Back to Journals » Cancer Management and Research » Volume 11
High Inflammatory Factor Grading Predicts Poor Disease-Free Survival in AJCC Stage I-II Hepatocellular Carcinoma Patients After R0 Resection
Authors Zhang M , Chua MS , Hu J, Li H, Zhang S, Wu L, Han B
Received 9 September 2019
Accepted for publication 26 November 2019
Published 19 December 2019 Volume 2019:11 Pages 10623—10632
DOI https://doi.org/10.2147/CMAR.S230386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Mao Zhang,1 Mei-Sze Chua,2 Jie Hu,3 Haoran Li,1 Shun Zhang,1 Liqun Wu,1 Bing Han1
1Department of Hepatobiliary and Pancreatic Surgery, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China; 2Asian Liver Center, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA; 3Department of General Surgery, The Third Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: Bing Han; Liqun Wu
Department of Hepatobiliary and Pancreatic Surgery, The Affiliated Hospital of Qingdao University, No.16 Jiangsu Road, Qingdao, Shandong 266003, People’s Republic of China
Tel +86-186 6180 8308
Fax +86-532-8291 1323
Email [email protected]; [email protected]
Purpose: In this study, we established the inflammatory factor grade system (IFGs) based on the hepatocellular carcinoma (HCC) microenvironment to investigate the role of inflammatory factor grade (IFG) in predicting the prognosis of patients with American Joint Committee on Cancer (AJCC) stage I-II.
Patients and methods: We enrolled 87 HCC patients with AJCC stage I-II who underwent R0 resection between 2000 and 2012 and had paraffin-embedded specimens. Immunohistochemistry (IHC) was performed to investigate the expression of 12 inflammatory factors and then to establish the IFGs (grade A or B) based on the IHC data. Subsequently, Kaplan-Meier and Cox univariate/multivariate survival analyses were performed to examine the potential prognostic significance.
Results: Higher IFG (IFG-B) is significantly associated with greater tumor size (P=0.037), and IFG-B predicts a worse disease-free survival (DFS, P<0.001). Moreover, a platelet count (PLT) ≤100×109/L, tumor size ≥5 cm, poor tumor differentiation, and IFG-B are independent risk factors for DFS.
Conclusion: Overall, by establishing a grading system for the level of inflammatory factors in the HCC microenvironment, IFG-B can effectively predict poor DFS in AJCC stage I-II HCC patients after R0 resection.
Keywords: hepatocellular carcinoma, recurrence, prognosis, inflammation
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer mortality worldwide and the third leading cause of cancer mortality in China.1,2 The treatment for early stage HCC is mainly liver resection, ablation, or liver transplantation. Although the 5-year survival rate can reach up to 70%, the recurrence rate is over 80%.3 Therefore, early prediction and early intervention in HCC recurrence are important to improve the overall survival of patients.
In recent years, an increasing number of studies have found that the tumor microenvironment is one of the key factors underlying the progression of HCC; however, the mechanism of regulation remains unclear.4–6 In particular, inflammatory cytokines and related signaling pathways in the tumor microenvironment have been shown to play important roles in the occurrence and development of HCC,7 as well as in the assessment of the risk of recurrence after hepatectomy. Moreover, heterogeneity and invasiveness are important causes of HCC recurrence. Inflammatory factors in the HCC tumor microenvironment are potentially derived from chronic liver disease and liver damage, which can cause not only HCC but also high heterogeneity.8 Furthermore, inflammatory stimuli activate hepatic stellate cells and secrete extracellular matrix proteins of different compositions and distributions; HCC cells can bind to these proteins and cross tissue boundaries, making the cancer more aggressive.9
Based on the 2017 version of the American Joint Committee on Cancer (AJCC) staging,10 we enrolled 87 HCC patients with stage I-II who underwent R0 resection and had paraffin-embedded specimens. We first used immunohistochemistry (IHC) to detect the expression levels of common inflammatory factors in these 87 paraffin specimens. Then, we assigned an inflammatory factor grade (IFG) based on the IHC data and examined its ability to predict prognosis in HCC patients, especially recurrence.
Materials and Methods
Clinicopathological Information
Between 2000 and 2012, 87 HCC patients with AJCC stage I or II underwent R0 resection in the Affiliated Hospital of Qingdao University, comprising 71 males and 16 females with an age range of 31–83 years and a median age of 56.0 years. The basic characteristics of the patients are summarized in Table 1.
