Back to Journals » Patient Preference and Adherence » Volume 12
Heroin-dependent patient satisfaction with methadone as a medication influences satisfaction with basic interventions delivered by staff to implement methadone maintenance treatment
Authors Alcaraz S , Viladrich C , Trujols J , Siñol N, Pérez de los Cobos J
Received 31 January 2018
Accepted for publication 4 April 2018
Published 10 July 2018 Volume 2018:12 Pages 1203—1211
DOI https://doi.org/10.2147/PPA.S164181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Saul Alcaraz,1 Carme Viladrich,2 Joan Trujols,1,3 Núria Siñol,1 José Pérez de los Cobos1,3,4
1Addictive Behaviors Unit, Department of Psychiatry, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute Sant Pau (IIB Sant Pau), Barcelona, Spain; 2Department of Psychobiology and Methodology of Health Sciences, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, Spain; 3Biomedical Research Networking Center on Mental Health (CIBERSAM), Madrid, Spain; 4Department of Psychiatry and Legal Medicine, School of Medicine, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, Spain
Purpose: The aim of the present study was to test a structural equation model of patient satisfaction with different key facets of methadone maintenance treatment (MMT). In this model, the three dimensions of patient satisfaction with methadone as a medication (ie, personal functioning and well-being, anti-addictive effect on heroin, and anti-addictive effect on non-opioid substances) were expected to predict satisfaction with the basic interventions delivered by the staff of treatment centers to implement MMT.
Patients and methods: A sample of 210 heroin-dependent patients, resistant to MMT treatment (mean age =41.66 years, SD =6.50; 75.7% male), participated voluntarily in this study. Preliminary analysis based on exploratory structural equation modeling supported the expected three-factor measurement model of the scale to assess satisfaction with medications for addiction treatment – methadone for heroin addiction. Moreover, the 15 items measuring staff’s basic interventions were shown to be compatible with the expected single-factor measurement model. Then, both measurement models were included in a structural model.
Results: Results of this model show that patient satisfaction with the compatibility of methadone with personal functioning and well-being, as well as with the anti-addictive effects of methadone on non-opioid substances, predicts satisfaction with basic interventions conducted at methadone treatment centers (β=0.191 and β=0.152, respectively).
Conclusion: Our results provide further understanding regarding patient satisfaction with MMT, which could help professionals to better understand patient perspective and experience during MMT.
Keywords: exploratory structural equation modeling, satisfaction with medication, satisfaction with treatment, treatment quality, methadone non-responders
Introduction
Methadone maintenance treatment (MMT) is considered the gold standard of treatment of heroin dependence as different studies show that it reduces heroin use, non-opioid substance use, crime, HIV infection, and mortality.1–3 Nevertheless, some patients do not achieve the MMT goal of stopping compulsive use of heroin,4,5 which hinders the management of complications associated with heroin dependence. According to the World Health Organization, MMT outcomes could be improved through the assessment of patient satisfaction with this treatment.6
Patient satisfaction with treatment is a global measure of treatment quality reflecting patients’ evaluation of the actual experience of care received compared to their care expectations.7,8 The resulting subjective state includes a wide range of patient evaluations regarding the different therapeutic interventions received, such as skills and manners of physicians, psychologists, nurses or social workers, and perceived safety and effectiveness of medications. Specifically, Trujols et al conceptualized patient satisfaction in MMT as a global process involving four interrelated and overlapping dimensions:9 satisfaction with methadone as a medication (eg, effectiveness, impact on daily life/functioning), satisfaction with treatment (eg, non-pharmacological interventions, professional skills), methadone holding dose, and dose adequacy.
