Back to Journals » Journal of Blood Medicine » Volume 11
Hematological Abnormalities and Associated Factors Among Undernourished Under-Five Children Attending University of Gondar Specialized Referral Hospital, Northwest Ethiopia
Authors Getawa S , Getaneh Z , Melku M
Received 29 September 2020
Accepted for publication 5 December 2020
Published 18 December 2020 Volume 2020:11 Pages 465—478
DOI https://doi.org/10.2147/JBM.S284572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Solomon Getawa, Zegeye Getaneh, Mulugeta Melku
Department of Hematology and Immunohematology, School of Biomedical and Laboratory Science, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Solomon Getawa
Department of Hematology and Immunohematology, School of Biomedical and Laboratory Science, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Tel +251-914-665-736
Email [email protected]
Introduction: The hematopoietic system is one of the systems which can be affected by malnutrition, leading to impaired production of all blood cell lines. Undernourished children with hematological abnormalities like anemia are at higher risk of mortality. Therefore, this study aimed at determining the magnitude and associated factors of hematological abnormalities among undernourished under-five children attending at the University of Gondar Specialized Referral Hospital, Northwest Ethiopia.
Methods and Materials: An institution-based cross-sectional study was conducted on a total of 251 undernourished under-five children at University of Gondar Specialized Referral Hospital from January to May 2020. A convenient sampling technique was employed to select study participants. Data were collected using a pretested structured questionnaire. Blood samples were collected for complete blood count which were determined by Sysmex KX-21N analyzer. Stool sample was processed via direct wet mount. Thin and thick blood films were examined to assess malaria parasite. The data were entered to EpiData version 4.6.0.0 and analyzed using SPSS version 23 software. Bi-variable and multi-variable binary logistic regression model were fitted to identify factors associated with hematological abnormalities. A p-value < 0.05 in the multivariable analysis was considered as statistically significant.
Results: The overall magnitude of anemia, leukocytosis, thrombocytosis, thrombocytopenia, and leukopenia was 53.4%, 26.7%, 23.9%, 8%, and 2.8%, respectively. Being male, age 6– 23 months, high birth order, intestinal parasite infection, edema, not eating vegetables and fruits, and paternal occupation were found to be associated with anemia. Only the age of a child was associated with leukocytosis in undernourished children.
Conclusion: The current study demonstrated the predominant existence of anemia, leukocytosis, and thrombocytosis among undernourished under-five children. Therefore, early diagnosis, monitoring and setting intervention strategies for anemia especially among children under two years old are required to prevent further complication.
Keywords: anemia, leukocytosis, thrombocytosis, leukopenia, thrombocytopenia, children, undernutrition, Ethiopia
Introduction
Protein-energy malnutrition (PEM) is a pathological conditions resulted from lower ingestion of various proportions of protein, calories, and micronutrients.1 It is a major health concern particularly in developing countries, mainly in under-five children.2,3 Globally in 2019, 144 million (21.3%) and 47 million (6.9%) under-five children suffer from stunting and wasting, respectively. Of them, 40% and 27% of stunted and wasted children lived in Africa, respectively.4 The prevalence of stunting, wasting, and underweight was 37%, 7%, and 21%, respectively, in Ethiopia.5 Undernutrition includes underweight (Z score of weight-for-age (WAZ) <−2), stunting (Z score of height-for-age (HAZ) <−2), and wasting (Z score of weight-for-height (WHZ) <−2).6 Children suffering from stunting and wasting may not attain their full possible height and weight; and may have shown an impaired cognitive potential, weakened immunity, and developmental delays.4
PEM results in widespread alterations in organ and system function.1,7 In the hematological system, the changes affect all blood cell lines. This hematological alteration includes anemia, changes in reticulocyte count, leukocytosis, and changes in the hematopoietic microenvironment of the bone marrow (BM) have been documented in previous studies.1,3,8,9 In an animal model, PEM produces alterations in hematopoiesis that are caused by impairment of the BM stroma and alterations in the hematopoietic progenitors’ cell cycle with a higher number of cells in the G0/G1 phase.10 PEM alters granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor production by macrophages which leads to diminished immune response and blood cell formation and maturation.11 It also alters lymphohematopoietic organs like BM, spleen, and thymus which leads to anemia, leukopenia, alters the immune system, and increases susceptibility to infections.12
Low red blood cell (RBC) count resulting anemia has a common feature of PEM. The need for protein and energy during erythropoiesis by BM justifies the occurrence of anemia in malnutrition.13 A decrease in erythropoietin, occurring due to the reduced ingestion of protein and reduced RBC production in adaptation to a smaller lean body mass may also be responsible for anemia.14,15 Amazingly, the prevalence of anemia among this population reaches to 97%.16 Stunted, underweighted, and wasted children are more likely to be anemic than well-nourished children.17 Studies revealed that severely malnourished children with anemia are at higher risk (2.62 times) of mortality compared to non-anemic.18,19 It is a leading cause of pediatric morbidity, hospitalization, and mortality. Moreover, anemia affects the cognitive performance and physical growth of the children.20 Anemia in PEM is unlikely to respond to treatment unless the associated infection is brought under control.15 The cause of anemia in PEM children may be due to iron and folate deficiency,16,21,22 vitamin A deficiency, protein deficiency,23 hemolysis due to an alteration in the antioxidant mechanisms in erythrocytes, infection, and infestation with parasites.24,25
Leukopenia and leukocytosis that occurs in PEM as a result of an infection by establishing a malnutrition–infection relationship in which the poor nutritional status disrupts leukopoiesis.26 The occurrence of leukocytosis in PEM is usually accompanied by infectious processes or chronic disease.9,27 Leukopenia is present in children with PEM if they are not accompanied by other diseases. Leukopenia was observed in children with acute malnutrition in up to 9% of cases.28 It is characterized not only by a decrease in the leukocyte count but also by impaired processes carried out by the leukocytes. Mainly in newborns and small children, PEM provokes thymus atrophy that leads to leukopenia, decreases CD4/CD8 ratio, increases number of immature T cells in the periphery, and impaired phagocytosis by macrophages.29 This in turn may cause immune suppression and increase susceptibility to infection. This may put a child at greater risk of dying from such infections, increase hospitalization dates, and contribute to delayed recovery.1
In children with malnutrition, a decreased platelet count may be attributed to a decrease in BM activities which indirectly affect megakaryocyte functions.30,31 This may also be due to platelet activation with an infection like malaria which shortened the life span of platelet during platelet pathogen interaction and pathogens can induce removal of platelets from the circulation by triggering their clearance through phagocytosis.32 Thrombocytosis is another hematological alteration in undernourished children that could be associated with infection.33 In Ethiopia, undernutrition is a major public health problem and a preliminary diagnosis in 20% of pediatric hospital admissions.34 The fatal effect of the disease in relation to hematological features is one concern where attentions have not yet got. Therefore, this study aimed at determining hematological abnormalities and associated factors among undernourished under-five children attending at the University of Gondar Specialized Referral Hospital.