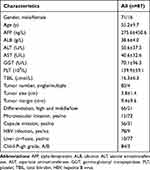 |
Table 1 The Basic Characteristics for the Study Participants |
All patients signed a written informed consent form before surgery. The inclusion criteria were as follows: (1) the patient underwent R0 resection; (2) the patient was diagnosed with HCC according to the European Society of Liver Research radiological standards and postoperative pathology; and (3) the HCC was AJCC stage I-II.10 The exclusion criteria were as follows: (1) the patient received anti-cancer treatment before surgery; (2) the patient experienced serious complications or death within 30 days after surgery; (3) the patient died of a cause unrelated to cancer; and (4) the patient lacked clinicopathological data. Pathological differentiation grades were classified according to the World Health Organization (WHO) tumor histological classification criteria. Liver function grading was performed using the Child-Pugh criteria and the most recent clinical data prior to surgery.
Immunohistochemistry Detection and Scoring Procedure
Briefly, the 87 HCC paraffin-embedded specimens were cut into 4-μm thick sections, dewaxed and rehydrated. After antigen extraction with citrate buffer (10 mM, pH 6), endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. Subsequently, the sections were stained overnight at 4 °C with primary antibodies (anti-human IL-6 (catalog no. Ab9324; Abcam, Cambridge, MA, USA), IL-6R (catalog no. Ab128008; Abcam), JAK2 (catalog no. Ab39636; Abcam), STAT3 (catalog no. Ab119352; Abcam), SOCS3 (catalog no. Ab53984; Abcam), IL-1α (catalog no. Ab9614; Abcam), IL-1β (catalog no. Ab9722; Abcam), IL-8 (catalog no. Ab106350; Abcam), TNF-α (catalog no. Ab6671; Abcam), NFκB (catalog no. Ab209795; Abcam), IKKβ (catalog no. 07-1479; Sigma-Aldrich, St. Louis, MO, USA), or P38MAPK (catalog no. Ab197348; Abcam). The MaxVision Kit (Fuzhou Maixin Biotechnology Development Co., Ltd., Fujian, China) was used to detect the primary antibodies, and the color was developed using 3,3ʹ‑diaminobenzidine chromogen substrate for 10 min. Then, the sections were counterstained with hematoxylin for 1 min. Finally, the tissue sections were dehydrated in an ethanol gradient, cleared, and scored.
Two pathologists at the Affiliated Hospital of Qingdao University (Qingdao, China) scored the extent of immunostaining based on the staining intensity and percentage of immune response cells. The staining intensity was graded as follows: no stain, pale yellow, brownish yellow, and tan were graded as 0, 1, 2, and 3, respectively. The degree of immune response was graded as follows: the percentage of positive cells <5%, 5%~25%, 26%~50%, 51%~74%, >75% were assigned 0, 1, 2, 3, and 4 points, respectively, and the two scores were added together. The specimens in the low-expression group had scores of ≤4 points, and those in the high-expression group had scores of >4 points.
Patient Follow-Up
Follow-up of all patients meeting the study criteria occurred in the form of outpatient visits, telephone calls, or letters. Disease-free survival (DFS) was defined as the date of surgery to the time of recurrence or the end of follow-up. Overall survival (OS) time was defined as the date of surgery to death or the end of follow-up. DFS and OS were calculated on a monthly basis, and the follow-up deadline was March 2017.
Statistical Analysis
Statistical analysis was performed using SPSS 24.0 and R software, and Student’s t-test was used to compare the mean values. The classification data were analyzed using Fisher’s exact test or the χ2 test. The survival analysis was performed using the Kaplan-Meier method, and the relationships between the expression of inflammatory factors and DFS or OS were compared by the log rank test. Univariate and multivariate analyses were used to determine the significance of prognostic factors. The data are presented as the mean ± S.D., and P<0.05 (two-tailed) was considered statistically significant.