Similarly, Shikiar and Rentz hypothesized that patient satisfaction with medication is related to satisfaction with other treatment interventions, such as physician–patient interactions or those physician recommendations that go beyond the medication prescribed.8 This hypothesis requires attention in the field of MMT, for both clinical and research reasons. It makes sense to think that physicians are concerned about whether patient satisfaction with methadone as a medication could be linked to patient satisfaction with the interventions delivered to implement MMT (eg, help offered to improve patients’ social relationships and self-care). Pérez de los Cobos et al explored this proposition using a single question to assess patient opinion about methadone as a medication and found that this opinion independently predicted patient satisfaction with MMT.10 Furthermore, previous research argued that satisfaction with methadone as a medication would be mediating the relation between polymorphisms of a gene involved in the metabolism of methadone (ie, cytochrome P-450 enzyme 2D6 [CYP2D6]) and satisfaction with basic interventions.11
Patient satisfaction in MMT has been primarily measured with generic instruments, such as the General Satisfaction Questionnaire12 or the Client Satisfaction Questionnaire.13 In addition, as patient satisfaction is a broad construct including different dimensions,9 it appears interesting to target each facet with instruments specifically designed to assess opioid maintenance treatments. For example, Pérez de los Cobos et al validated the Verona service satisfaction scale for methadone treatment (VSSS-MT) to measure patient satisfaction with the services received at methadone treatment centers.14 Similarly, the scale to assess satisfaction with medications for addiction treatment – methadone for heroin addiction (SASMAT-METHER) – was developed to assess patient satisfaction with methadone as a medication.15
The SASMAT-METHER is a 17-item questionnaire targeting three dimensions of patient satisfaction with methadone as a medication: personal functioning and well-being, anti-addictive effect on heroin, and anti-addictive effect on non-opioid substances.16 Personal functioning and well-being assess patient satisfaction regarding methadone compatibility with other activities, its influence on overall physical health, its impact on enjoying pleasant things of life, and the tolerability of its adverse effects. The other two subscales evaluate patient satisfaction with methadone in reducing consumption, craving, withdrawal, thoughts about, and attention paid to 1) heroin or 2) non-opioid substances. Previous research has not only offered evidence supporting the psychometric properties of this instrument but also advocated that further studies are needed to replicate its reliability and factor structure.15
The aim of the present study was to test the hypothesis of heroin-dependent patient satisfaction with methadone as a medication impacting their satisfaction with the basic interventions delivered by the staff to implement MMT. To do so, we tested a structural equation model (SEM) where the three dimensions of the SASMAT-METHER (ie, personal functioning and well-being, anti-addictive effect on heroin, and anti-addictive effect on non-opioid substances) were predictors of satisfaction with the basic interventions factor of the VSSS-MT (ie, satisfaction with skills and manners of physicians and nurses, help received to improve self-care and inter-personal relationships, and overall satisfaction with services delivered at MMT centers). The study sample comprised of non-responders to MMT (ie, patients who are unable to stop substance use)17 because, in this group of patients, the impact of satisfaction with methadone on satisfaction with basic interventions could hinder the implementation of any clinical interventions aimed at treating heroin dependence. As a secondary purpose, we provide further evidence regarding the measurement model of the SASMAT-METHER.
Patients and methods
Participants and procedure
The sample in this study included 210 patients who met DSM-IV criteria for heroin dependence18 and had received MMT for at least the previous 3 months. Exclusion criteria included mental disorders that could hinder patient assessment (eg, neurocognitive disorders, substance intoxication) and difficulty answering the survey due to limited literacy or poor Spanish language proficiency. All patients were recruited at the Addictive Behaviors Unit of Sant Pau Hospital (Barcelona) by using accidental sampling. All participants had been referred from other methadone-treatment centers in Barcelona and were at the Addictive Behaviors Unit of Sant Pau Hospital for clinical assessment and/or short-term treatments at the time of the study. No compensation was offered to patients for his/her participation in this research. The study protocol was approved by the institutional review board of Sant Pau Hospital.
Two research assistants who were not part of the clinical staff recruited the participants and conducted their assessment. Participants read the information of the investigation and signed an informed consent before beginning their data collection. Participation was voluntary and confidentiality was guaranteed. Completion of the instruments lasted ~45 minutes. The survey began in February 2007 and ended in June 2015.
