Back to Journals » Cancer Management and Research » Volume 12
High Expression of CD44 Predicts a Poor Prognosis in Glioblastomas
Authors Si D, Yin F, Peng J, Zhang G
Received 4 October 2019
Accepted for publication 15 January 2020
Published 3 February 2020 Volume 2020:12 Pages 769—775
DOI https://doi.org/10.2147/CMAR.S233423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Daolin Si,1 Fei Yin,1 Jing Peng,1 Guangying Zhang2
1Department of Pediatric Neurology, Xiangya Hospital, Central South University, Changsha, Hunan Province 410008, People’s Republic of China; 2Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan Province 410008, People’s Republic of China
Correspondence: Guangying Zhang
Department of Oncology, Xiangya Hospital, Central South University, 87 Xiangya Road, Kaifu District, Changsha, Hunan Province 410008, People’s Republic of China
Tel +86-731- 89753043
Fax +86-731- 89753332
Email [email protected]
Purpose: Glioblastoma multiforme (GBM) is the most common of the malignant and invasive gliomas. High grade glioma is prone to relapse and has a poor prognosis. However, there is a big difference in terms of survival time with the same grade glioma. Cluster of differentiation 44 (CD44) is an indicator of cancer stem cells with abnormal expression in many malignant tumors, however the expression in GBM is unknown.
Methods: Tissue specimens were collected from 62 GBM patients to investigate CD44 expression and their prognosis was followed-up. Chi-square test was used to identify the association between CD44 staining and clinical characteristics of the patients. Kaplan-Meier analysis was performed to draw survival curves and Cox regression analysis to confirm the independent prognostic factors of GBM patients.
Results: In total, 38.7% (24/62) of the patients had high CD44 staining. The median survival times were 3.5 months and 18.5 months for high and low expressions of CD44, respectively. Kaplan-Meier analysis revealed that tumor location, the extent of tumor resection, adjuvant chemotherapy, and CD44 expression were related to overall survival time of GBM patients (P<0.05). Multivariate analysis showed that non-usage of adjuvant chemotherapy (HR=4.097, 95% CI=1.489– 11.277, P=0.006) and CD44 overexpression (HR=3.216, 95% CI=1.452– 7.125, P=0.004) were independent unfavorable prognostic factors for GBM patients.
Conclusion: The results demonstrate that high expression of CD44 acts as a poor prognosis indicator in GBM patients.
Keywords: glioblastoma, CD44, immunohistochemistry, survival times
Introduction
Glioma is a highly heterogeneous disease at the molecular level with different survival times, even among the patients with the same grade.1,2 GBM (regarded as grade IV glioma) is the highest grade of glioma, with a median survival time of approximately 12–15 months even with conventional treatment.3 Due to the highly invasive and malignant nature of GBM, the treatment remains challenging, with a high recurrence rate. So we mainly focus on the prognostic factors of GBM. Searching for a potential therapeutic target for GBM may enable a more effective strategy against GBM.
CD44 is a transmembrane glycoprotein and hyaluronic acid receptor. It has been reported that CD44 is involved in epithelial-mesenchymal transition (EMT) and tumor invasion.4 Verhaak et al5 classified GBM into four categories: proneural, neural, classical, and mesenchymal after analyzing the Cancer Genome Atlas for GBM. The adhesion molecule CD44 predicts resistance to chemotherapy in mesenchymal-like glioma.6,7 CD44 may play a crucial role in the prognosis of GBM patients. And CD44 inhibitions have been suggested as a therapeutic strategy for many malignant tumors, but the role for GBM remains controversial.8 Therefore, we analyzed CD44 expression in tissue sections of 62 GBM patients using immunohistochemistry.
Our study aimed to explore the expression of CD44 in highly malignant GBM. To know the expression of CD44 in GBM may lay the foundation to explore inhibitors for CD44-high GBM.
