Back to Journals » International Journal of Nanomedicine » Volume 14
High drug payload nanoparticles formed from dexamethasone-peptide conjugates for the treatment of endotoxin-induced uveitis in rabbit
Authors Yu X, Zhang R, Lei L, Song Q, Li X
Received 4 July 2018
Accepted for publication 29 November 2018
Published 14 January 2019 Volume 2019:14 Pages 591—603
DOI https://doi.org/10.2147/IJN.S179118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Xinxin Yu, Renshu Zhang, Lei Lei, Qianqian Song, Xingyi Li
Institute of Biomedical Engineering, School of Ophthalmology and Optometry and Eye Hospital, Wenzhou Medical University, Wenzhou 325027, People’s Republic of China
Purpose: To develop and demonstrate the effectiveness of a novel dexamethasone (Dex) nanoformulation for treating uveitis.
Materials and methods: We designed and screened a dexamethasone-peptide conjugate (Dex-SA-FFFE), formed via a biodegradable ester bond linkage, that could spontaneously form high drug payload nanoparticles in aqueous solution for treating uveitis.
Results: An in vitro release study indicated that Dex and Dex-SA-FFFE sustainably released from Dex-SA-FFFE nanoparticles over a 48 h study period. Meanwhile, the formed Dex-SA-FFFE nanoparticles hardly caused cytotoxicity in human corneal epithelial cell at drug concentrations up to 1 mM after 24 h of incubation but reduced cell viability after 48 h and 72 h of incubation. An in vitro anti-inflammatory efficacy assay showed that the Dex-SA-FFFE nanoparticles exhibited a comparable anti-inflammatory efficacy to that of Dex in lipopolysaccharide (LPS)-activated RAW264.7 macrophages via significant decreases in the secretion of various pro-inflammatory cytokines (e.g., nitric oxide, tumor necrosis factor-α, interleukin-6). Topical instillation of Dex-SA-FFFE nanoparticles showed good ocular tolerance without causing changes in corneal thickness and intraocular pressure during the entire study period. Furthermore, topical instillation of Dex-SA-FFFE nanoparticles displayed a comparable in vivo therapeutic efficacy to that of dexamethasone sodium phosphate (Dexp) aqueous solutions in an endotoxin-induced uveitis (EIU) rabbit model.
Conclusion: Based on these results, it is reasonable to believe that the proposed Dex-SA-FFFE nanoparticles might have great application for the treatment of anterior uveitis.
Keywords: drug-peptide conjugate, self-assembly, ocular inflammation, in vivo, nanoparticle
Introduction
Uveitis, a term encompassing a variety of intraocular inflammatory disorders, is one of the leading causes of blindness in developed countries.1–3 Even when the underlying cause is unknown, steroids (e.g., dexamethasone [Dex] and triamcinolone acetonide) are still first-line treatment in the clinical therapy of uveitis.4–8 Because they can induce significant systemic side effects, treatments using systemic steroids are extremely limited.4,7,9 Topical steroid treatments in the form of eye drops, suspensions, or ointments are less likely to cause significant side effects than systemic steroid treatment and are more acceptable for the patient; however, they also suffer from low ocular bioavailability owing to the rapid precorneal clearance and poor corneal permeability of the drugs.9–12
In the past several decades, a variety of strategies including the use of adhesive additives, micro/nanotechnologies, and hydrogelation have been explored to extend the precorneal residence and increase the corneal permeability of steroids, thus enhancing ocular bioavailability.13–17 For instance, Kalam reported a Dex sodium phosphate (Dexp)-loaded chitosan/hyaluronic acid nanoparticle synthesized via an ionotropic gelation technique to improve precorneal retention and corneal permeability.18 Nagai et al designed an ophthalmic formulation containing Dex-loaded solid nanoparticles for extending precorneal retention and enhancing ocular bioavailability after topical instillation.19 Similarly, a cationic nanocrystal formulation composed of Dex acetate nanocrystals and polymyxin B was proposed through a self-developed small-scale method for increasing the mucoadhesion of drugs after topical ocular delivery.20 More recently, we proposed a Dex prodrug supramolecular hydrogel formed by a pH hydrolytic strategy for ophthalmic drug delivery. This hydrogel, acting as a “self-delivery” system, combined the advantages of nanoparticles and hydrogels to greatly increase corneal permeability and significantly prolong precorneal retention.21