Instruments
Scale to assess satisfaction with medications for addiction treatment–methadone for heroin addiction
Participants responded to SASMAT-METHER in order to assess their satisfaction with methadone for heroin treatment.15 All items were introduced with the stem: “What is your overall opinion about ….” SASMAT-METHER includes three factors (number of items presented in brackets): personal functioning and well-being (seven items), anti-addictive effect on heroin (five items), and anti-addictive effect on other substances (five items). Examples of items for each scale are as follows: “… the impact of taking methadone on your overall physical health?” “… the efficacy of methadone to stop thinking about heroin?”, and “… the efficacy of methadone in reducing craving for other substances of abuse?”, respectively. Response categories for SASMAT-METHER items are ordered using a 5-point Likert scale (1=terrible, 2=generally unsatisfactory, 3=mixed, 4=generally satisfactory, and 5=excellent). Four items also include a “not applicable” response option: 1) one evaluates satisfaction with the efficacy of methadone to interfere with the effects of heroin; 2) one assesses compatibility between methadone and other medications; or 3) two assess compatibility of methadone with work/study activities. Similarly, the five items assessing the anti-addictive effect of methadone on secondary substances of abuse (eg, cocaine) may not be applicable if these secondary substances were not consumed at least 20 times during the treatment period. All response options of the SASMAT-METHER are presented with alternate directionality. SASMAT-METHER scores are obtained by taking an average of the applicable items and thus the scores for each scale can range from 1 to 5.
Basic interventions implemented by the staff of treatment centers
Patient satisfaction with the services delivered by methadone-treatment centers, which are essential for methadone administration, was measured with the basic interventions subscale of the VSSS-MT.14 This subscale is the first factor of the VSSS-MT and comprises 15 items. The basic interventions dimension assesses the satisfaction with the activity of those professionals who are essential to deliver the pharmacotherapeutic component of MMT (ie, doctors and nurses), along with the help received in two areas that are particularly deteriorated in heroin-dependent patients: interpersonal relationships and self-care. However, basic interventions do not include other components of MMT, such as psychosocial treatment (eg, individual or family therapy) or the activity of other professionals (eg, psychologists or social workers). The basic interventions items were introduced by using the stem: “What is your overall feeling about the ...” and an example of an item is: “... ability of physicians (internists or psychiatrists) to listen to and understand your problems?” Participants responded to the items by using a 5-point Likert scale (1=terrible, 2=generally unsatisfactory, 3=mixed, 4=generally satisfactory, 5=excellent). Response categories were presented with alternate directionality. The score of basic interventions is obtained by taking an average of item responses, and thus the scale score ranges from 1 to 5.
Data analysis
Data were analyzed using SPSS version 17 (SPSS Inc., Chicago, IL, USA) and Mplus software version 7.0.19 Preliminary analyses included the study of missing values, distribution of our data, and scale reliability. Scale reliability was assessed using McDonald’s coefficient omega (ω).20 Subsequently, we analyzed the factor structure of the questionnaires used in this study in order to provide evidence supporting their internal structure. The measurement model of SASMAT-METHER was tested comparing a series of nested measurement models: Model 1 was based on an exploratory structural equation modeling (ESEM) approach where items were allowed to load into three correlated factors including cross-loadings;21 Model 2 assumed a confirmatory factor analysis (CFA) structure where each item loaded into one of the three correlated factors and cross-loadings were fixed to zero; Model 3 was also based on a CFA approach and tested for a three uncorrelated factor structure (ie, correlations between factors were constrained to 0); and Model 4 assessed a single-factor structure. Comparison between Model 1 and Model 2 was a test of the tenability of all item cross-loadings being 0. Comparison between Model 2 and Model 3 assessed the presence of correlations between SASMAT-METHER factors. Finally, comparison between Model 2 and Model 4 was a test of discriminant validity between factors. Regarding the basic interventions measurement model, we tested a single-factor structure. Consequently, we assessed its discriminant validity by testing a global measurement model including the SASMAT-METHER measurement model as well as the basic interventions factor.
