Back to Journals » Clinical Interventions in Aging » Volume 16
Hemodynamic Characteristics Associated with Recurrence of Middle Cerebral Artery Bifurcation Aneurysms After Total Embolization
Authors Yuan J, Huang C, Li Z, Jiang X, Zhao X, Lai N, Xia D , Wu D, Zhang B, Wang X, Fang X
Received 25 June 2021
Accepted for publication 8 September 2021
Published 6 December 2021 Volume 2021:16 Pages 2023—2032
DOI https://doi.org/10.2147/CIA.S326635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Jinlong Yuan,1,* Chenlei Huang,2,* Zhenbao Li,1 Xiaochun Jiang,1 Xintong Zhao,1 Niansheng Lai,1 Dayong Xia,1 Degang Wu,1 Bingbing Zhang,1 Xuanzhi Wang,3 Xinggen Fang1
1Department of Neurosurgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu, People’s Republic of China; 2Department of Clinical Laboratory, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu, People’s Republic of China; 3Department of Neurosurgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinggen Fang
Department of Neurosurgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Zheshan West Road 2, Wuhu, 241001, People’s Republic of China
Email [email protected]
Objective: Hemodynamic parameters are associated with the recurrence of intracranial aneurysms (IAs). Studies showed that high velocity and wall shear stress (WSS) were associated with IAs recurrence after endovascular treatment; nevertheless, factors such as small sample size, locations of IAs, and types of IAs (ie, sidewall or bifurcation) were neglected. The purpose of this study was to identify the hemodynamic characteristics associated with recurrence of middle cerebral artery bifurcation aneurysms (MCABAs) after total embolization by the method of computer fluid dynamics (CFD).
Methods: Following inclusion criteria, we included 92 MCABAs treated with coils only after total embolization from January 2014 to January 2019. We segmented into recurrent and non-recurrent groups according to follow-up digital subtraction angiography (DSA). The MCABA models, including pre-operatively and immediate post-operatively, were reconstructed using 3D-DSAs. The hemodynamic parameters pre-operatively and immediately post-operatively between the groups were calculated and analyzed.
Results: There were no significant differences between the recurrent and non-recurrent groups for spatially averaged wall shear stress (SAWSS), maximum wall shear stress (MWSS), velocity, or oscillatory shear index (OSI) at the neck pre-operatively. In the recurrent group, the WSS (22.02± 5.11 vs 37.43± 8.27 pa, p < 0.001), MWSS (42.59± 17.02 vs 66.98± 18.61 pa, p=0.014), velocity (0.86± 0.19 vs 1.44± 0.61 m/s, p=0.01) preoperatively were significantly higher than postoperative values. By contrast, in the non-recurrent group, the WSS (26.53± 8.18 vs 22.29± 8.64pa, p=0.002), MWSS (42.71± 14.01 vs 37.15± 15.56 pa, p=0.013), velocity (1.08± 0.43 vs 0.23 (0.52, 0.57) m/s, p < 0.001) postoperatively were significantly lower than preoperative values. The OSI, whether in the recurrent group or the non-recurrent group, did not differ significantly between groups (p=0.79 and p=0.19).
Conclusion: Higher WSS (SAWSS, MWSS) and flow velocity at the aneurysm neck after embolization might be related to recurrence of bifurcation IAs. These might be applied to clinical post-embolization management for the evaluation of bifurcation IAs recanalization.
