Back to Journals » International Medical Case Reports Journal » Volume 10
Hematopoietic stem cell transplantation in a severe refractory Crohn’s disease patient with intestinal stoma: a case report
Authors Ruiz MA , Kaiser Junior RL , de Quadros LG, Caseiro GHX, Oliveira AF, Peña-Arciniegas T, Piron-Ruiz L , Kaiser FSL , Oliveira VL
Received 13 April 2017
Accepted for publication 25 August 2017
Published 24 October 2017 Volume 2017:10 Pages 353—359
DOI https://doi.org/10.2147/IMCRJ.S139552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Milton Artur Ruiz,1 Roberto Luiz Kaiser Junior,2 Luiz Gustavo de Quadros,2 Gustavo Henrique Xavier Caseiro,2 Aderson Francisco Oliveira,1 Tatiana Peña-Arciniegas,1 Lilian Piron-Ruiz,1 Fernanda Soubhia Liedtke Kaiser,2 Vera Lucia Oliveira2
1Bone Marrow Transplant Department, Associação Portuguesa de Beneficencia de São José do Rio Preto, 2Kaiser Clinica, Centro Médico Avançado de São José do Rio Preto, Sao Paulo, Brazil
Background: Hematopoietic stem cell transplantation (HSCT) can be used in the treatment of patients with refractory Crohn’s disease (CD) when no alternative treatment is available. However, HSCT increases the risk of infections, in particular during the aplasia of mobilization and conditioning. Moreover, intestinal stomas in CD augment the risk of morbidity in immunocompromised patients and under aplastic conditions. The objective of this report was to describe the results of the first year after HSCT in a CD patient with an intestinal stoma.
Methods: The patient was assessed in respect to disease symptoms and endoscopic findings before the procedure and 30, 90, 180, and 365 days after HSCT.
Results: No complications were observed during mobilization and conditioning with sufficient CD34+ cells being harvested in just one apheresis session. Toxicity was restricted to the hematological series. Scores of all the CD indexes and the quality of life of the patient improved. However, two of three endoscopic scores remained unchanged even though improvements were found in the appearance of the lesions.
Conclusion: HSCT may be an alternative treatment for refractory CD in patients with an intestinal stoma, and a priori, carefully selected patients with stomas should not be excluded as candidates for this procedure.
Keywords: Crohn’s disease, intestinal stoma, hematopoietic stem cell transplant, stem cell therapy, autologous HSCT, autoimmune diseases, nonmyeloablative HSCT
Introduction
Crohn’s disease (CD), a chronic, refractory inflammatory bowel disease that can coexist with other autoimmune diseases, affects the whole digestive tract causing intestinal and extraintestinal manifestations.1 Intestinal stomas constructed from the small bowel or colon are commonly needed by patients with CD. Temporary stomas are used to allow an anastomosis to heal or an inflammation to subside.2
Complications of ileostomy, including abscess, stenosis, allergy, edema, irritative or mechanical trauma, hemorrhage, dermatitis, necrosis, folliculitis, parastomal herniation, prolapse, retraction, and parastomal varices, can occur soon after or many years after the procedure.3 According to Leong et al, the incidence of stomal complications reported in a 20-year follow-up of CD patients was about 59%; the most common complications were skin problems (34%), intestinal obstruction (23%), retraction (17%), and parastomal herniation of the oblique muscles (16%).4
The risk of a permanent stoma in CD patients with colitis and anorectal involvement is significantly reduced when surgical and biological treatments are combined.5
Hematopoietic stem cell transplantation (HSCT) was first used by Drakos et al in 1993 in a patient with CD who evolved to Hodgkin’s lymphoma.6
The Autoimmune Disease Working Party of the European Society for Blood and Marrow Transplantation (EBMT) suggests that patients are candidates for HSCT when active disease, refractoriness, and the imminent need of further surgery are proven.7 Comorbidities and infectious diseases are contraindications to HSCT, but there is no mention in the literature about the possibility of performing the procedure when CD patients have stomas.
Even so, HSCT is a procedure that increases the risk of infection, in particular during the aplasia of the mobilization and conditioning phases.8 The objective of this report was to describe the results of the first year after HSCT in a CD patient with an intestinal stoma.