Materials and Methods
Patients
We collected 62 tissue specimens from newly diagnosed GBM patients in Xiangya Hospital, Central South University between May 2012 and October 2015. The research was approved by the Ethics Committee of our institution and was in agreement with the Declaration of Helsinki. Written informed consent was obtained for all patients. If any patients had passed away, the consent was signed by their next of kin. Classification of glioma was done according to World Health Organization (WHO) 2007 criteria. We divided tumor location into five groups: frontal, parietal, temporal, occipital, and others (basal ganglia, thalamus, epiphysis, ventricles, corpus callosum, brainstem, and insular lobe). Patients were first treated with surgery when they were diagnosed as GBM. They received adjuvant radiotherapy (intensity modulated radiation therapy, IMRT) and concurrent temozolomide (TMZ) chemotherapy after surgery, but with or without adjuvant chemotherapy. The standard radiotherapy was one fraction daily, 5 days per week. The total radiotherapy dose was 59.4–64.2 Gy in 30 fractions. Dose limits and plan evaluation were as defined by the European Organization for Research and Treatment of Cancer (EORTC).9,10 The concurrent TMZ dose was 75 mg/m2/day and adjuvant TMZ was 150–200 mg/m2/1–5 days every 28 days for six cycles. Overall survival (OS) was defined as the duration from initial operation to death or last follow-up.
Immunohistochemistry
We used 3-μm GBM tissue slices to perform immunohistochemistry assay, tissue samples were fixed in formalin and embedded in paraffin. It was confirmed that every tissue slice was diagnosed as GBM using H&E staining. The tissue slices were deparaffinized with xylene and rehydrated with alcohol routinely, then heated in citrate buffer for antigen retrieval by pressure cooker. They were washed in phosphate buffer saline (PBS) after natural cooling; 3% hydrogen peroxide was used to regulate endogenous peroxidase inactivation for 20 minutes, and 5% normal goat serum to prevent non-specific staining for 30 minutes. The specimens were incubated with anti-CD44 antibody (Abcam ab51037, a dilution of 1:100) overnight at 4°C. They were incubated with secondary antibodies for 30 minutes at room temperature on the next day. Finally, we used 3, 3-diaminobenzidine and hydrogen peroxide chromogen substrate to expose the immunoreaction. The hematoxylin was applied to counterstain. The negative controls were incubated with non-immune isotypic antibodies.
Evaluation of CD44 Staining
The immunohistochemistry (IHC) staining was evaluated by two pathologists, blinded to the clinicopathological diagnosis of the patients before. CD44 expression was given scores ranging from 0–4 according to staining intensity and the percentage of staining cells. Details were as follows: 0=low or weak stained in 10% of cells or less; 1=weak stained in 11–30% of cells; 2=weak stained in more than 30% of cells or moderate stained in less than 30% of cells; 3=moderate stained in 30–60% of cells; 4=moderate or strong stained in more than 60% of cells.11 The average staining scores were obtained based on ten randomly selected fields in each slice. According to the above staining criteria, the scores (0–2) and (3–4) were regarded as low and high CD44 expression, respectively.
Statistical Analysis
SPSS 23.0 software was used for statistical analyses. The relationship between CD44 expression and clinicopathologic features was calculated by Chi-square. Kaplan-Meier method was used to draw survival curves while group comparison was performed by long-rank test. The Cox regression analyses were used to confirm independent prognostic factors of GBM patients. Meanwhile, hazard ratio (HR) and their corresponding 95% confidence interval (CI) were calculated. The probability value of less than 0.05 indicated statistical significance.
Results
Clinical Features
In this study, 36 of the patients were males and 26 were females. Age ranged from 9–69 years. The tumor diameter varied from 2.0 to 8.1 cm (17 cases were smaller than 6.0 cm and the other 45 cases were larger than 6.0 cm). Moreover, tumor invasion of 17 GBM patients involved more than one brain lobe. Forty-five patients underwent gross total resection while the other 17 patients had either subtotal resection or biopsies were taken during surgical operation. Seventeen patients had recurrence, while 45 had no recurrence. In total, 50 patients received adjuvant TMZ chemotherapy, while 12 patients were unwilling to receive TMZ chemotherapy after surgery. We followed up all patients to October 2018. Unfortunately, 48 patients died during this time frame. The survival time of the 62 patients ranged from 1 to 72 months and the median survival time was 13 months.