Although significant advances in pharmaceutical technologies for ophthalmic steroid delivery have been achieved, the development of a novel, superior system with high drug payload for ocular steroid delivery is still urgently required. More recently, drug-peptide conjugates as an effective prodrug strategy represent an important class of therapeutic formulation owing to their high drug payload and lower amount of inert materials.22–27 Since the peptide is derived from amino acids, it is highly biodegradable and biocompatible and should not elicit unexpected side effects after in vivo application. Moreover, the diversity of amino acid combinations permits the facile preparation of various types of peptide-drug conjugates.28–31 For instance, Li et al described a hydrogelator, composed of a D-amino acid and a nonsteroidal anti-inflammatory drug, that had enhanced selectivity as a cyclooxygenase-2 inhibitor.32 Cui et al reported a series of drug-peptide conjugates that could self-assemble into various nanostructures (e.g., nanofiber and nanosphere) to improve the drug’s water solubility, cellular uptake, and potency against cancerous cells.33–35
Encouraged by the results of these studies, herein, we designed and screened a Dex-peptide conjugate (Dex-SA-FFFE), formed via a cleavable ester bond linkage (Figure 1), that could spontaneously form high drug payload nanoparticles in aqueous solution. Owing to the absence of a drug vehicle, the formed Dex-SA-FFFE nanoparticles significantly alleviate the safety concerns associated with vehicles and give a precise and relatively high drug payload. Furthermore, the absence of any organic solvents and surfactants during the fabrication procedure might be beneficial for practical applications. To assess the potential therapeutic efficacy of Dex-SA-FFFE nanoparticles, in vitro anti-inflammatory assays in lipopolysaccharide (LPS)-activated RAW264.7 macrophages and in vivo anti-inflammatory tests in an endotoxin-induced uveitis (EIU) rabbit model were performed.
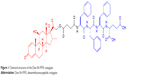  | Figure 1 Chemical structure of the Dex-SA-FFFE conjugate. |
Materials and methods
Materials
Succinated Dex (Dex-SA) was synthesized by using a method described in our previous study.21 The 2-chlorotrityl chloride resin and N-Fmoc-protected amino acids were provided by GL Biochem (Shanghai) Ltd., (Shanghai, China). Porcine liver esterase, cell counting kit-8 (CCK-8), and LPS were purchased from Sigma-Aldrich Co., (St Louise, MO, USA). Dexp and dialysis bags (molecular weight cutoff: 3,500) were provided by Dalian Meilun Biology Technology Co., Ltd., (Liaoning Province, China). All other chemical reagents used are of analytical grade.
Synthesis of Dex-SA-FFFE
According to a previous study, a Dex-SA-FFFE was synthesized by a classic solid-phase peptide synthesis method using 2-chlorotrityl chloride resin and N-Fmoc-protected amino acids.32 The 2-chlorotrityl chloride resin was first allowed to swell in dry dichloromethane (DCM) for 20 minutes, and then the first amino acid was loaded onto the resin in a dimethyl formamide (DMF) solution of Fmoc-protected amino acids (1.5 equiv) and N,N-diisopropylethylamine ([DIEA]; two equiv) for 2 hours. After washing with DCM five times, the unreactive sites of resin were blocked by the blocking solution (DCM/methanol/DIEA =17:2:1) for 10 minutes. Thereafter, the Fmoc protective group was removed by the addition of 20% piperidine (in DMF), followed by the coupling of Fmoc-protected amino acids (two equiv) to the free amino group on the resin using DIEA (two equiv) and O-benzotriazol-1-yl-tetramethyluronium hexafluorophosphate (HBTU) (one equiv) as coupling agents in DMF for 2 hours. These two steps were repeated to elongate the peptide chain. Finally, Dex-SA was coupled onto the peptide using DIEA (two equiv) and HBTU (one equiv) as the coupling agent in DMF for 2 hours, and the resultant Dex-SA-FFFE was cleaved from the resin with a washing solution (trifluoroacetic acid/triisopropylsilane/H2O=95/2.5/2.5). The obtained crude product was purified by reverse phase high-performance liquid chromatography (HPLC) and characterized by mass spectrometry (MS). MS (m/z): [M+H]+ calculated for C58H67FN4O14: 1,063.46; found: 1,063.4 (Figure S1).