Methods
Following the recommendations of the CAse REport guidelines for reporting case reports or case series, we describe herein the case report of a patient with CD and a permanent intestinal stoma who was submitted to HSCT.9
CD activity was assessed by subjectively reported symptoms and the results of laboratorial and endoscopic evaluations using the Crohn’s disease activity index (CDAI),10 Harvey–Bradshaw index (HBI), and Craig Crohn’s severity index (CSI). The following endoscopic evaluation instruments were also used during the assessment for the purpose of identifying colonoscopic changes: Crohn’s disease endoscopic index of severity (CDEIS), simple endoscopic score for Crohn’s disease (SES-CD),11 and Rutgeerts’ score to evaluate the anastomosis after ileocolic resection.12 Clinical evaluations and laboratory tests were performed before transplantation and 30, 90, 180, and 365 days after the procedure.
The remission scores of the above indexes are as follows: CDAI <150, HBI <5, CSI <17, CDEIS <3, SES-CD <2, Rutgeerts’ scale <i1, and Truelove–Witts index <10. Furthermore, the patient answered two questionnaires, the Medical Outcomes Study Questionnaire Health Survey – Short Form 36 (SF-36)13 and the Inflammatory Bowel Disease Questionnaire (IBDQ), to investigate possible improvements in the quality of life (QoL) of the patient after the procedure.13
Ethical considerations
The patient, on meeting the criteria for HSCT, was given information regarding the risks of the procedure and the expected evolution and prognosis. She accepted to participate in the study of the Auto Crohn Project and signed an informed consent form prior to any data collection giving permission to publish case details and images. The project was approved by the Institutional Review Board of the Hospital Associaçao Portuguesa de Beneficencia in Sao Jose do Rio Preto, Sao Paulo, Brazil, and the Auto Crohn Project was registered as a clinical trial (NCT #03000296).
Case report
A 40-year-old woman reported digestive symptoms with constipation in 1990 followed by a fecaloma. Ten years later, she presented significant weight loss, accompanied by vomiting, fetid diarrhea, and increased abdominal volume. The diagnosis of CD was reached 1 year later with the worst disease being identified in the colon. Five years later, the patient was submitted to surgeries to repair retrovaginal fistulas.
Throughout this period, she was treated with anti-inflammatory drugs, immunosuppressant drugs, and biological agents including infliximab, adalimumab, and certolizumab pegol, but without clinical improvement. Eventually she became dependent on corticosteroids. In 2010, after the loss of response to infliximab, she evolved with sigmoid stenosis and perirectal fistulas. She was submitted to total colectomy with the rectal stump being preserved and ileostomy with the cut end diverted to the right, where it remains until today.
After this surgery, she continuously suffered from ulcers and lesions in the ileum, and changes were seen in the entire achievable extension of colonoscopy, which were subsequently confirmed by computed tomography enterography. In the initial examination before HSCT, the patient reported severe abdominal pain for which she took narcotics; she had many internal hemorrhages with bloody stools. Furthermore, she presented fatigue, anorexia, nausea, and vomiting, together with joint and epigastric pain.
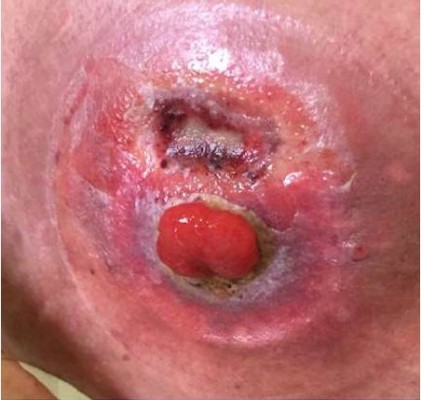
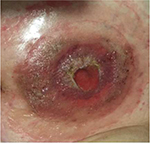
At the physical examination, she was extremely emaciated, ~12 kg less than her ideal weight. She had an intestinal stoma with intense peristomal dermatitis (Figure 1). She had elevated CDAI, HBI, and CSI scores proving active disease and impaired QoL based on the SF-36 and IBDQ. It was difficult to perform colonoscopy through the ileostomy because of the peristomal skin injuries. Moreover, this examination identified distal ileal stenosis, and so digital dilatation was used to permit the passage of the device. Multiple deep and serpiginous ulcers were observed in addition to other stenoses (Figure 2). Endoscopic scores were high (CDEIS =34; SES-CD =11) characterizing severe endoscopic activity, and the Rutgeerts score (i4) indicated disease recurrence after surgery (Table 1). Esophagogastroduodenoscopy was normal. Laboratory data showed anemia, the inflammatory activity test was positive with elevated erythrocyte sedimentation rate and C-reactive protein and β2-microbulin serum levels, calprotectin was positive, and albumin and vitamin D levels were below normal.