CD44 Expression and Clinicopathological Features
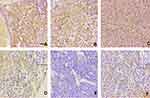
CD44 protein in GBM tissues mainly accumulated in the cell membrane but also some of cells had high expression in the cytoplasm. We found that CD44 staining scored one in 22 cases, two in 16 cases, three in 9 cases, and four in 15 cases. According to immunostaining evaluation criteria, the 0–2 scores were regarded as low CD44 expression and 3–4 scores were regarded as high CD44 expression. The high expression of CD44 was observed in about 38.7% (24/62) of the GBM patients. The representative images of CD44 staining is shown in Figure 1. Statistical analysis revealed that CD44 expression was associated with the number of tumor invading brain lobes (P=0.002). In contrast, there were no significant differences in terms of age, gender, tumor diameter, tumor location, recurrence, or extent of surgical resection. The relationship between CD44 expression and clinicopathological characteristics of the 62 GBM patients is summarized in Table 1. In addition, 1 year and 3 years survival rates of patients with high CD44 staining after surgery were significantly lower than those of patients with low staining (1 year, 29.17% vs 71.05% and 3 years, 16.67% vs 31.58%; see Table 2). We can see that only the 1 year survival rate achieved statistical significance. That may be due to poor prognosis and short survival times of GMB patients.
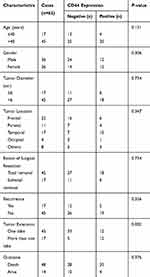 |
Table 1 The Correlation Between CD44 Expression and Clinicopathological Characteristics in Glioblastoma |
 |
Table 2 The Relationship Between CD44 Expression and Survival Rate (1 -Year and 3 Years After Surgical Operation) of GBM Patients |
The Prognostic Value of CD44 Expression in Glioblastoma Patients
The Kaplan-Meier was performed to evaluate prognostic factors including age, gender, the extent of resection, tumor diameter, tumor location, recurrence, tumor invasion range, adjuvant chemotherapy, and CD44 expression in GBM patients. Patients with high CD44 staining had a poor prognosis as compared to those with low CD44 staining (P=0.036). The Log rank test data is listed in Table 3. The median survival time of CD44 staining was 18.5 months for low staining and 3.5 months for high staining. According to univariate regression analysis, tumor location (HR=1.220, 95% CI=1.006–1.478, P=0.043), adjuvant chemotherapy (HR=3.537, 95% CI=1.725–7.253, P=0.001), and CD44 expression (HR=2.111, 95% CI=1.181–3.772, P=0.012) significantly affected OS of GBM patients. The corresponding information has been detailed in Table 4. Multivariate regression analysis revealed that non-usage of adjuvant chemotherapy and high expression of CD44 were independent poor prognosis factors for GBM patients. The survival time of GBM patients with high CD44 expression is significantly shorter than those with low CD44 expression, indicating that GBM patients with high CD44 expression had a poor prognosis. The difference was statistically significant (Figure 2).
 |
Table 3 The Kaplan-Meier Analyses of the Overall Survival of 62 Glioblastoma Patients. |
 |
Table 4 Cox Regression Analysis of Univariate Analysis and Multivariate Analysis with Glioblastoma Patients |
 |
Figure 2 Kaplan-Meier survival analysis shows the relationship of CD44 expression and overall survival times in 62 glioblastoma patients. |
Discussion
The grave prognosis of high-grade glioma is due to its invasive nature, high relapse rate, and resistance to therapy. GBM is an extremely malignant glioma which constantly ends up with therapeutic failure and has a short survival (within ~15 months).12,13 Nevertheless, glioma is a highly heterogeneous disease at the molecular level. There is a difference in terms of survival time among the patients with same grade glioma. Therefore, the grave prognosis and heterogeneity in GBM mandates an urgent need for identification of better prognostic indicators and therapeutic targets.
