van Dijk L, Egberts AC, Heerdink ER. Initiation of antidepressant therapy: do patients follow the GP’s prescription? Br J Gen Pract. 2009;59(559):81–87. | ||
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment. Arch Intern Med. 2000; 160(14):2101–2107. | ||
Curtis JR, Xi J, Westfall AO, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47(3):334–341. | ||
Fusfeld L, Aggarwal J, Dougher C, et al. Assessment of motivating factors associated with the initiation and completion of treatment for chronic hepatitis C virus (HCV) infection. BMC Infect Dis. 2013; 13:234. | ||
Mutsatsa SH, Joyce EM, Hutton SB, et al. Clinical correlates of early medication adherence: West London first episode schizophrenia study. Acta Psychiatr Scand. 2003;108(6):439–446. | ||
Arcury TA, Quandt SA. Qualitative methods in arthritis research: sampling and data analysis. Arthritis Care Res. 1998;11(1):66–74. | ||
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. | ||
Leventhal H, Cameron L. Behavioral theories and the problem of compliance. Patient Educ Couns. 1987;10(2):117–138. | ||
Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2:328–335. | ||
Rogers RW. A protection motivation theory of fear appeals and attitude change. J Psychol. 1975;91(1):93–114. | ||
Ajzen I. Attitudes, personality and behavior. Open University Press; 2005. | ||
Bandura A. Social foundations of thought and action. A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. | ||
Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9): 1102–1114. | ||
Weinstein ND, Sandman PM. The Precaution Adoption Process Model. In: Glanz K, Rimer BK, Lewis FM, eds. Health Behavior and health education: theory, research and practice. San Francisco: Jossey-Bass; 2002:121–143. | ||
Flay BR, Petraitis J. The theory of triadic influence: A new theory of health behaviour with implications for preventive interventions. In: Albrecht GS, ed. Advances in medical sociology: Reconsideration of models of health behavior change. Greenwich, CT: JAI Press; 1994:19–44. | ||
McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002;7(1):45–50. | ||
Nicassio PM, Wallston KA, Callahan LF, Herbert M, Pincus T. The measurement of helplessness in rheumatoid arthritis. The development of the arthritis helplessness index. J Rheumatol. 1985;12(3):462–467. | ||
Moss-Morris R, Weinman J, Petrie K, Cameron L, Buick D. The revised illness perception questionnaire. Psychol Health. 2002;17(1):1–16. | ||
van Lankveld W, Näring G, van der Staak C, van ‘t Pad Bosch P, van de Putte L. De ontwikkeling van de CORS: Coping met reuma stressoren [Development of the CORS: Coping with rheumatic stressors]. Gedrag Gezondheid. 1993;21:40–48. Dutch. | ||
Stomp-van der Berg SG, Vlaeyen JW, ter Kuile MM, Spinhoven P, van Breukelen G, Kole-Snijders AM. Pijn Coping en Cognitie Lijst (PCCL) [Pain Coping and Cognition List]. Maastricht: Pijnkenniscentrum, 2001. Dutch. | ||
Folkman S, Lazarus RS. An analysis of coping in a middle-aged community sample. J Health Soc Behav. 1980;21(3):219–239. | ||
Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ Monogr. 1978;6(2):160–170. | ||
Geiser DS. A comparison of acceptance-focused and control-focused psychological treatments in a chronic pain treatment center. Reno, NV: University of Nevada-Reno, 1992. | ||
Barelds DP, Luteijn F, van Dijk H, Starren J. Nederlandse Persoonlijkheidsvragenlijst 2 [Dutch Personality Questionnaire 2]. 2013. Amsterdam: Stichting Werkgroep Nederlandse Persoonlijkheidsvragenlijst. Boom test uitgevers. Dutch. | ||
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: The development and evaluation of a new method for assissing the cognitive representation of medication. Psychology and Health. 1999;14(1):1–24. | ||
Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychological Medicine. 1997;27:363–370. | ||
Cameron KA, Ross EL, Clayman ML, et al. Measuring patients’ self-efficacy in understanding and using prescription medication. Patient Educ Couns. 2010;80(3):372–376. | ||
de Klerk E. Measurement of Patient Compliance on Drug Therapy: an Overview. In: Vingerhoets A, ed. Advances in Behavioral Medicine Assessment. Harwood: Academic Publishers; 2001:215–244. | ||
Wagner GJ, Ghosh-Dastidar B. Electronic monitoring: adherence assessment or intervention? HIV Clin Trials. 2002;3(1):45–51. | ||
Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11(6):1189–1197. | ||
Lee JY, Kusek JW, Greene PG, et al. Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Am J Hypertens. 1996;9(8):719–725. | ||
de Klerk E, van der Heijde D, Landewé R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol. 2003;30(1):44–54. | ||
de Jong PH, Quax RA, Huisman M, et al. Response to glucocorticoids at 2 weeks predicts the effectiveness of DMARD induction therapy at 3 months: post hoc analyses from the tREACH study. Ann Rheum Dis. 2013;72(10):1659–1663. | ||
Hornung N, Ellingsen T, Attermann J, Stengaard-Pedersen K, Poulsen JH. Patients with rheumatoid arthritis treated with methotrexate (MTX): concentrations of steady-state erythrocyte MTX correlate to plasma concentrations and clinical efficacy. J Rheumatol. 2008;35(9): 1709–1715. | ||
van der Heijde DM, van’t Hof MA, van Riel PL, van Leeuwen MA, van Rijswijk MH, van de Putte LB. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis. 1992;51(2):177–181. | ||
Boers M, Jacobs JW, van Vliet Vlieland TP, van Riel PL. Consensus Dutch health assessment questionnaire. Ann Rheum Dis. 2007;66(1): 132–133. | ||
van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol. 2009;36(10):2164–2170. | ||
Van Houwelingen JC, Le Cessie S. Predictive value of statistical models. Stat Med. 1990;9(11):1303–1325. | ||
Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–781. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