  | Figure 1 Dermatitis around the stoma before hematopoietic stem cell transplantation. |
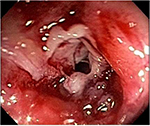  | Figure 2 Pretransplant lesions. |
HSCT was suggested even though initially there was a concern related to the stoma and the risks involved in performing this procedure. Without any other option of treatment and with further surgery imminent, the patient accepted to be included in the Auto Crohn Project of the institution.
Mobilization phase
A central venous catheter was inserted prior to starting the procedure. Cyclophosphamide (Cy; 50 mg/kg/day) was administered for mobilization followed 5 days later by granulocyte colony-stimulating factor (G-CSF; 10 mcg/kg/day) which was maintained until harvesting the peripheral blood stem cells (PBSCs). Concomitant to the administration of Cy, 2-mercaptoethanesulfonic acid (MESNA) was administered at the same dose as Cy. The prophylactic therapy during this phase included antiparasitic, antiviral, and antifungal drugs. During neutropenia (neutrophils <0.5×109/L), the patient received intravenous cefepime (1 g bid). The goal was to achieve 3.5×106 CD34+ cells/kg in order to perform the HSCT safely. The harvested PBSCs were cryopreserved at −87°C (−124.6°F) with no product selection or manipulation. At the end of the harvesting, the patient rested for 10 days before the conditioning phase.
Conditioning phase
The aim of conditioning was to provide sufficient immunoablation to reset the immune system. The conditioning regimen consisted of Cy (200 mg/kg/total dose) and rabbit antithymocyte globulin (rATG; 6.5 mg/kg/total dose) administered for 4 days prior to the reinfusion of the PBSCs. Methyl prednisolone (500 mg/day) was also administered prior to the administration of rATG in order to prevent adverse reactions. The risk of toxicity leading to conditions such as hemorrhagic cystitis was decreased through the administration of MESNA at the same dose as the Cy as described for the mobilization period. During neutropenia, G-CSF was administered at a dose of 10 μg/kg/day and continued until the absolute neutrophil and platelet counts reached 0.5×109/L and 20×109/L, respectively, on 2 consecutive days.
Supportive care in mobilization and conditioning phases
During the 4 days prior to the infusion of PBSCs, sulfamethoxazole and trimethoprim at 400 mg and 80 mg per os (PO), respectively, were administered tid for prophylaxis against Pneumocystis jirovecii. Other medications prophylactically administered included ciprofloxacin (500 mg PO bid), metronidazole (250 mg PO tid), acyclovir (200 mg PO tid), and fluconazole (150 mg PO bid). Once neutropenia occurred, intravenous cefepime (1 g intravenously bid) was administered. The criterion for the transfusion of blood components was determined by hematocrit or hemoglobin values <25% and 7.0 g/dL, respectively, whereas the administration of platelets was indicated when the count was <20×109/L. All blood components were deleucotized and irradiated prior to transfusion.
Data were collected during the different stages of the HSCT procedure: at hospital admission before mobilization and during the follow-up phase at 30, 90, 180, and 365 days posttransplant.
Results
Table 1 summarizes the patient’s data. Note that the CDAI, Truelove–Witts, HBI, and CSI scores improved within 30 days of HSCT and remained better throughout the 1-year follow-up. The IBDQ improved in all domains, and the SF-36 improved in all domains with significant results for general health perception, vitality, social functioning, emotional aspects, and mental health. In fact, the QoL of the patient remains better 1 year after HSCT. All pain disappeared after the procedure.
A total of 19.4×106 CD34+ cells/kg were harvested in one apheresis session on Day 10 of mobilization and engraftment, defined as neutrophil recovery (neutrophil count >0.5×109 cells/L) for 2 consecutive days, occurred on the 10th day after HSCT. The toxicity of HSCT was restricted to the hematological series with the patient requiring one unit of packed red blood cells during mobilization and two units during the conditioning phase. Moreover, the patient required three units of platelet concentrates in the conditioning phase.