In this study, all measurement models and the structural model were estimated using the weighted least squares mean and variance adjusted (WLSMV) estimator, an estimator that is appropriate for Likert scales.19 Geomin rotation was used to define the correlated factors in the ESEM model. We employed different fit indices to test the fit of the measurement models to the data: χ2 statistic, root mean square error of approximation (RMSEA)22 including its 90% CI, comparative fix index (CFI),23 and Tucker-Lewis index (TLI).24 A non-significant χ2 value indicates a good fit between the observed and the implied covariance matrices. The threshold of acceptable fit for the RMSEA is ≤0.08 (for an excellent fit, ≤0.06).25 Moreover, for its CI, <0.05 (lower bound) and <0.08 (upper bound) are acceptable, and 0 (lower bound) and <0.05 (upper bound) are good.26 In addition, CFI and TLI values >0.95 are considered as indicators of excellent fit.26 It should be noted that those interpretation criteria were initially proposed for CFA models with quantitative indicators, but they have been subsequently applied to ESEM models and categorical indicators.27
The comparison between SASMAT-METHER nested measurement models was based on χ2 along with CFI, TLI, and RMSEA differences. According to previous research, the more parsimonious model should be selected only when changes in CFI are <0.01 and increases in RMSEA are <0.015.28,29 Changes in the TLI were evaluated following the guidelines associated with CFI changes.30
Then, in order to provide evidence supporting the discriminant validity of the latent factors that would be included within the structural model, we tested the correlations between these factors. Correlation coefficients were interpreted using Zhu’s criterion:31 0–0.19 =no correlation, 0.20–0.39 =low correlation, 0.40–0.59 =moderate correlation, 0.60–0.79 =moderately high correlation, and ≥0.80 =high correlation. As a final step of our data analyses, we constructed an SEM to evaluate the relationship between SASMAT-METHER factors and basic interventions. Specifically, it was hypothesized that all three SASMAT-METHER factors (ie, personal functioning and well-being, anti-addictive effect on heroin, and anti-addictive effect on other substances) would positively predict patients’ perceptions of the basic interventions conducted at their methadone-treatment centers. The fit of the SEM to the data was assessed using the same criteria explained earlier, although these criteria should be treated with a degree of caution.32
Results
Preliminary analyses and scale reliability
Table 1 presents the description of participants. As can be observed, the sample was mostly male. Percentages of missing data were below 5% for most of the items (Table 2). According to Graham’s criterion,33 our patterns of missing data were assumed not to have consequences on subsequent data analyses. However, three items had a percentage of missing values >5% (item 7 of personal functioning and well-being, items 2 and 14 of basic interventions). In those cases, missing values corresponded to the option “not applicable” in the questionnaire.14,15 In this study, we used pairwise deletion of missing data. In general, participants chose mid and mid to high values to evaluate their satisfaction with methadone and methadone-treatment centers. In addition, descriptive statistics for the scales tested in the study are presented in Table 4.
Evidence supporting scale reliability was obtained by calculating McDonald’s coefficient omega (ω).17 Values for each scale were as follows: ωpersonal functioning and well-being =0.880, ωanti-addictive effect on heroin =0.875, ωanti-addictive effect on other substances =0.927, and ωbasic interventions =0.945.
Measurement models
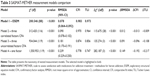
In order to provide evidence supporting the internal structure of the SASMAT-METHER, we tested a hierarchy of nested measurement models (Table 3). The ESEM measurement model (Model 1) showed an acceptable fit to the data (χ2 [df] =200.546 [88], p<0.001, RMSEA [90% CI] =0.078 [0.064–0.092], CFI =0.983, TLI =0.973) and significantly outperformed the other models. The ESEM model also exhibited salient factor loadings (>0.40)34 and non-remarkable cross-loadings, the highest being 0.276 (Table 2). Although the three-factor CFA correlated model (Model 2) also exhibited a good fit to the data, the ESEM model was preferred and then selected to be used in all subsequent analyses (comparison between Models 1 and 2: Δχ2 [Δdf] =115.675 [28], p<0.001, ΔRMSEA =0.012, ΔCFI =−0.013, ΔTLI =−0.009).