CD44 is a transmembrane glycoprotein and hyaluronic acid receptor.14 Several studies found that CD44 was involved in angiogenesis, proliferation, invasion, and migration, and may promote EMT in glioma. Orian-Rousseau15 reported that CD44 could combine with vascular endothelial growth factor receptors to block angiogenesis and inhibit proliferation in hepatocyte. Mani et al16 detected that CD44 had been implicated in EMT and contributed to breast cancer invasion. Breyer et al8 indicated that CD44 could be an important mediator of glioma cell migration into brain parenchyma. Blocking the related signaling pathway of CD44 expression contributes to inhibiting tumor cells growth and promoting apoptosis.17,18 Fu et al19 found that knockdown of tissue transglutaminase and protein tyrosine kinase attenuated tumor growth and induced apoptosis on CD44-high glioma.20
The therapeutic outcome and prognosis of different CD44 expression in GBM are not consistent among patients with the same treatment regimen. In the study, we found high CD44 expression in tissues of 24 cases of 62 GBM tissues. One year survival rate of patients with high CD44 staining after operation was significantly lower than those with low staining. The CD44 expression affects OS significantly in GBM patients. CD44 expression was an independent prognosis factor in GBM patients. Some studies have found that increased CD44 immunostaining is correlated with an advanced grade of glioma.21 The poor prognosis and treatment resistance of GBM promote the exploration of novel therapeutic approaches. CD44 is related to resistance to therapies, and an inhibitor of CD44 has the potential to sensitize cells to chemotherapy or radiotherapy.22 Gao et al23 found that CD44-high ovarian cells are resistant to paclitaxel-induced cancer cells apoptosis. The same is true of doxorubicin resistance of CD44-high breast cancer cells.24 Fernando et al25 found that knockdown of CD44 in liver tumor cells sensitized them to sorafenib-induced cancer cell death. Molecular targeted therapy with rare injury to normal cells that is safe and tolerable is a strategy for tumor treatment. Targeted drugs have been aimed mainly at specific receptors, kinase and molecular structure. However, the role of CD44 inhibition for GBM remains controversial.26 CD44 consists of 20 exons on chromosome 11, as various exons combination, standard and variants isoforms of CD44 were generated.27 CD44 standard isoforms (CD44s) were expressed in primary gliomas, while CD44 variant isoforms (CD44v) were expressed in intracranial metastatic tumors and participated in inflammatory response.28,29 We encourage more studies to be done on expression of different CD44 sub-types in tumor tissues.
In addition, some studies found that CD44 expressed in different types of cells, including stromal cells, cancer cells, and cancer stem cells.16,30,31 Glioma stem cells are responsible for tumor initiation, relapse, and therapeutic resistance. Also the CD44 is a common component of cancer stem cells. CD44 expression in GBM stem cells and CD44 involvement in cancer stem cells niche signaling may be another reason for its correlation with poor prognosis. Judit et al32 have observed that the depletion of CD44 expression by TGF-β inhibitors can prevent tumor proliferation and recurrence in glioma stem cells. GBM is the highest grade of glioma with a short survival time. The number of WHO IV glioma cases is small. Our study was restricted by the amount of samples. In our current work, we are expanding the sample size and researching related signaling pathways of CD44-high GBM.
Our results suggest that CD44 expression may be a crucial prognostic indicator of GBM. Investigation of CD44 expression may provide a theoretical basis for the development of anticancer drugs of CD44-high GBM.
Abbreviations
GBM, Glioblastoma multiforme; CD44, Cluster of differentiation 44; WHO, World Health Organization; EMT, mesenchymal transition; IHC, Immunohistochemistry; PBS, phosphate buffer saline; HR, hazard ratio; CI, confidence interval; OS, overall survival; CD44s, CD44 standard isoform; CD44v, CD44 variant isoform; EORTC, European Organization for Research and Treatment of Cancer; IMRT, intensity modulated radiation therapy; TMZ, temozolomide.
Acknowledgments
This work was supported by the Natural Science Foundation of China (grant number 81672510) and Clinical Specialty Project of Xiangya Hospital.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi:10.1056/NEJMoa043330
2. Wang FYH, Kang CS, Wang-gou SY, Huang CH, Feng CY, Li XJ. EGFL7 is an intercellular EGFR signal messenger that plays an oncogenic role in glioma. Cancer Letters. 2017;384:9–18. doi:10.1016/j.canlet.2016.10.009
3. Liu Q, Zhang C, Yuan J, et al. PTK7 regulates Id1 expression in CD44-high glioma cells. Neuro-Oncology. 2015;17(4):505–515. doi:10.1093/neuonc/nou227