No complications were observed during mobilization and conditioning, not even fever during neutropenia (4 days during mobilization and 8 days in the conditioning phase). However, the inflammation of the stoma injury was more intense on the day of grafting (Figure 3).
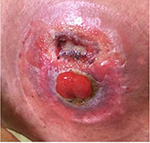  | Figure 3 An increased degree of inflammation of the stomal lesion seen on the day of grafting. |
Table 2 summarizes the results of laboratory tests and the scores of the endoscopic tests. No relevant change was seen in respect to the patient’s weight, before and 1 year after HSCT. There was a slight improvement in parameters indicating the inflammatory status (C-reactive protein, erythrocyte sedimentation rate, and β2-microglobulin), and the hemoglobin and albumin levels are now within reference ranges.
The CDEIS of 34 before HSCT was classified as severe endoscopic activity. However, 1 year later, this score had improved significantly, characterizing moderate activity. Conversely, the score of Rutgeerts’ scale remained the same 1 year after HSCT as before the procedure. The examination performed through the ileostomy prior to HSCT was very difficult because the colonoscopy did not progress sufficiently to gain an adequate view due to the external lesions and stenosis. One year later, there was a marked improvement, and it was possible to see the lesions better. Thus, as the score related to the circumference of the ulcers did not change substantially, the disease persists from the endoscopic point of view even though it was much easier to perform the examination, and there was a better endoscopic appearance showing healing (Figure 4).
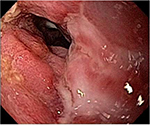  | Figure 4 Healing of lesions 1 year after transplant. |
Discussion
The strengths of this case study are the results observed in respect to the QoL, the symptoms related to the stoma, and the absence of complications during HSCT with improvement in the intestinal stoma lesions. An important feature was that apart from the expected hematological toxicity, no complications such as fever or infection during aplasia or worsening of the patient’s stoma lesions were observed. There was much concern because it is known that intestinal stomas can increase morbidities in immunocompromised patients and under aplastic conditions with descriptions of cases of neutropenic enterocolitis in some patients.14–16
Improvements were seen in several domains of the QoL instruments, particularly in the SF-36, where general health perception, vitality, and emotional and mental aspects were significantly better within 30 days and 1 year after the procedure.
Some limitations of this case study should be considered, in particular relating to the scores determining disease activity such as the CDAI and HBI. Often a colostomy makes it difficult to gauge the number of daily evacuations with the information obtained from patients being vague and sometimes restricted to the number of bag exchanges during the day or to the feces discarded by the patient. Another issue to be evaluated relates to the evolution of colonoscopy over this period with no significant changes in two of the three endoscopic scores used. However, the patient reported the disappearance of asthenia, pain, disease flares, and all associated symptoms with improvements in all scores of disease activity showing that 1 year after the procedure the patient was in clinical remission, but without endoscopic remission.
Autologous HSCT in CD patients with no alternative treatment has provided beneficial results, with patients being selected using the criteria proposed by the EBMT.17–23 Refractoriness to anti-inflammatory, immunosuppressant, and biological agents; disease activity; and the imminence of further surgery are used to indicate a patient for HSCT according to these criteria. The procedure is considered safe with low toxicity, most often restricted to the hematological series in the mobilization and conditioning phases. In the current case, the patient was suffering from complications due to a stoma. This was taken into account in the indication of the procedure because of the possible risk of infections during aplasia.8 Different from other studies, a low dose of Cy was used for mobilization with harvesting by leukapheresis.21,23 The patient had no problems in obtaining sufficient CD34+ cells, and she should be considered a supermobilizer. No relevant toxicities occurred at this stage, and a small amount of blood components was used as mentioned above. No side effects were observed in the conditioning phase either with Cy or with rATG as described in other reports.21 However, the important factor was the improvements in the stoma injury without problems during the procedure with the patient happy throughout the HSCT and during the follow-up. Adequate care and guidance with the use of a better quality wafer and pouch solved the stoma injury with this improving patient’s QoL greatly 1 year after the procedure (Figure 5).