Then, we tested the global measurement model including all the indicators considered in this study. SASMAT-METHER was defined using the ESEM three-factor structure and the basic interventions as a single-factor structure. The global measurement model showed good fit to the data, thus supporting the discriminant validity of the basic interventions factor: χ2 (df) =773.497 (430), p<0.001, RMSEA (90% CI) =0.062 (0.055–0.069), CFI =0.967, and TLI =0.962.
Correlations between factors
As a previous step before testing the SEM, we offer evidence supporting the discriminant validity of all factors analyzed. Thus, we present the correlations between the latent factors that would be included within the structural model (Table 4). As can be observed, correlations between SASMAT-METHER factors and basic interventions were low (ie, 0.20–0.39), whereas correlations between SASMAT-METHER factors ranged from low to moderate (0.40–0.59).
Testing a model of patient satisfaction with MMT
The results of the structural model tested in this study are presented in Figure 1. In this model, SASMAT-METHER factors were defined using an ESEM approach, and basic interventions were specified as a single-factor structure. The structural model hypothesized that patient satisfaction with methadone as a medication was an antecedent of patient satisfaction with the basic interventions conducted at treatment centers. The model exhibits a good fit to the data: χ2 (df) =773.497 (430), p<0.001, RMSEA (90% CI) =0.062 (0.055–0.069), CFI =0.967, and TLI =0.962. As can be observed, patient satisfaction with the effects of methadone on their personal functioning and well-being, as well as methadone anti-addictive effect on non-opioid substances, significantly and positively predict patient satisfaction with the basic interventions conducted at their methadone treatment centers (β=0.191 and β=0.152, respectively). All three SASMAT-METHER factors combined explain 13.4% of the basic interventions variance.
Discussion
In the present study, we tested a structural model of satisfaction with MMT in a sample of heroin-dependent patients, who were non-responders to methadone treatment. This model assumed that patient satisfaction with methadone as a medication would influence patient satisfaction with the basic MMT interventions delivered by the staff. Our results partially support the hypothesized model. Specifically, patient satisfaction with their own personal functioning and well-being as well as with the anti-addictive effects of methadone on non-opioid substances of abuse positively, but weakly, predicts satisfaction with the interventions conducted at MMT centers, whereas satisfaction with the anti-addictive effects of methadone on heroin does not. These results are consistent with Trujols et al’s theoretical model, regarding the capability of heroin-dependent patients to discriminate between their experiences with methadone medication and their judgment of the clinical staff who delivers this medication.9
Shikiar and Rentz pointed out that patient satisfaction with medication could have an impact on satisfaction with other areas of patients’ treatment.8 In this study, two dimensions of patient satisfaction with methadone, namely personal functioning and well-being and anti-addictive effects of methadone on non-opioid substances, predict patient experiences with the staff interventions received. These relations are relatively small but are of clinical importance. In fact, the moderate percentage of explained variance may be interpreted as a favorable result. Our initial concern was that satisfaction with methadone could have a strong impact on satisfaction with the interventions conducted by clinical staff. However, our results show that this relation is not strong, suggesting that patient relationships with clinical staff might probably be safeguarded even when medication is not effective.
In addition, we also expected that satisfaction with methadone regarding its anti-addictive effects on heroin would be the main predictor of patient experiences with MMT basic interventions. The assumption was based on the idea that the primary therapeutic effect of methadone administration is decreasing the use of heroin and reducing the severity of heroin addiction.2,3 However, the path between both variables was not statistically significant, suggesting that patients could be satisfied or dissatisfied with the staff interventions regardless of their satisfaction with the pharmacological effects of methadone on heroin. As the study began in 2007, a possible explanation is that patients knew that methadone was the only option for maintenance treatment regardless of whether they were satisfied with it or not (ie, the Spanish Ministry of Health first regulated prescription buprenorphine-naloxone in 2010) and understood that physicians were unable to suggest a different medication. Another explanation may be related to the statistical analysis. In this study, the correlation matrix showed that satisfaction with opioid effects had a positive, statistically significant association with satisfaction with basic interventions. However, when we included this variable in the SEM, its relevance decreased. As its correlation with personal functioning and well-being had the greatest value, we speculate that some degree of collinearity between both variables could be responsible for this result.