Keywords: computational fluid dynamic, hemodynamics, middle cerebral artery bifurcation aneurysms, recurrence
Introduction
Intracranial aneurysms (IAs) are pathological dilatations of the intracranial arteries in approximately 3–7% of the population.1,2 Subarachnoid hemorrhage (SAH) often results from IAs rupture and leads to high mortality and morbidity rates. Although the annual rupture rate of IAs is about 1%, the consequences are catastrophic.3 They are most often treated with microsurgical clipping or endovascular treatment (EVT). Following the rapid development of embolization technologies and materials, especially the publication of the international subarachnoid aneurysm trial, an increasing number of IAs have been treated with EVT as the first line of therapy. Nevertheless, the recurrence rate of 20–40% remains a significant problem.4,5 Although the embolization of aneurysms reaches the standard of Raymond I (complete occlusion), the recurrence rate nevertheless ranges from 12.7% to 14% during the follow-up period.6 Multiple parameters such as aneurysms size, hypertension, degree of embolization, and location are associated with recanalization of IAs; however, hemodynamics plays a fundamental role in the recurrence of IAs.7–9 Computer fluid dynamics (CFD) based on patient-specific models found that high velocity and wall shear stress (WSS) around the neck may contribute to recurrence after EVT, whether total or partial occlusion.9–11 However, several essential factors were neglected in previous studies. First, the sample sizes were small, leading to statistical bias. Second, IAs were not divided into sidewall and bifurcation aneurysms. To the best of our knowledge, hemodynamic parameters differ between sidewall and bifurcation aneurysms. Hemodynamic studies based on sidewall aneurysms or bifurcation aneurysms might provide accurate results. Furthermore, hemodynamic parameters are affected by location. Hemodynamic studies at the same location might weaken the influence.
To address these problems, we performed the research on hemodynamic characteristics associated with the recurrence of MCABAs. Previous studies indicated that MCABAs accounted for 18–36% of all IAs, with an annual rupture rate of 0.36%.12 Moreover, to exclude the influence of stents and the degree of embolization on hemodynamics, we selected MCABAs after total embolization. As far as we know, this is the first hemodynamic study with a large sample size associated with the recurrence of bifurcation aneurysms after total embolization.
Materials and Methods
Patient Selection
We reviewed our database retrospectively, including imaging data and medical records. Two hundred sixty-five consecutive patients with 310 MCA aneurysms were diagnosed and treated with EVT at our institution from January 2014 to January 2019. For ruptured MCA aneurysms, coil embolization only was preferred to reduce thrombotic complications. For unruptured MCA aneurysms, stents assisted coils were selected to reduce the recurrence of aneurysms. Inclusion criteria were as follows: (1) middle cerebral artery bifurcation saccular aneurysms; (2) follow-up period more than 3 months; (3) the aneurysms were treated with coil embolization only; (4) the degree of aneurysms embolization reached the standard of Raymond I (complete occlusion); (5) the quality of 3D DSA imaging was suitable for CFD analysis; Exclusion criteria were as follows: (1) fusiform, dissected, M1, and MCA distal aneurysms; (2) follow-up period less than 3 months; (3) aneurysms were treated with stent-assisted or stent embolization only; (4) the degree of aneurysms embolization was Raymond II (neck remnant) or Raymond III (partial occlusion). (5) the quality of DSA images was below an acceptable standard. A total of 92 MCABAs met the selection criteria (Figure 1). The Research Ethics Committee of Wannan Medical College approved the study conducted following the Declaration of Helsinki. The participants and their family members provided written informed consent.
 |
Figure 1 Flowchart of the population selection of the MCABAs. |
Patient-Specific Model Reconstruction
All three-dimensional images of aneurysms and parent arteries, including current and no-recurrent groups, were obtained by DSA (Siemens, Artis Zee Floor VC14). Each MCABA model used three-step reconstruction models, including preoperative, immediately postoperative, and follow-up models. After 360° rotation with 15 mL contrast medium injection, 266 images were gained, immediately reconstructed into patient-specific IAs geometry, and outputted in stereolithography format on the Syngo X Workplace workstation. We obtained the pulsatile velocity waveform from a healthy person using transcranial Doppler and subsequently collected the flow spectrum envelope to get the average flow velocity waveform of the entire cardiac cycle using MATLAB 14.0 (MathWorks, Natick, MA, US).