Formation of Dex-SA-FFFE nanoparticles
Thirty milligrams of Dex-SA-FFFE powder was suspended in 1 mL of phosphate buffered saline (PBS, pH=7.4), followed by the addition of 1 mM Na2CO3 aqueous solution (one molar equivalent to Dex-SA-FFFE). The resulting suspension was heated up to 95°C and cooled down to room temperature to generate Dex-SA-FFFE nanoparticles.
Characterization
The size distribution and zeta potential of Dex-SA-FFFE nanoparticles were analyzed by a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK).
The micromorphology of Dex-SA-FFFE nanoparticles was observed by a transmission electron microscope (TEM, Tecnai F20; FEI, Hillsboro, OR, USA). Ten microliters of Dex-SA-FFFE nanoparticles was pipetted onto the grid and stained with 0.5 wt% phosphotungstic acid before TEM observation.
In vitro release study
In vitro release profiles of the drugs (Dex and Dex-SA-FFFE) from the Dex-SA-FFFE nanoparticles were investigated in both PBS (pH =7.4) and PBS (pH =7.4) containing 20 U/mL porcine liver esterase at 37°C. Briefly, 0.15 mL of Dex-SA-FFFE nanoparticles (23.4 mg/mL Dex-SA-FFFE) was sealed into a dialysis bag (molecular weight cutoff: 3,500) and immersed into 5 mL of either PBS or PBS containing 20 U/mL porcine liver esterase at 37°C for the period of in vitro release study. At predetermined time points, a 1-mL aliquot of the release medium was collected and the amount of the released drugs was quantified by HPLC with a reversed-phase C18 column (ZORBAX Eclipse XDB-C18, 150×4.6 mm i.d., 5 μm; Agilent Technologies, Santa Clara, CA, USA). The residual release medium was completely replaced with 5 mL of either freshly prepared PBS or PBS containing 20 U/mL porcine liver esterase at regular intervals of the study. The mobile phase for HPLC analysis was composed of acetonitrile and 0.1% triethylamine/phosphoric acid (55/45; v/v). Aliquots of 20 μL of tested samples were injected for HPLC analysis. The eluent was detected at 240 nm by a diode array detector.
In vitro cytotoxicity assay
To assess the in vitro cytotoxicity of Dex-SA-FFFE nanoparticles, CCK-8 assay was used to detect the viability of human corneal epithelial cell (HCEC) line after exposure to various drug concentrations. HCEC line was purchased from Chinese Academy of Sciences Cell Bank (Shanghai, China). Briefly, cells were seeded into 96-well plates (5×103 cells/well) with 100 μL of cell culture medium per well and incubated in air containing 5% CO2 at 37°C. Thereafter, 100 μL of cell culture medium containing different concentrations of Dex-SA-FFFE nanoparticles ranging from 0 to 2 mM was added to each well. After 24, 48, and 72 hours of incubation, 100 μL of the CCK-8 solution was added to each well, followed by the incubation for another 2 hours. Cells that were not manipulated were used as control. The absorbance of each well was recorded by a microplate reader (model SpectraMax M5; Molecular Devices LLC, Sunnyvale, CA, USA) at 450 nm. Finally, cell viability was calculated using the following equation: cell viability (%) = absorbance of tested sample/absorbance of control sample ×100. All experiments were performed in triplicate (mean ± SD, six wells for each drug concentration).
In vitro anti-inflammation test
To evaluate the in vitro anti-inflammatory effect of Dex-SA-FFFE nanoparticles, we measured the pro-inflammatory cytokines in the culture medium after exposure to different drug concentrations in LPS-activated RAW264.7 macrophages. RAW264.7 macrophages were provided by Chinese Academy of Sciences Cell Bank. Briefly, RAW264.7 macrophages were seeded into 24-well plates at a density of 1×105 cells/well and incubated overnight in air containing 5% CO2 at 37°C. In addition, the cells were pre-treated with 0.01 mM or 0.1 mM drug (Dex-SA-FFFE nanoparticles or Dex in DMSO) for 2 hours, followed by challenge with 1 μg/mL LPS for 24 hours. Thereafter, the cell culture medium was collected, and the levels of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were measured by the Griess assay and an ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), respectively.