Results
Establishment of Inflammatory Factor Score and Grade
The patients were divided into high and low expression groups according to the immunohistochemical results (Figure 1A–E). The Kaplan-Meier survival analysis showed that five inflammatory factors, namely, interleukin-6 (IL-6), interleukin-6 receptor (IL-6R), interleukin-1 beta (IL-1β), inhibitor kappa B kinase beta (IKKβ), and p38 mitogen-activated protein kinase (P38MAPK), were associated with the postoperative HCC recurrence (P<0.05, Table 2). The patients with high levels of expression of IL-6 and IL-6R had poor DFS (Figure 2A and B, P<0.05); therefore, the patients with low levels of expression of these two factors were assigned 1 point, and those with high levels of expression were assigned 2 points. Patients with high levels of IL-1β, IKKβ and P38MAPK expression had good DFS (Figure 2C–E, P<0.05) and were assigned 1 point, and patients with low expression levels were assigned 2 points. The scores of the five inflammatory factors for each HCC patient were added together, and the resulting total scores ranged from 5 to 10. Patients with scores of 5 to 7 were assigned IFG-A, and patients with scores of 8 to 10 were assigned IFG-B (Table 3).
 |
Table 2 The Median DFS Rates of the Low and High Expression Groups of Different Inflammatory Factors |
 |
Table 3 The Assignment of IHC Scores and Establishment of IFG |
Clinicopathological Factors Associated with IFG
Among all clinicopathological factors studied, we found that IFG was only positively correlated with tumor size (P=0.037, Table 4); i.e., a higher IFG indicated a larger tumor size.
 |
Table 4 Basic Characteristics of the Study Participants in the IFG-A and IFG-B Groups |
Relationship Between IFG and HCC Prognosis
The mean follow-up time was 44.7 ± 24.1 months (4.0–114.5). The DFS rates of patients at 1-, 3-, and 5- years were 50.6%, 33.3%, and 18.4%, respectively, and the median DFS was 12.9 months (95% CI: 1.7–24.1). The median DFS for the IFG-A and IFG-B groups were 26.8 months (95% CI: 10.7–42.9) and 7.5 months (95% CI: 4.7–10.4), respectively, and the corresponding 1-, 3-, and 5-year DFS rates were 62.7%, 45.78%, 25.4% and 25.0%, 7.1%, 3.6%, respectively. The DFS of the IFG-B group was significantly shorter than that of the IFG-A group, and the difference between the two groups was statistically significant (P<0.001, Figure 3A).
The 1-, 3-, and 5-year OS rates were 90.8%, 64.4%, and 29.9%, respectively, and the median OS was 67.3 months (95% CI: 34.4–100.2). The median OS rates of the IFG-A and IFG-B groups were 67.3 months (95% CI: 32.4–102.2) and 40.6 months (95% CI: 24.4–56.8), respectively, and the corresponding 1-, 3-, and 5-year OS rates were 89.8%, 64.4%, 33.9% and 92.9%, 64.3%, 21.4%, respectively. There were no significant differences between the IFG-A and IFG-B groups in terms of OS (P=0.2, Figure 3B).
Univariate and Multivariate Analyses of DFS
Univariate analysis showed that the aspartate aminotransferase (AST) level, platelet (PLT) count, tumor number, tumor size, tumor differentiation, and IFG were associated with DFS, and the differences were statistically significant (P<0.05, Table 5). Multivariate Cox regression analysis was performed with the statistically significant factors identified by univariate analysis. The results showed that PLT ≤100×109/L (HR: 0.522, 95% CI: 0.299–0.909, P=0.022), tumor size ≥5 cm (HR: 2.362, 95% CI: 1.317–4.236, P=0.004), poor tumor differentiation (HR: 1.966, 95% CI: 1.105–3.498, P=0.022), and IFG-B (HR: 2.535, 95% CI: 1.468–4.378, P=0.001) are independent risk factors for DFS (Table 5).
 |
Table 5 Univariate and Multivariate Analyses of Disease-Free Survival |
Univariate and Multivariate Analyses of OS
Univariate analysis showed that AST level, PLT count, and differentiation degree were associated with OS, and the differences were statistically significant (P<0.05, Table 6). Multivariate Cox regression analysis was performed with these factors that were significant in the univariate analysis, with the results showing that AST >42 U/L (HR: 1.933, 95% CI: 1.031–3.623, P=0.040) and poor tumor differentiation (HR: 2.402, 95% CI: 1.268–4.500, P=0.007) were independent risk factors for OS (Table 6).