Hemodynamic Models Reconstruction
We used GEOMAGIC STUDIO 12.0 (Geomagic, US) to smooth and move unimportant minor vascular branches and then imported them into ICEM CFD 14.0 (ANSYS, Lebanon, NH, US) to create volume grids. We set the maximum element size to 3mm. The total number of element grids in each model was between 1,100,000 and 1,60,000. For accurate WSS, all surface data were gridded to create approximately 1 million finite volume tetrahedral element grids with four layers of prism elements. Then, ANSYS CFX 14.0 (ANSYS, Inc.) was used to define blood fluid properties, blood vessel boundary conditions, and calculation steps for hemodynamic calculation. The blood fluid was calculated using the Navier-Stoke formula. Blood flow was assumed to be laminar, incompressible, and Newtonian. The density and dynamic viscosity of blood were 1050 kg/m3 and 0.00345 pa·s, respectively. The vessel wall was considered as having a no-slip boundary condition and a rigid wall. The inlet was set to a pulsatile velocity profile derived from a healthy individual with transcranial Doppler. The outlet was treated as having an opening boundary condition with zero static pressure. It was assumed that the IAs filled with coils were completely independent of the blood flow. Each 0.8s cardiac cycle was divided into 800 steps with times of 0.001s. The maximum blood flow velocity and WSS appeared at the end of the systolic period with the highest blood pressure (t = 0.2s). We simulated three cardiac cycles to obtain stable data, and the final cycle was used as output.
The Hemodynamic and Morphological Parameters Definition and Calculation
Twelve morphological parameters were calculated. The size, aspect ratio (AR) and the size ratio (SR) were described by Dhar et al.13 Neck size was the diameter of the neck. The V1 was the angle between two M2 segments and the V2 was defined as the smaller angle between M1 and one M2 segment of MCA. The V3 was the larger angle between M1 and the other M2 segment of MCA. We also defined the LA ratio as V3/V2, larger/smaller angle ratio. The D1 was the diameter of M1. The D2 was defined as the diameter of the smaller M2 branch, and the D3 was the diameter of the larger M2 branch. As before, the DA ratio was defined as D3/D2. The eight morphological parameters were described by Zhang et al and calculated by the method of Ingebrigtsen et al.12,14
All hemodynamic parameters, including velocity, normalized wall shear stress (NWSS), the percentage of low WSS area (LSA%), maximum wall shear stress (MWSS), spatially averaged wall shear stress (SAWSS), oscillatory shear index (OSI), and relative residence time (RRT) were calculated as described previously.8,10,11
Statistical Analysis
Statistical analyses were performed using SPSS20.0 (SPSS Inc, Chicago, Illinois). The Shapiro–Wilks W or Kolmogorov–Smirnov D test was used to determine whether a parameter was normally distributed. Normally distributed data were expressed as mean ± SD and non-normally distributed data were presented as medians (25%, 75%). The independent sample t-test (for normally distributed data), nonparametric Mann–Whitney U-test (for non-normally distributed data), and chi-square test were used to calculate the statistical significance of all parameters between the groups. For preoperative and postoperative data, paired t-tests (for normally distributed data) or the Mann–Whitney U-test (for non-normally distributed data) were used to calculate the differences between the two groups. P-values less than 0.05 were considered statistically significant.
Results
The Clinical Features of Patients with MCABAs in the Recurrent and Non-Recurrent Groups
We divided the 92 MCABAs, including 61 females and 31 males with mean age 55.59 ± 9.86 years, into two groups: recurrent group (n = 12) and non-recurrent group (n = 80). A total of 184 3D models, 92 preoperative MCABAs models, 92 postoperative models, were constructed for CFD analysis. In the recurrent group, there were seven females and five males with mean age 58.83 ± 6.56 years. In the non-recurrent group, there were 54 females and 26 males with mean age of 55.11 ± 10.21 years. There were no significant differences between groups for age (p = 0.11), gender (p = 0.53), hypertension (p = 0.21), smoking (p = 0.84), diabetes mellitus (p = 0.48), hyperlipidemia (p = 0.86), atherosclerosis (p = 0.74), or follow-up period (p = 0.69) (p < 0.05). The clinical features of 92 MCABAs in the recurrent and non-recurrent groups are displayed in Table 1. Table 1 shows clinical features of 92 MCABAs in the recurrent and non-recurrent groups.