4. Toole BP. Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res. 2010;15(24):7462–7468. doi:10.1158/1078-0432.CCR-09-0479
5. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell. 2010;17(1):98–110. doi:10.1016/j.ccr.2009.12.020
6. Rebecca LK, Stacy ADG, Benjamin LB, et al. Biphasic dependence of glioma survival and cell migration on CD44 expression level. Cell Reports. 2017;18:23–31. doi:10.1016/j.celrep.2016.12.024
7. Bhat KP, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-kB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. doi:10.1016/j.ccr.2013.08.001
8. Breyer R, Hussein S, Radu DL, et al. Disruption of intracerebral progression of C6 rat glioblastoma by in vivo treatment with anti-CD44 monoclonal antibody. J Neurosurg. 2000;92(1):140–149. doi:10.3171/jns.2000.92.1.0140
9. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10(5):459–466. doi:10.1016/S1470-2045(09)70025-7
10. Preusser M, de Ribaupierre S, Wöhrer A, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. doi:10.1002/ana.22425
11. Liu SC, Tsang NM, Chiang WC, et al. Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Invest. 2013;123(12):5269–5283. doi:10.1172/JCI63428
12. Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2(8):616–626. doi:10.1038/nrc866
13. Zhang GY, Li ZZ, Si DL, Shen LF. Diagnostic ability of intraoperative ultrasound for identifying tumor residual in glioma surgery operation. Oncotarget. 2017;8(42):73105–73114. doi:10.18632/oncotarget.20394
14. Yan YM, Zuo XS, Wei DY. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cell Transl Med. 2015;4(9):1033–1043. doi:10.5966/sctm.2015-0048
15. Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer. 2010;4(6):1271–1277. doi:10.1016/j.ejca.2010.02.024
16. Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi:10.1016/j.cell.2008.03.027
17. Feng CZ, Zhang Y, Yin JB, et al. Regulatory factor X1 is a new tumor suppressive transcription factor that acts via direct downregulation of CD44 in glioblastoma. Neuro-Oncology. 2014;16(8):1078–1085. doi:10.1093/neuonc/nou010
18. Wiranowska M, Ladd S, Moscinski LC, et al. Modulation of hyaluronan production by CD44 positive glioma cells. Int J Cancer. 2010;127(3):532–542. doi:10.1002/ijc.v127:3
19. Fu J, Yang QY, Ke S, et al. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro-Oncology. 2013;15(10):1353–1365. doi:10.1093/neuonc/not079
20. Zhang C, Yuan XR, Li HY, et al. Anti-cancer effect of metabotropic glutamate receptor 1 inhibition in human glioma U87 cells: involvement of PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 2015;35:419–432. doi:10.1159/000369707
21. Pietras A, Katz AM, Ekström EJ, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–369. doi:10.1016/j.stem.2014.01.005
22. Han XX, Yi JT, Xun Y, et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Therapy. 2015;8:3783–3792.
23. Gao Y, Foster R, Yang X, et al. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget. 2015;6(11):9313–9326. doi:10.18632/oncotarget.v6i11
24. Xie GZ, Yao QW, Liu Y, et al. IL-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int J Oncol. 2012;40(4):1171–1179. doi:10.3892/ijo.2011.1275
25. Fernando J, Malfettone A, Cepeda EB, et al. A mesenchymal-like phenotype and expression of CD44 predict lack of apoptotic response to sorafenib in liver tumor cells. Int J Cancer. 2015;136(4):161–172. doi:10.1002/ijc.29097
26. Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res. 2011;9(12):1573–1586. doi:10.1158/1541-7786.MCR-11-0156
27. Rodrigo JP, Dominguez F, Alvarez C, et al. Clinicopathologic significance of expression of CD44s and CD44v6 isoforms in squamous cell carcinoma of the supraglottic larynx. Am J Clin Pathol. 2002;118(1):67–72. doi:10.1309/F50H-6MLG-R7LM-2XFT
28. Xiao DS, Huang J, Pan Y, et al. Chromatin remodeling factor LSH is upregulated by the LRP6-GSK3β-E2F1 axis linking reversely with survival in gliomas. Theranostics. 2017;7(1):132–143. doi:10.7150/thno.17032
29. Erb U, Megaptche AP, Gu XY, Büchler MW, Zöller M. CD44 standard and CD44v10 isoform expression on leukemia cells distinctly influences niche embedding of hematopoietic stem cells. J Hematol Oncol. 2014;7(29):1–19. doi:10.1186/1756-8722-7-29
30. Daniel VB, Gulay F, Paul MD, et al. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intratumor heterogeneity. PLoS One. 2017;12(2):e0172791. doi:10.1371/journal.pone.0172791
31. Wang X, Du Z, Liu X, et al. Expression of CD44 standard form and variant isoforms in human bone marrow stromal cells. Saudi Pharm J. 2017;25(4):488–491. doi:10.1016/j.jsps.2017.04.011
32. Judit A, Andrea SB, Alba GJ, et al. TGF-β receptor inhibitors target the CD44high/Id1 high glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668. doi:10.1016/j.ccr.2010.10.023
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.