 |
Table 6 Univariate and Multivariate Analyses of Overall Survival |
Discussion
In this study, we used 87 patients with AJCC stage I-II HCC who underwent R0 resection to study the correlation of IFG with survival parameters and prognosis. We detected the expression of 12 common inflammatory factors and established a grading system (designated the IFGs) based on the expression of the five factors significantly correlated with recurrence. We found that IFG is positively associated with tumor size and that patients with higher IFG (IFG-B) levels had worse DFS. Moreover, PLT ≤100×109/L, tumor size ≥5 cm, poor tumor differentiation level, and IFG-B are independent risk factors for DFS after HCC resection, and importantly, the IFG has the strongest association with DFS.
The clinical grading systems for HCC prognosis include Barcelona clinic liver cancer (BCLC) staging,11 Okuda staging,12 tumor-node-metastasis (TNM) staging,13 and albumin-bilirubin (ALBI) grading,14 and they consider factors such as tumor size, number of lesions, vascular invasion, extrahepatic metastases, and common clinical and pathological factors such as Child-Pugh classification. However, none of these grading systems take into account the inflammatory status of the tumor microenvironment.
Of the 12 inflammatory factors screened, we further studied the five factors that were associated with recurrence after R0 resection in AJCC stage I-II HCC patients. Although many studies have reported the involvement of these inflammatory factors in cancer, no study has examined the relationship between the expression levels of these inflammatory factors in tumor tissues and patient prognosis. Studies have shown that high levels of IL-6 in peripheral blood are closely related to a poor prognosis in HCC patients; the higher the IL-6 level is, the worse the prognosis.15–17 In addition, in multiple myeloma patients, higher IL-6R values correlate with poorer disease outcomes.18 Our study found that patients with high expression levels of IL-6 or IL-6R in the HCC microenvironment had significantly shorter postoperative recurrence times (Figure 2A and B, P<0.05), which is consistent with the findings previously reported in the literature. Okamoto et al studied the relationship between IL-1β and matrix metalloproteinase (MMP)-3 gene polymorphisms and hepatitis C virus (HCV)-related HCC prognosis and found that the simultaneous presence of the IL-1β-31T allele and MMP-3-5A allele is a risk factor for poor prognosis.19 At present, there are no reports on IL-1β expression and HCC prognosis, but we found that DFS was significantly prolonged in patients with high levels of IL-1β expression in tumor tissues (Figure 2C, P=0.014). Jiang et al found that the level of tumor apoptosis was elevated in IKKα- and IKKβ-knockout HCC models, and lung metastasis and the growth of subcutaneous xenografts in mice were significantly inhibited.20 However, He et al reported that IKKβ inhibits chemically induced HCC by inhibiting hepatocyte death and compensatory proliferation.21 Our study found that high IKKβ expression significantly prolongs DFS (Figure 2D, P=0.038). The P38MAPK signaling pathway is an important component of the MAPK cascade and performs different biological functions by mediating signal transduction.22 P38MAPK and extracellular regulated protein kinases (ERKs) are closely related to HCC invasion, migration, and apoptosis.23–25 Our study found that DFS was significantly prolonged in the P38MAPK high expression group and that P38MAPK may play a role as a tumor suppressor gene (Figure 2E, P=0.0033).