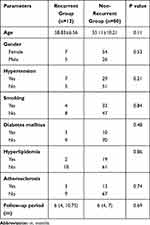 |
Table 1 Clinical Features of 92 MCABAs in the Recurrent and Non-Recurrent Groups |
Hemodynamic and Morphological Analysis of 92 MCABAs Before Embolization
Compared with the non-recurrent group, the recurrent group was more likely to have larger size, neck size, higher AR, SR, V1, V2, V3, D2, D3, and LA. However, these results did not achieve statistical significance (p = 0.38, p = 0.10, p = 0.62, p = 0.53, p = 0.78, p = 0.90, p = 0.84, p = 0.73, p = 0.83, and p = 0.76 respectively). Nevertheless, in terms of D1 and DA, even though the recurrent group was characterized with lower D1 and DA than with no-recurrent group, the difference did not reach statistical difference (p = 0.28 and p = 0.95, respectively). These results are presented in Table 2. Table 2 displays morphological and hemodynamic parameters of 92 MCABAs in the recurrent and non-recurrent groups preoperatively.
 |
Table 2 Morphological and Hemodynamic Parameters of 92 MCABAs in the Recurrent and Non-Recurrent Groups Preoperatively |
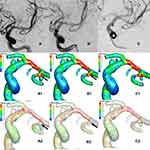
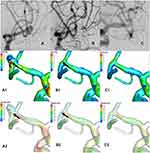
Whether the recurrent or non-recurrent groups, the blood flow patterns from M1 to the MCABAs sac were almost identical. The blood flow from M1 directly impacted the wall of the aneurysm sac with relatively high WSS where complex vortices began to form and then flowed down at a lower rate (Figure 2 A1, A2 and Figure 3 A1, A2).
Concerning hemodynamic parameters in the aneurysmal sac, compared with the non-recurrent group, the recurrent group tended to have higher NWSS, OSI, RRT, and lower LSA%; however, there were no statistically significant differences (p = 0.3, p = 0.92, p = 0.94 and p = 0.55, respectively) (Table 2).
In the terms of hemodynamic factors on the aneurysm neck preoperatively, the SAWSS (22.02 ± 5.11 pa vs 26.53 ± 8.18pa), MWSS (42.59 ± 17.02 pa vs 42.71 ± 14.01 pa), flow velocity (0.86 ± 0.19 m/s vs 1.08 ± 0.43 m/s), and OSI (0.07±0.03 VS 0.074±0.019) in the recurrent group were lower than those in the non-recurrent group, however, without significant differences (p = 0.068, p = 0.978, p = 0.104, p = 0.88 respectively). The results are presented in Table 3. Table 3 displays hemodynamic parameters of 92 MCABAs between the recurrent and non-recurrent groups pre-operatively and immediately post-operatively.
 |
Table 3 Hemodynamic Parameters of 92 MCABAs Between the Recurrent and Non-Recurrent Groups Preoperatively and Immediately Postoperatively |
Hemodynamic Analysis of 92 MCABAs Postoperatively
The blood flow pattern of the recurrent and the non-recurrent groups immediately after total embolization was almost identical. Owing to the disappearance of the aneurysmal sac, the flow pattern changed substantially. The blood flow from M1 to the MCABA sac was blocked by the filling material (coils), which streamlined the flow to the distal M2 (Figures 2 B2 and 3 B2). In the non-recurrent group, the flow pattern after the follow-up period was the same as that immediately after complete occlusion (Figure 3 C2) and Figure 3 C1 showed the MWSS at follow-up of the non-recurrent MCABAs. Nevertheless, in the recurrent group after the follow-up period, streamlined flow into a new aneurysm sac formed at the neck of the previous aneurysm, where a new complex vortex began to form and then flowed downward the aneurysm or into distal M2 at a lower rate (Figure 2 C2) and Figure 2 C1 showed the MWSS at follow-up of the recurrent MCABAs. The SAWSS, MWSS, and velocity at the neck postoperatively in the recurrent group were significantly higher than those in the non-recurrent group (all the pvalues < 0.001). Despite the result that the OSI at the neck in the recurrent group was moderately higher than those in the non-recurrent group, there was no statistical significance (p=0.52) (Table 3).