The present study also offers further evidence regarding the measurement model of the SASMAT-METHER.15 Specifically, results support the selection of a three-factor model based on ESEM. This model exhibited a satisfactory fit to the data, with all three factors being correctly identified. It should be noted that the model with three correlated factors based on CFA also showed a satisfactory fit to the data. In addition, we also provided evidence supporting the internal consistency of the SASMAT factors through optimal omega coefficient values. Our results strengthen the line of previous research developed with this instrument16,35 and support the SASMAT-METHER as valid instrument to assess patient satisfaction with methadone as a medication.
Limitations and future research
First, the present investigation was cross-sectional in nature; therefore, longitudinal designs are warranted to confirm the theoretical relations tested in this study.8,9 Second, the data assessing basic treatment interventions were based upon a self-report instrument (ie, VSSS-MT), which may not be an accurate reflection of how professionals really behave. However, it was the perception of those behaviors that was of interest in studies about patient satisfaction with treatment. Third, the sampling method was accidental and limited to Spanish non-responders to MMT, and thus may not ensure representativeness of all opioid-dependent patients. However, such sampling method allowed us to only include participants who were in stabilized clinical condition at the time of assessment, which surely reduced the influence of potential substance intoxications or withdrawals on patients’ opinions. Still, future research is needed to replicate the findings of the present study by using other samples, such as responders to MMT who are following outpatient treatment and/or patients in MMT from different countries.
Conclusion
The present study represents the first attempt to define how heroin-dependent patient satisfaction with methadone as a medication influences satisfaction with the basic interventions implemented at treatment centers. Specifically, our structural model suggests that those patients satisfied with the effects of methadone on their personal functioning and well-being, as well as with its anti-addictive effects on non-opioid substances, would be inclined to be more satisfied with the basic interventions received at treatment centers. In the current study, we also extend the existing literature by providing further evidence supporting the measurement model of the SASMAT-METHER, highlighting its role as a remarkable evaluation tool to measure satisfaction with methadone as a medication.
Acknowledgments
The authors are grateful to the patients who kindly participated in the study and to Saiko Allende and Isabel Blásquiz for their secretarial support. Funding for this study was provided by grants PS09/01072 and PI06/0531 from Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (Spanish Ministries of Economy and Competitiveness, and Health, Social Services and Equality). Funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Author contributions
JPC and JT designed the study and wrote the protocol. NS and SA performed the data collection. SA and CV conducted the statistical analysis. SA wrote the first draft of the manuscript, and all authors substantially contributed to and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. JPC has received grant support from RB Pharmaceuticals Ltd to conduct research and educational activities not related to the present research.
Disclosure
The authors report no conflicts of interest in this work.