In the process of assigning IHC scores for the inflammatory factors studied, we assigned 2 points for the high expression of factors that promote recurrence and 2 points for the low expression of factors that inhibit recurrence to integrate the expression trends of several inflammatory factors. Malaguarnera et al reported that higher levels of IL-6 were associated with tumor size and cancer aggressiveness in patients with HCC,26 and we found that IFG was positively correlated with tumor size, which is consistent with the reports in the literature. At the same time, we conducted long-term follow-up of all patients for more than 5 years (4.0–114.5 months). We revealed that the median DFS of the IFG-A group was significantly higher than that of the IFG-B group (Figure 3A, P<0.001). However, there was no significant difference in median OS between the IFG-A and IFG-B groups (Figure 3B, P=0.2), which may be due to recurrence patterns between groups, treatment after relapse, and tumor size. These factors may affect the OS after recurrence in HCC patients.27
In addition, the multivariate analysis revealed that PLT ≤100×109/L (HR: 0.522, 95% CI: 0.299–0.909, P=0.022) is an independent risk factor for postoperative recurrence in patients with AJCC stage I-II HCC, which is consistent with the findings of Amano et al28 and Kaneko et al29 The results of this study showed that tumor size ≥5 cm (HR: 2.362, 95% CI: 1.317–4.236, P=0.004) was an independent risk factor for postoperative recurrence, which may be related to tumor compression, the infiltration of surrounding tissue vessels, and the increased risk of vascular invasion.30 When the degree of tumor differentiation is lower, tumor invasiveness is enhanced, and the tumor is more prone to recurrence and metastasis. Hubert et al found that pathological grade is an independent risk factor for the OS and DFS rates after liver cancer resection.31 We also found that tumor differentiation is an independent risk factor for DFS (HR: 1.966, 95% CI: 1.105–3.498, P=0.022) and OS (HR: 2.402, 95% CI: 1.268–4.500, P=0.007) in HCC patients after R0 resection. IFG-B (HR: 2.535, 95% CI: 1.468–4.378, P=0.001) is also an independent risk factor for DFS. Importantly, by comparing HR values, we found that IFG had the strongest association with DFS, indicating that IFG is a promising predictor of recurrence in HCC patients after R0 resection.
Conclusions
This study established IFGs in HCC patients with AJCC stage I-II and demonstrated that IFG-B can predict poor DFS after R0 resection in HCC patients. Therefore, IFG-B HCC patients with AJCC stage I-II require more frequent follow‑up observations to prevent HCC recurrence, thereby improving the long-term survival of HCC patients. However, our study had some limitations. First, our results have a certain scope of application and cannot be generalized to other populations. Second, the number of samples included in this study is limited; our conclusions must be validated through larger multicenter and prospective studies in the future.
Acknowledgments
We thank the patients and their families for their cooperation and participation in the study, as well as the pathologists Dr. Feng Hou and Dr. Wenjuan Yu (Affiliated Hospital of Qingdao University, Qingdao, China) involved with IHC analysis for this study. In addition, the study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Funding
This work was supported by the Science and Technology for People’s Livelihood Project of Qingdao under Grant No. 18-6-1-89-nsh; Key Research and Development Plan of Shandong Province under Grant No. 2018GSF118233; and Science and Technology Plan of Qingdao City Shinan District under Grant No. 20184018YY.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
3. Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):181–200. doi:10.1055/s-2005-871198
4. Tu T, Budzinska MA, Maczurek AE, et al. Novel aspects of the liver microenvironment in hepatocellular carcinoma pathogenesis and development. Int J Mol Sci. 2014;15(6):9422–9458. doi:10.3390/ijms15069422
5. Giannelli G, Rani B, Dituri F, et al. Moving towards personalised therapy in patients with hepatocellular carcinoma: the role of the microenvironment. Gut. 2014;63(10):1668–1676. doi:10.1136/gutjnl-2014-307323
6. Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144(3):512–527. doi:10.1053/j.gastro.2013.01.002
7. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. doi:10.1172/JCI31537
8. Jeng KS, Chang CF, Jeng WJ, et al. Heterogeneity of hepatocellular carcinoma contributes to cancer progression. Crit Rev Oncol Hematol. 2015;94(3):337–347. doi:10.1016/j.critrevonc.2015.01.009
9. Villa E, Critelli R, Lei B, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a Prospective Study. Gut. 2016;65(5):861–869.
10. Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): a Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117(4):644–650. doi:10.1002/jso.24908
11. Kamiyama T, Orimo T, Wakayama K, et al. Survival outcomes of hepatectomy for stage B Hepatocellular carcinoma in the BCLC classification. World J Surg Oncol. 2017;15(1):156. doi:10.1186/s12957-017-1229-x
12. Attallah AM, Omran MM, Attallah AA, et al. Simplified HCC-ART score for highly sensitive detection of small-sized and early-stage hepatocellular carcinoma in the widely used Okuda, CLIP, and BCLC staging systems. Int J Clin Oncol. 2017;22(2):332–339. doi:10.1007/s10147-016-1066-x
13. Liu C, Duan LG, Lu WS, et al. Prognosis evaluation in patients with hepatocellular carcinoma after hepatectomy: comparison of BCLC, TNM and Hangzhou criteria staging systems. PLoS One. 2014;9(8):e103228. doi:10.1371/journal.pone.0103228
14. Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346. doi:10.1016/j.jhep.2016.09.008
15. Jang JW, Oh BS, Kwon JH, et al. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60(3):686–693. doi:10.1016/j.cyto.2012.07.017
16. Ohishi W, Cologne JB, Fujiwara S, et al. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int j Cancer. 2014;134(1):154–163. doi:10.1002/ijc.v134.1
17. Soresi M, Giannitrapani L, D’Antona F, et al. Interleukin-6 and its soluble receptor in patients with liver cirrhosis and hepatocellular carcinoma. World j Gastroenterol. 2006;12(16):2563–2568. doi:10.3748/wjg.v12.i16.2563
18. Wierzbowska A, Urbanska-Rys H, Robak T. Circulating IL-6-type cytokines and sIL-6R in patients with multiple myeloma. Br J Haematol. 1999;105(2):412–419. doi:10.1111/bjh.1999.105.issue-2
19. Okamoto K, Ishida C, Ikebuchi Y, et al. The genotypes of IL-1 beta and MMP-3 are associated with the prognosis of HCV-related hepatocellular carcinoma. Intern Med. 2010;49(10):887–895. doi:10.2169/internalmedicine.49.3268
20. Jiang R, Xia Y, Li J, et al. High expression levels of IKKalpha and IKKbeta are necessary for the malignant properties of liver cancer. Int j Cancer. 2010;126(5):1263–1274. doi:10.1002/ijc.24854
21. He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17(3):286–297. doi:10.1016/j.ccr.2009.12.048
22. Yokota T, Wang Y. p38 MAP kinases in the heart. Gene. 2016;575(2 Pt 2):369–376. doi:10.1016/j.gene.2015.09.030
23. Sathish Kumar P, Viswanathan MBG, Venkatesan M, Balakrishna K. Bauerenol, a triterpenoid from Indian Suregada angustifolia: induces reactive oxygen species-mediated P38MAPK activation and apoptosis in human hepatocellular carcinoma (HepG2) cells. Tumour Biol. 2017;39(4):1010428317698387. doi:10.1177/1010428317698387
24. Bai ZT, Wu ZR, Xi LL, et al. Inhibition of invasion by N-trans-feruloyloctopamine via AKT, p38MAPK and EMT related signals in hepatocellular carcinoma cells. Bioorg Med Chem Lett. 2017;27(4):989–993. doi:10.1016/j.bmcl.2016.12.073
25. Lin JJ, Su JH, Tsai CC, et al. 11-epi-Sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling pathways. Mar Drugs. 2014;12(9):4783–4798. doi:10.3390/md12094783
26. Malaguarnera M, Di Fazio I, Laurino A, et al. Role of interleukin 6 in hepatocellular carcinoma. Bull Cancer. 1996;83(5):379–384.
27. Zhang M, Zhang S, Yang Z, et al. Association between the expression levels of IL-6 and IL-6R in the hepatocellular carcinoma microenvironment and postoperative recurrence. Oncol Lett. 2018;16(6):7158–7165. doi:10.3892/ol.2018.9557
28. Amano H, Tashiro H, Oshita A, et al. Significance of platelet count in the outcomes of hepatectomized patients with hepatocellular carcinoma exceeding the Milan criteria. J Gastrointest Surg. 2011;15(7):1173–1181. doi:10.1007/s11605-011-1538-2
29. Kaneko K, Shirai Y, Wakai T, et al. Low preoperative platelet counts predict a high mortality after partial hepatectomy in patients with hepatocellular carcinoma. World j Gastroenterol. 2005;11(37):5888–5892. doi:10.3748/wjg.v11.i37.5888
30. Löhe F, Angele MK, Rentsch M, et al. Multifocal manifestation does not affect vascular invasion of hepatocellular carcinoma: implications for patient selection in liver transplantation. Clin Transplant. 2007;21(6):696–701. doi:10.1111/j.1399-0012.2007.00707.x
31. Hubert C, Sempoux C, Rahier J, et al. Prognostic risk factors of survival after resection of hepatocellular carcinoma. Hepato-Gastroenterology. 2007;54(78):1791–1797.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