Intraocular biocompatibility
We investigated the intraocular biocompatibility and ocular irritation of Dex-SA-FFFE nanoparticles in healthy Japanese big-eared white rabbits after topical instillation. Japanese big-eared white rabbits (weight: 2.0–2.5 kg) were provided by Wenzhou Medical University Laboratory Animal Center (Wenzhou, China). All animal experiments were conducted with the Guidelines for Care and Use of Research Animals established by Wenzhou Medical University and approved by the Ethical Committee of Wenzhou Medical University. Prior the in vivo applications, the formed Dex-SA-FFFE nanoparticles were sterilized by 0.22 μm filter membrane. The right eyes of the rabbits were topically instilled with 50 μL of Dex-SA-FFFE nanoparticles (30 mg/mL Dex-SA-FFFE) four times daily (6 mg Dex-SA-FFFE per eye daily) for three successive days, while the left eyes were topically instilled with 50 μL of PBS (pH =7.4). Clinical signs including conjunctival hyperemia, corneal edema, and lens opacity were monitored daily by a slit-lamp (SLM-4ER; Chongqing Kanghua Ruiming S&T Co., Ltd., Chongqing, China). The integrity of the corneal epithelium was checked by a fluorescein staining assay. Meanwhile, changes in intraocular pressure and corneal thickness were measured by a hand-held tonometer (Tono-Pen AVIA®, Reichert Technologies, Depew, NY, USA) and an iVue (Optovue, Fremont, CA, USA), respectively.
EIU rabbit model and medication
We assessed the in vivo anti-inflammatory efficacy of Dex-SA-FFFE nanoparticles in an EIU rabbit model. Eighteen rabbits were intravitreally injected with 100 ng of LPS to establish the EIU model and then randomly divided into three groups (n=6): 1) eyes topically instilled with 50 μL of Dex-SA-FFFE nanoparticles (30 mg/mL Dex-SA-FFFE) four times daily; 2) eyes topically instilled with 50 μL of Dexp (14.6 mg/mL Dexp; one molar equivalent to Dex-SA-FFFE) solution four times daily; and 3) eyes topically instilled with 50 μL of PBS (pH =7.4) four times daily. After 24 hours, the clinical signs of an inflammatory response in the anterior chamber (e.g., miosis, iris hyperemia, and protein leakage) were observed with a slit lamp (SLM-4ER; Chongqing Kanghua Ruiming S&T Co., Ltd) by an experienced ophthalmologist. Additionally, 100 μL of aqueous humor was collected with a 30-gauge insulin syringe, followed by the quantification of inflammatory cells, TNF-α, and IL-6 levels in the sample by a cell counting assay and ELISA kits (Shanghai MLBIO Biotechnology Company, China and R&D, USA). Finally, the rabbits from each group were sacrificed, and whole eyeballs were harvested for pathological observation by the hematoxylin and eosin (H&E) staining assay.
Statistical analysis
The data were analyzed by SPSS version 22 (IBM Corporation, Armonk, NY, USA) and expressed as the mean and Standard deviation (SD). The significant differences between control groups and experimental groups were analyzed by one-way ANOVA, and a P-value <0.05 was considered to indicate statistical significance.
Results and discussion
Dex-SA-FFFE nanoparticle formation and characterization
Since prodrugs provide a versatile approach to improve the clinical usefulness of many therapeutic agents by improving water solubility and permeability, sustained release, drug targeting, and the metabolic stability of the parent drug, prodrug design should be considered at the early stages of preclinical development.36–39 Amino acid prodrugs have been illustrated to significantly improve the oral delivery of drugs that have poor solubility and permeability (e.g., valacyclovir and valganciclovir).40,41 More importantly, the diversity of amino acids offers a great opportunity to finely tune the amphiphilicity of drugs, thus altering their self-assembly behavior in aqueous solution to generate various nanostructures (e.g., nanotubes, nanospheres, and nanofibers).35,42 In this article, we designed and screened a Dex-SA-FFFE prodrug via a cleavable ester bond linkage (Figure 1), which could spontaneously self-assemble into nanoparticles in an aqueous solution with prodrug concentrations up to 100 mg/mL (Figures S2 and S3). Unlike the conventional encapsulation approach, Dex-SA-FFFE nanoparticles formed by the prodrug itself significantly alleviate the safety concerns associated with carriers (e.g., biocompatibility and biodegradability) and provide a precise and relatively high drug payload.