Hemodynamic Pre- and Post-Operative Comparison in the Recurrent and Non-Recurrent Groups
In the recurrent group, the SAWSS (22.02 ± 5.11 pa vs 37.43 ± 8.27pa, p < 0.001), MWSS (42.59 ± 17.02pa vs 66.98 ± 18.61, p=0.014), velocity (0.86 ± 0.19 m/s vs 1.44 ± 0.61 m/s, p = 0.01) postoperatively were significantly higher than preoperative values (Figure 2 A1, B1 A2 and B2). By contrast, in the non-recurrent group, the SAWSS (26.53 ± 8.18 pa vs 22.29 ± 8.64 pa, p = 0.002), MWSS (42.71 ± 14.01 pa vs 37.15 ± 15.56, p = 0.013), velocity (1.08 ± 0.43 m/s vs [0.23 {0.52, 0.57}] m/s, p < 0.001) postoperatively were significantly lower than preoperative values (Figure 3 A1, B1, A2 and B2). There were no significant differences in OSI in the recurrent and the non-recurrent groups (Table 3).
Discussion
The persistence of endovascular embolization, whether coils only or stent-assisted coils embolization, has been a significant problem in treating IAs. The study on hemodynamic characteristics associated with MCABAs recurrence after total embolization showed that higher WSS and velocity at the aneurysm neck after embolization might be related to the recurrence of bifurcation aneurysms recurrence.
Wall shear stress, the frictional force produced by blood flowing tangentially on the vessel wall, is an extremely important hemodynamic parameter related to aneurysm initiation, progress, rupture and occurrence. For IAs formation, Metaxa et al reported that high WSS and positive WSS gradient, when they exceed a specific threshold, were associated with IAs initiation using eight rabbit models.15 Meng et al also demonstrated that high WSS and steep WSS gradients were conducive to destructive remodeling of the apical vessel wall and aneurysm initiation.16 The recurrence of aneurysms was similar to the initiation of aneurysms. For recurrence of incompletely occluded IAs, Irie et al and Liu et al reported that high WSS region and high blood flow velocity was found near the remnant neck coinciding with the location of aneurysm recanalization basing on a longitudinal hemodynamic analysis using CFD.17,18 Luo et al compared the hemodynamic parameters between stable and recanalized cases with partially occluded IAs. Also, they showed that the WSS in the remnant neck of unconsolidated embolized aneurysms were susceptible to the following recanalization at higher rates than other regions, indicating that higher WSS might play an essential role in IAs recurrence. Even for recurrence of completely occluded IAs,9 Li and Park et al still reported that higher WSS of the treated aneurysmal neck contributed to aneurysm recurrence after endovascular treatment.10,21 Higher WSS exceeding a physiological threshold could trigger cascades of biochemical signals through endothelium-mediated mechanotransduction and brought about internal elastic lamina loss, thinning of the media, and dilatation formation.19 In the present study, for MCABAs, also showed that the WSS, including SAWSS and MWSS, at the neck preoperatively were significantly higher than postoperative values in the recurrent group. However, in the non-recurrent group, we found that the WSS at the neck preoperatively was significantly lower than preoperative values in the recurrent group. The results of MCBAs were following previous studies.