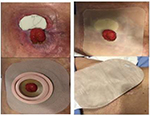  | Figure 5 Treatment of stomal lesion. |
The main “take-away” lesson of this case report is that HSCT can be performed in refractory CD cases with intestinal stomas when no alternative treatment exists, but only by an experienced team using a multidisciplinary approach to care for the patient.
Conclusion
Thus, the presence of an intestinal stoma, even with peristomal lesions and dermatitis, does not seem to prejudice the outcome of HSCT. HSCT may be an alternative treatment for refractory CD associated with an intestinal stoma, and a priori, patients with stomas should not be excluded from performing this procedure. Now the patient says that she is much better and has a new perspective of life.
Disclosure
The authors report no conflicts of interest in this work.
References
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–1605. | ||
Martin ST, Vogel JD. Intestinal stomas: indications, management, and complications. Adv Surg. 2012;46:19–49. | ||
Harilingam M, Sebastian J, Twum-Barima C, et al. Patient-related factors influence the risk of developing intestinal stoma complications in early post-operative period. ANZ J Surg. Epub 2015 Dec 3. | ||
Leong AP, Londono-Schimmer EE, Phillips RK. Life-table analysis of stomal complications following ileostomy. Br J Surg. 1994;81:727–729. | ||
Coscia M, Gentilini L, Laureti S, et al. Risk of permanent stoma in extensive Crohn’s colitis: the impact of biological drugs. Colorectal Dis. 2013;15(9):1115–1122. | ||
Drakos P, Nagler A, Or R. Case of Crohn’s Disease in bone marrow transplantation. Am J Hematol. 1993;43(2):157–158. | ||
Snowden JA, Saccardi R, Farge D. Autoimmune diseases. In: Apperley J, Carreras E, Gluckman E, Masszi T, editors. EBMT-ESH Handbook on Haematopoietic Stem Cell Transplantation, 6th ed; ESH EBMT 2012. | ||
Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012;71(4 Pt 2):439–444. | ||
Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guidelines development. J Clin Epidemiol. 2014;67:46–51. | ||
Naber AH, de Jong DJ. Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials. Neth J Med. 2003;61(4):105–110. | ||
Khanna R, Nelson SA, Feagan BG, et al. Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev. 2016;(8):CD010642. | ||
Daperno M, Comberlato M, Bossa F, et al. Inter-observer agreement in endoscopic scoring systems: preliminary report of an ongoing study from the Italian Group for Inflammatory Bowel Disease (IG-IBD). Dig Liver Dis. 2014;46(11):969–973. | ||
Ciccocioppo R, Klersy C, Russo ML, et al. Validation of the Italian translation of the Inflammatory Bowel Disease Questionnaire. Dig Liver Dis. 2011;43(7):535–541. | ||
Rokhsar S, Harrison EA, Shaul DB, Phillips JD. Intestinal stoma complications in immunocompromised children. J Pediatr Surg. 1999;34:1757–1761. | ||
Ullery BW, Pieracci FM, Rodney JRM, Barie PS. Neutropenic enterocolitis. Surg Infect. 2009;10(3):307–314. | ||
Martin ST, Vogel JD. Intestinal stomas indications, management and complications. Adv Surg. 2012;46:19–49. | ||
Cassinotti A, Annaloro C, Ardizzone S, et al. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn’s disease. Gut. 2008;57:211–217. | ||
Burt RK, Craig RM, Milanetti F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116(26):6123–6132. | ||
Hommes DW, Duijvestein M, Zelinkova Z, et al. Long-term follow-up of autologous hematopoietic stem cell transplantation for severe refractory Crohn’s disease. J Crohns Colitis. 2011;5(6):543–549. | ||
Snowden JA, Ansari A, Sachchithanantham S, et al. Autologous stem cell transplantation in severe treatment-resistant Crohn’s disease: long-term follow-up of UK patients treated on compassionate basis. QJM. 2014;107(11):871–877. | ||
Jauregui-Amezaga A, Rovira M, Marín P, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut. 2016;65(9):1456–1462. | ||
Ruiz MA, Kaiser Junior RL, Gouvea Faria MA, de Quadros LG. Remission of refractory Crohn’s disease after autologous hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2015;37(2):136–139. | ||
Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoietic stem cell transplantation for refractory Crohn’s disease: a randomized clinical trial. JAMA. 2015;314(23):2524–2534. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