As presented in Figure 2A, the formed Dex-SA-FFFE nanoparticles displayed a uniform spherical shape with a diameter of ~30 nm. Conversely, dynamic light scattering (DLS) analysis indicated that the mean diameter of the formed Dex-SA-FFFE nanoparticles was 150±3.4 nm with polydispersity index of 0.26±0.09 (Figure 2B). The formed Dex-SA-FFFE nanoparticles were negatively charged (−17.8±1.2 mV) owing to the ionized carboxylate groups of the amino acid residues present on the surface of the nanoparticles. The discrepancy between particle sizes measured by TEM and DLS might be ascribed to the high vacuum conditions of TEM as well as to hydrodynamic and electrokinetic effects during the DLS measurements.43 Additionally, the pH value and osmotic pressure of formed Dex-SA-FFFE nanoparticles are 7.3 and 298±2 mOsm·L−1, respectively.
  | Figure 2 (A) TEM image of Dex-SA-FFFE nanoparticles and (B) size distribution of Dex-SA-FFFE nanoparticles. |
In vitro release study
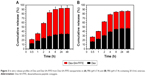
The drug release profiles of Dex and Dex-SA-FFFE from the Dex-SA-FFFE nanoparticles in PBS (pH =7.4) and PBS (pH =7.4) containing 20 U/mL esterase at 37°C are presented in Figure 3A and B. It is clearly observed that almost 100% of the total drug (Dex or Dex-SA-FFFE) was completely released from the Dex-SA-FFFE nanoparticles in either PBS (pH =7.4) or PBS containing 20 U/mL esterase during the 48-hour study. Despite the absence of esterase, Dex-SA-FFFE underwent obvious hydrolysis in PBS (pH =7.4) to release Dex at each time point (Figure 3A). This result seems to indicate that Dex-SA-FFFE was susceptive to the weak alkali condition, leading to the hydrolysis of Dex-SA-FFFE and the release of its native drug form (Dex). Not surprisingly, with the addition of 20 U/mL of esterase in PBS (pH =7.4), a higher ratio of Dex in the released drug was observed, which indicated that esterase could greatly accelerate the hydrolysis of Dex-SA-FFFE (Figure 3B).
In vitro cytotoxicity
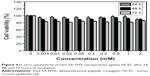
Figure 4 shows the cell viability of Dex-SA-FFFE nanoparticles in HCEC after 24, 48, and 72 hours of incubation. The results indicated that Dex-SA-FFFE nanoparticles were not toxic against HCEC at drug concentrations up to 1 mM after 24 hours of incubation. However, HCEC showed reduced cell viability after 48 and 72 hours of incubation, which might be ascribed to the cleavage and subsequent hydrolysis of Dex-SA-FFFE by esterase to release the native, potentially toxic, Dex.
In vitro anti-inflammation test
To assess the in vitro anti-inflammatory efficacy of Dex-SA-FFFE nanoparticles with respect to its native drug form (Dex), we monitored the secretion of pro-inflammatory cytokines such as NO, IL-6, and TNF-α in LPS-activated RAW264.7 macrophages. As depicted in Figure 5, RAW264.7 macrophages challenged with LPS significantly elevated the levels of NO, IL-6, and TNF-α compared to control cells. Treatments of both Dex and Dex-SA-FFFE nanoparticles at dosages of 0.01 and 0.1 mM were able to remarkably inhibit NO, IL-6, and TNF-α production compared to the LPS group (P<0.05). However, there was no significant difference between the Dex group and the Dex-SA-FFFE nanoparticles group at the tested concentrations. These results seem to imply that the Dex-SA-FFFE nanoparticles displayed nearly identical anti-inflammatory efficacy as the native drug (Dex). Early studies have illustrated that the inhibition of IL-6 and TNF-α production has a protective effect in the control of ocular inflammation.5,6,10 Therefore, it is reasonable to believe that the anti-inflammatory activity of the proposed Dex-SA-FFFE nanoparticles is not compromised in terms of its effects on pro-inflammatory cytokines (e.g., TNF-α, and IL-6) and that Dex-SA-FFFE nanoparticles might be a promising candidate for the therapy of ocular inflammatory disorders.