Flow velocity is another hemodynamic parameter associated with IAs recurrence. Luo et al showed that higher blood flow velocity near the remnant neck of partially embolized aneurysms was associated with IAs recurrence.9 To control individual potential differences, Zhang et al used the reduction ratio (RR) of velocity as a normalized parameter instead of flow velocity.20 They found that, compared with the non-recanalization, the RR of velocity of the aneurysm neck was lower in the recanalization group. This suggested that elevated velocity after embolization was linked to IAs recurrence. There are many possible mechanisms explaining how high flow velocity causes IAs recurrence. First, comparatively high blood flow velocity might weaken the local blood clotting and thrombosis formation in the IAs sac. Second, the higher blood flow velocity at the neck implies a greater impact of local coils inside the IAs. These are more likely to cause compaction of the coils and final IAs recurrence. Furthermore, high flow velocity might prevent neointima from forming at the neck of IAs, which is considered as critical for IAs recurrence. In the present study, we also found that blood flow velocity in the recurrent group was higher than in the non-recurrent group. It was in line with the results of these studies.
There are some potential limitations in our study. First, although we compared hemodynamic between recurrent and non-recurrent groups using patient-specific MCABAs models using CFD, we did not accurately simulate the actual coils in the aneurysm sac during treatment. With the development of CFD, we believed that we could draw accurate results. Second, while we applied patient-specific MCABAs models in the CFD simulation, the inlet boundary conditions were not patient-specific and rigid wall, laminar flow, and Newtonian blood flow were used in patient-specific MCABA models for CFD analysis. In a way, these treatments might affect the accuracy of the results. Third, to establish MCABAs models, some small unimportant vessel branches distant from the MCABAs were deliberately managed. Such treatment might compromise our conclusions. Fourth, our study was a single-center study. Multicenter studies with a larger sample size were needed to validate our results. Fifth, according to the inclusion criteria, we totally selected 92 MCABAs. This was the first hemodynamic study with a large sample size associated with the recurrence of bifurcation aneurysms after total embolization; however, because of the recurrence rate ranged from 12.7% to 14% during when the embolization of aneurysms reached the standard of Raymond I,6 we compared groups of patients using substantial numbers (80 patients from the non-recurrent group and 12 patients). These facts might bias the results and conclusion. Sixth, in addition to hemodynamic parameters, some of these parameters, the application of stents or flow diversion, packing density, genetic factors, and histopathological mechanisms might play essential roles in the recurrence of aneurysms. The connection between these factors and aneurysm recurrence should be thoroughly investigated in the following studies. Finally, because of the complexity and time-consuming process of CFD research, the protocols need to be simplified to improve the simplicity and effectiveness of the process.
Conclusion
We studied hemodynamic characteristics associated with MCABAs recurrence after total embolization and found that higher WSS (SAWSS, MWSS) and blood flow velocity at the aneurysm neck after embolization might be related to recurrence of bifurcation aneurysms. These findings might aid assessment of the recanalization of bifurcation aneurysms for clinical post-embolization.
Acknowledgments
This work was supported by grants from Young and Middle-Aged Natural Science Foundation of Wannan Medical College (No. WK2021F26), Domestic Visiting Scholar Program for Excellent Young Talents in Colleges and Universities of Anhui Province (No. gxgnfx2021125), the Science Research Project of Professional of the First Affiliated Hospital of Wannan Medical College (No.YR201911), Anhui Provincial Natural Science Foundation (No. 2008085QH421), the Fundamental Research Funds for the Central Universities (WK9110000156), the key Scientific Research Projectof Wannan Medical College (No. WK2019ZF02) and Key Project of Natural Science Research of Anhui Universities of China (KJ2020A0610). Jinlong Yuan and Chenlei Huang are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest in this article.