References
Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence [review]. Cochrane Database Syst Rev. 2003;3:CD002208. | ||
Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375:357–368. | ||
Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–1740. | ||
Gossop M, Marsden J, Stewart D, Kidd T. The National Treatment Outcome Research Study (NTORS): 4–5 year follow-up results. Addiction. 2003;98:291–303. | ||
White WL, Campbell MD, Spencer RD, Hoffman HA, Crissman B, DuPont RL. Patterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retention. J Psychoactive Drugs. 2014;46:114–122. | ||
World Health Organization (WHO). Evaluation of psychoactive substance use disorder treatment: Workbook 6 client satisfaction evaluations. Geneva: World Health Organization; 2000. | ||
Crow R, Gage H, Hampson S, et al. The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature. Health Technol Assess. 2002;6:1–244. | ||
Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7:204–215. | ||
Trujols J, Siñol N, Pérez de los Cobos J. Methadone maintenance treatment: the need to distinguish between holding dose, dose adequacy, satisfaction with methadone as a medication, and satisfaction with treatment. J Clin Psychopharmacol. 2010;30:95–96. | ||
Pérez de los Cobos J, Trujols J, Valderrama JC, Valero S, Puig T. Patient perspectives on methadone maintenance treatment in the Valencia Region: dose adjustment, participation in dosage regulation, and satisfaction with treatment. Drug Alcohol Depend. 2005;79:405–412. | ||
Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190–194. | ||
Gabbay MB, Clarke S, Willert E, Esmail A. Shared care methadone clinics a survey of patient satisfaction behaviour change and staff views. Addict Res. 1999;7:129–147. | ||
Marchand K, Palis H, Peng D, et al. The role of gender in factors associated with addiction treatment satisfaction among long-term opioid users. J Addict Med. 2015;9:391–398. | ||
Pérez de los Cobos J, Valero S, Haro G, et al. Development and psychometric properties of the Verona Service Satisfaction Scale for methadone-treated opioid-dependent patients (VSSS-MT). Drug Alcohol Depend. 2002;68:209–214. | ||
Pérez de los Cobos J, Trujols J, Siñol N, Batlle F. Development and validation of the scale to assess satisfaction with medications for addiction treatment-methadone for heroin addiction (SASMAT-METHER). Drug Alcohol Depend. 2014;142:79–85. | ||
Pérez de los Cobos J, Trujols J, Siñol N, Duran-Sindreu S, Batlle F. Satisfaction with methadone among heroin-dependent patients with current substance use disorders during methadone maintenance treatment. J Clin Psychopharmacol. 2016;36:157–162. | ||
Pérez de los Cobos J, Alcaraz S, Siñol N, et al. Satisfaction with methadone and opioid receptor genes polymorphisms in treatment-refractory heroin-dependent patients. J Clin Psychopharmacol. 2017;7:378–380. | ||
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association; 1994. | ||
Muthén LK, Muthén BO. Mplus Editor (version 7.0). Los Angeles: Muthén Muthén; 2012. | ||
McDonald RP. Theoretical foundations of principal factor analysis and alpha factor analysis. Br J Math Stat Psych. 1970;23:1–21. | ||
Asparouhov T, Muthén B. Exploratory structural equation modeling. Struct Equ Modeling. 2009;16:397–438. | ||
Steiger JH, Lind JC. Statistically based tests for common factors. Paper presented at: Annual Meeting of the Psychometric Society; June; 1980; Iowa City, IA. | ||
Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. | ||
Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. | ||
Browne MW, Cudeck R. Alternative ways of assessing model fit. In: K Bollen, J Long, editors. Testing Structural Equation Models. Newbury Park: Sage; 1993:136–162. | ||
Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. | ||
Marsden J, Eastwood B, Ali R, et al. Development of the addiction dimensions for assessment and personalised treatment (ADAPT). Drug Alcohol Depend. 2014;139:121–131. | ||
Chen FF. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct Equ Modeling. 2007;14:464–504. | ||
Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct Equ Modeling. 2002;9:233–255. | ||
Marsh HW, Muthén B, Asparouhov A, et al. Exploratory structural equation modeling, integrating CFA and EFA: application to students’ evaluations of university teaching. Struct Equ Modeling. 2009;16:439–476. | ||
Zhu W. Sadly, the earth is still round (p<0.005). J Sport Health Sci. 2012;1:9–11. | ||
Kline RB. Principles and Practice of Structural Equation Modeling. 3rd ed. New York: The Guilford Press; 2011. | ||
Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. | ||
Brown TA. Confirmatory Factor Analysis for Applied Research. New York: The Gilford Press; 2006. | ||
Alcaraz S, Trujols J, Siñol N, Duran-Sindreu S, Batlle F, Pérez de los Cobos J. Exploring predictors of response to methadone maintenance treatment for heroin addiction: the role of patient satisfaction with methadone as a medication. Heroin Addict Relat Clin Probl. 2017;19:35–40. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.