Intraocular biocompatibility
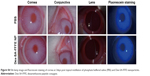
The intraocular biocompatibility of the Dex-SA-FFFE nanoparticles after topical instillation have been investigated in detail. As shown in Figure S4, the eyes treated by Dex-SA-FFFE nanoparticles did not show any apparent ocular irritation (eg, corneal edema, conjunctival swelling, and lens opacity) during the entire study period. Fluorescein sodium staining indicated that the architecture of the corneal epithelium layer was kept normal and intact, without any obvious defects. Together with the general observation, there are no significant changes in the corneal thickness or intraocular pressure after topical instillation of Dex-SA-FFFE nanoparticles (Figures S5 and S6). Based on these results, we could conclude that the proposed Dex-SA-FFFE nanoparticles are a well-tolerated formulation for ocular therapy.
EIU rabbit model and medication
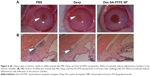
At 24 hours after intravitreal injection of endotoxin in rabbits, the eyes from the PBS group exhibited a severe inflammatory cellular response in the anterior chamber, whereas the eyes from the Dexp and Dex-SA-FFFE nanoparticles groups displayed a slight inflammatory response with minimal inflammatory exudate in the anterior chamber (Figure 6A). This result clearly indicated that treatments using both Dexp and Dex-SA-FFFE nanoparticles could significantly reduce the severity of clinical signs. Early studies have demonstrated that the main cause for the initiation of inflammatory symptoms in uveitis is the breakdown of the blood–aqueous humor barrier, and inflammation may subsequently occur owing to the migration of white blood cells such as leukocytes.44–46 We, therefore, quantified inflammatory cells in the aqueous humor at 24 hours of intravitreal injection of endotoxin (Figure 7A). The inflammatory cell count in the aqueous humor of eyes from the PBS group was (104.5±11) ×105 cells. The eyes treated by Dexp and Dex-SA-FFFE nanoparticles showed significantly lower inflammatory cell counts (P<0.05), but there was no significant difference between the Dexp group and the Dex-SA-FFFE nanoparticles group.
In line with clinical observations, histopathologic observations (Figure 6B) indicated that there were severe inflammatory changes with extensive inflammatory cell infiltration of the anterior chamber in the eyes treated by PBS. Conversely, the eyes treated by Dexp and Dex-SA-FFFE nanoparticles showed very minimal inflammatory changes with an absence of inflammatory cell infiltration in the anterior chamber. Notably, the Dex-SA-FFFE nanoparticles exhibited nearly identical in vivo anti-inflammatory efficacy as Dexp, indicating that the conjugation of Dex to peptide did not compromise the biological activity of Dex.
Although the exact pathogenic mechanisms underlying uveitis are poorly understood, several cytokines, including TNF-α, IL-1, IL-2, and IL-6 have been regarded as very important mediators in the pathogenesis of uveitis.2,5–8,10 We, thereafter, measured the levels of the cytokines TNF-α and IL-6 in the aqueous humor at 24 hours after intravitreal injection of endotoxin (Figure 7B and C). Both Dexp and Dex-SA-FFFE nanoparticles significantly downregulated TNF-α and IL-6 levels in the aqueous humor compared to the PBS treatment (P<0.05).
Conclusion
In this paper, we proposed the formation of a Dex-SA-FFFE, via an ester bond linkage, that could self-assemble into nanoparticles, acting as a “self-drug” delivery system for treating anterior uveitis. Both Dex and Dex-SA-FFFE were sustainably released from Dex-SA-FFFE nanoparticles in PBS or PBS containing 20 U/mL esterase in a 48-hour study, and the addition of esterase in PBS significantly influenced the ratio of Dex/Dex-SA-FFFE in the released drug, as indicated by the in vitro release study. In vitro cytotoxicity assays indicated that the formed Dex-SA-FFFE nanoparticles hardly caused cytotoxicity in HCEC cells at drug concentrations below 1 mM within 24 hours. Meanwhile, Dex-SA-FFFE nanoparticles displayed a comparable in vitro anti-inflammatory efficacy to that of Dex and significantly inhibited NO, IL-6, and TNF-α production in LPS-activated RAW264.7 macrophages. On the other hand, the formed Dex-SA-FFFE nanoparticles showed good ocular tolerance, as they appeared to exert no deleterious influence on corneal thickness or intraocular pressure in in vivo ocular biocompatibility tests. More importantly, Dex-SA-FFFE nanoparticles provided nearly identical in vivo anti-inflammatory efficacy as an aqueous solution of Dexp and downregulated the levels of cytokines (TNF-α and IL-6) in aqueous humor, indicating that the conjugation of Dex to peptide did not compromise the biological activity of Dex in vivo. In conclusion, the proposed Dex-SA-FFFE nanoparticles might have great application for the treatment of anterior uveitis.