References
1. Li MH, Chen SW, Li YD, et al. Prevalence of unruptured cerebral aneurysms in Chinese adults aged 35 to 75 years: a cross-sectional study. Ann Intern Med. 2013;159:514–521. doi:10.7326/0003-4819-159-8-201310150-00004
2. Korja M, Lehto H, Juvela S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke. 2014;45(7):1958–1963. doi:10.1161/STROKEAHA.114.005318
3. Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi:10.1056/NEJMoa1113260
4. Gallas S, Januel AC, Pasco A, et al. Long-term follow-up of 1036 cerebral aneurysms treated by bare coils: a multicentric cohort treated between 1998 and 2003. AJNR Am J Neuroradiol. 2009;30(10):1986–1992. doi:10.3174/ajnr.A1744
5. Sluzewski M, Menovsky T, van Rooij WJ, et al. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24(2):257–262.
6. Grunwald IQ, Papanagiotou P, Struffert T, et al. Recanalization after endovascular treatment of intracerebral aneurysms. Neuroradiology. 2007;49(1):41–47. doi:10.1007/s00234-006-0153-5
7. Yuan J, Huang C, Lai N, et al. Hemodynamic and morphological analysis of mirror aneurysms prior to rupture. Neuropsychiatr Dis Treat. 2020;16:1339–1347. doi:10.2147/NDT.S254124
8. Xiang J, Natarajan SK, Tremmel M, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 2011;42(1):144–152. doi:10.1161/STROKEAHA.110.592923
9. Luo B, Yang X, Wang S, et al. High shear stress and flow velocity in partially occluded aneurysms prone to recanalization. Stroke. 2011;42(3):745–753. doi:10.1161/STROKEAHA.110.593517
10. Li C, Wang S, Chen J, et al. Influence of hemodynamics on recanalization of totally occluded intracranial aneurysms: a patient-specific computational fluid dynamic simulation study. J Neurosurg. 2012;117(2):276–283. doi:10.3171/2012.5. JNS111558.
11. Sheng B, Wu D, Yuan J, et al. Hemodynamic characteristics associated with paraclinoid aneurysm recurrence in patients after embolization. Front Neurol. 2019;10:429. doi:10.3389/fneur.2019.00429
12. Zhang XJ, Hao WL, Zhang DH, et al. Asymmetrical middle cerebral artery bifurcations are more vulnerable to aneurysm formation. Sci Rep. 2019;9(1):15255. doi:10.1038/s41598-019-51734-4
13. Dhar S, Tremmel M, Mocco J, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008;63(2):
14. Ingebrigtsen T, Morgan MK, Faulder K, et al. Bifurcation geometry and the presence of cerebral artery aneurysms. J Neurosurg. 2004;101(1):108–113. doi:10.3171/jns.2004.101.1.0108
15. Metaxa E, Tremmel M, Natarajan SK, et al. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke. 2010;41(8):1774–1782. doi:10.1161/STROKEAHA.110.585992
16. Meng H, Wang Z, Hoi Y, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007;38(6):1924–1931. doi:10.1161/STROKEAHA.106.481234. Epub 2007 May 10.
17. Irie K, Anzai H, Kojima M, et al. Computational fluid dynamic analysis following recurrence of cerebral aneurysm after coil embolization. Asian J Neurosurg. 2012;7(3):109–115. doi:10.4103/1793-5482.103706
18. Liu J, Jing L, Wang C, et al. Recanalization, regrowth, and delayed rupture of a previously coiled unruptured anterior communicating artery aneurysm: a longitudinal hemodynamic analysis. World Neurosurg. 2016;89:
19. Meng H, Tutino VM, Xiang J, et al. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35(7):1254–1262. doi:10.3174/ajnr.A3558
20. Zhang Q, Jing L, Liu J, et al. Predisposing factors for recanalization of cerebral aneurysms after endovascular embolization: a multivariate study. J Neurointerv Surg. 2018;10(3):252–257. doi:10.1136/neurintsurg-2017-013041
21. Park W, Song Y, Park KJ, et al. Hemodynamic characteristics regarding recanalization of completely coiled aneurysms: computational fluid dynamic analysis using virtual models comparison. Neurointervention. 2016;11(1):30–36. doi:10.5469/neuroint.2016.11.1.30
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.