Acknowledgments
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No LR18H300002) and the National Natural Science Foundation of China (Grant No 31671022).
Disclosure
The authors report no conflicts of interest in this work.
References
Reddy AK, Engelhard SB, Shah CT, Sim AJ, Thorne JE. Medical malpractice in uveitis: a review of clinical entities and outcomes. Ocul Immunol Inflamm. 2018;26(2):242–248. | ||
Samudre SS, Lattanzio FA, Williams PB, Sheppard JD. Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther. 2004;20(6):533–547. | ||
Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23(5):705–717. | ||
Rodríguez Villanueva J, Rodríguez Villanueva L, Guzmán Navarro M, Villanueva JR, Villanueva LR, Navarro MG. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int J Pharm. 2017;516(1–2):342–351. | ||
Ghasemi H. Roles of IL-6 in Ocular Inflammation: A Review. Ocul Immunol Inflamm. 2018;26(1):37–50. | ||
Impellizzeri D, Ahmad A, Bruschetta G, et al. The anti-inflammatory effects of palmitoylethanolamide (PEA) on endotoxin-induced uveitis in rats. Eur J Pharmacol. 2015;761:28–35. | ||
Lin P, Suhler EB, Rosenbaum JT. The future of uveitis treatment. Ophthalmology. 2014;121(1):365–376. | ||
Zheng C, Lei C, Chen Z, et al. Topical administration of diminazene aceturate decreases inflammation in endotoxin-induced uveitis. Mol Vis. 2015;21:403. | ||
Cao J, Naeem M, Noh J-K, Lee EH, Yoo JW. Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol Res. 2015;23(5):485–492. | ||
Barbosa Saliba J, Vieira L, Fernandes-Cunha GM, et al. Anti-Inflammatory Effect of Dexamethasone Controlled Released From Anterior Suprachoroidal Polyurethane Implants on Endotoxin-Induced Uveitis in Rats. Invest Ophthalmol Vis Sci. 2016;57(4):1671–1679. | ||
Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–512. | ||
Mcghee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. | ||
Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197–1216. | ||
Liu S, Jones L, Gu FX. Nanomaterials for ocular drug delivery. Macromol Biosci. 2012;12(5):608–620. | ||
Yuan X, Marcano DC, Shin CS, et al. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;9(2):1749–1758. | ||
Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr Eye Res. 2010;35(7):537–552. | ||
Swaminathan S, Vavia PR, Trotta F, Cavalli R. Nanosponges encapsulating dexamethasone for ocular delivery: formulation design, physicochemical characterization, safety and corneal permeability assessment. J Biomed Nanotechnol. 2013;9(6):998–1007. | ||
Kalam MA. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int J Biol Macromol. 2016;89:127–136. | ||
Nagai N, Nakazawa Y, Ito Y, Kanai K, Okamoto N, Shimomura Y. A Nanoparticle-Based Ophthalmic Formulation of Dexamethasone Enhances Corneal Permeability of the Drug and Prolongs Its Corneal Residence Time. Biol Pharm Bull. 2017;40(7):1055–1062. | ||
Romero GB, Keck CM, Müller RH, Bou-Chacra NA. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;107:215–222. | ||
Zhang Z, Yu J, Zhou Y, et al. Supramolecular nanofibers of dexamethasone derivatives to form hydrogel for topical ocular drug delivery. Colloids Surf B Biointerfaces. 2018;164:436–443. | ||
An L, Shah Gilani MR, Liang G, Gilani HS, Rehan M. Peptide-based nanostructures for cancer diagnosis and therapy. Curr Med Chem. 2014;21(21):2453–2466. | ||
Du X, Zhou J, Shi J, Xu B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev. 2015;115(24):13165–13307. | ||
Mei B, Miao Q, Tang A, Liang G. Enzyme-instructed self-assembly of taxol promotes axonal branching. Nanoscale. 2015;7(38):15605–15608. | ||
Sundar S, Chen Y, Tong YW. Delivery of therapeutics and molecules using self-assembled peptides. Curr Med Chem. 2014;21(22):2469–2479. | ||
Vemula PK, Wiradharma N, Ankrum JA, Miranda OR, John G, Karp JM. Prodrugs as self-assembled hydrogels: a new paradigm for biomaterials. Curr Opin Biotechnol. 2013;24(6):1174–1182. | ||
Yang C, Wang Z, Ou C, Chen M, Wang L, Yang Z. A supramolecular hydrogelator of curcumin. Chem Commun. 2014;50(66):9413–9415. | ||
Gynther M, Laine K, Ropponen J, et al. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem. 2008;51(4):932–936. | ||
Pochopin NL, Charman WN, Stella VJ. Amino acid derivatives of dapsone as water-soluble prodrugs. Int J Pharm. 1995;121(2):157–167. | ||
Santer V, del Río Sancho S, Lapteva M, Kalia YN. Targeted intracorneal delivery-Biodistribution of triamcinolone acetonide following topical iontophoresis of cationic amino acid ester prodrugs. Int J Pharm. 2017;525(1):43–53. | ||
Adelli GR, Bhagav P, Taskar P, et al. Development of a Δ9-Tetrahydrocannabinol Amino Acid-Dicarboxylate Prodrug With Improved Ocular Bioavailability. Invest Ophthalmol Vis Sci. 2017;58(4):2167–2179. | ||
Li J, Kuang Y, Gao Y, du X, Shi J, Xu B. D-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J Am Chem Soc. 2013;135(2):542–545. | ||
Lin R, Cui H. Supramolecular nanostructures as drug carriers. Curr Opin Chem Eng. 2015;7:75–83. | ||
Ma W, Cheetham AG, Cui H. Building Nanostructures with Drugs. Nano Today. 2016;11(1):13–30. | ||
Wang Y, Cheetham AG, Angacian G, Su H, Xie L, Cui H. Peptide-prug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliver Rev. 2011;110:112–126. | ||
Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–270. | ||
Rautio J, Kärkkäinen J, Sloan KB. Prodrugs – Recent approvals and a glimpse of the pipeline. Eur J Pharm Sci. 2017;109:146–161. | ||
Hamada Y. Recent progress in prodrug design strategies based on generally applicable modifications. Bioorg Med Chem Lett. 2017;27(8):1627–1632. | ||
Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C. Prodrug approach: An overview of recent cases. Eur J Med Chem. 2017;127:810–827. | ||
Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89(6):781–789. | ||
Dias C, Nashed Y, Atluri H, Mitra A. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC-MS. Curr Eye Res. 2002;25(4):243–252. | ||
Vig BS, Huttunen KM, Laine K, Rautio J. Amino acids as promoieties in prodrug design and development. Adv Drug Deliv Rev. 2013;65(10):1370–1385. | ||
Dobrovolskaia MA, Patri AK, Zheng J, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009;5(2):106–117. | ||
Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5(5):567–581. | ||
Ratay ML, Bellotti E, Gottardi R, Little SR. Modern Therapeutic Approaches for Noninfectious Ocular Diseases Involving Inflammation. Adv Healthc Mater. 2017;6(23):1700733. | ||
Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye – Part I – Barriers and determining factors in ocular delivery. Eur J Pharm Biopharm. 2017;110:70–75. |
Supplementary materials
  | Figure S1 LC-MS spectra of Dex-SA-FFFE. |
  | Figure S2 Appearance of Dex-SA-FFFE suspension before (left) and after (right) heating-cooling circle. |
  | Figure S3 LC-MS spectra of Dex-SA-FFFE nanoparticles formed after heating-cooling circle. |
  | Figure S5 The changes of intraocular pressure after instillation of phosphate buffered saline (PBS, pH=7.4) and Dex-SA-FFFE nanoparticles. |
  | Figure S6 The changes of corneal thickness after instillation of phosphate buffered saline (PBS, pH=7.4) and Dex-SA-FFFE nanoparticles. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.