Methods and Materials
Study Design, Period and Area
An institution-based cross-sectional study was conducted at the University of Gondar Specialized Referral Hospital from January to May 2020. The hospital is located in the central Gondar zone, Gondar town, Amhara Regional State. It is located at 738 km far from the capital city of Ethiopia, Addis Ababa, and 180 km far from the capital city of the region, Bahir Dar. The town is situated at 2133 meters elevation above sea level. Currently, the town has one referral hospital, five government health centers, and more than 45 private clinics. The hospital provides different medical services for more than 7 million people in the zones and peoples of the neighboring zones through different wards and outpatient departments. It has 512 beds, of which 70 beds are allocated to the pediatric ward. This ward has a separate room or center for the treatment of malnourished children.35
Population
All undernourished under-five children who were attending at the University of Gondar Specialized Referral Hospital were taken as the source population whereas, undernourished under-five children who attended at the University of Gondar Specialized Referral Hospital during the study period, and willing to participate in the study were considered as the study population.
Inclusion and Exclusion Criteria
All under-five children diagnosed as undernourished and fulfill criteria for severe acute malnutrition (SAM) treatment (WHZ less than −3 z-score); for moderate acute malnutrition (MAM) treatment (WHZ ≥-3 to less than −2 z-score), children with z-score below −2 standard deviations for their WAZ and HAZ, and before receiving treatment at the University of Gondar Specialized Referral Hospital were included in the study. But undernourished children who had been receiving iron and vitamin supplements in the last four weeks, children who received a blood transfusion in the last three months, and children who had been confirmed chronic diseases like renal failure, cancer, and liver diseases were excluded. Children with tuberculosis and human immunodeficiency virus were also excluded from the study.
Sample Size Determination and Sampling Technique
The sample size calculation was based on a single population proportion formula, [n = (Z α/2)2 p (1-p)/d2]. By taking the prevalence of anemia (61%) among undernourished (stunted) children which was conducted in Ethiopia.17 The total sample size was calculated as follows: n= (Z α/2)2p (1-p)/d2 = (1.96)2 x 0.61 (1–0.61)/(0.05)2 then ni =366. But, the estimated number of undernourished children attending the hospital is <10,000. For such situation, finite population correction formula is recommended and the sample size was adjusted as follows: nf = ni/1+ni/N = 366/1+366/800 = 251. Where, d = margin of error between the sample and the population (d=5%), ni = initial sample size, nf = final sample size, N = total sample size of the target population, Z = 95% confident interval, Zα/2= 1.96 and P=61%. A convenience sampling technique was used to select study participants.
Operational Definitions
Anemia: defined based on the altitude adjusted hemoglobin (Hb) values less than 11 g/dl for children <5 years old and its severity is classified as Mild anemia: if Hb is between 10 and 10.9 g/dl, Moderate anemia: if Hb level is between 7 and 9.9 g/dl and Severe anemia: if Hb level of less than 7 g/dl.36 Morphologically based on mean corpuscular volume (MCV), anemia is classified as microcytic (MCV <80 fl), normocytic (MCV between 80 and 100 fl), and macrocytic (MCV>100 fl).37 Thrombocytopenia is defined as platelet count below 150,000/mm3 while thrombocytosis is defined as platelet count above 450,000/mm3. Moreover, leukopenia is defined as a WBC count below 4000/mm3 while, leukocytosis is defined as a WBC count above 12,000/mm3.38
Data Collection and Laboratory Methods
Socio-Demographic, Feeding, and Clinical Data
A written informed consent was obtained from the parents. A detailed and thorough history along with complete anthropometry and physical examination was done. Feeding characteristics were collected based on a food frequency questionnaire that was used previously in Ethiopia and modified based on the reviewed literature.39 The frequency variable coded as never, once a month, 2–3 times a month, once a week, 2–3 times a week, and at least once a day. Data on socio-demographic characteristics of children, feeding characteristics, breastfeeding status, complimentary food supplementation status, and characteristics of parents/guardian were collected using structured pre-tested questionnaire via face-to-face-interview of children’s parents/guardians. The aforementioned data were collected by trained clinical nurses working in the nutritional rehabilitation center.
Hematological Analysis
Under aseptic conditions, 3 mL venous blood was collected by venipuncture from superficial veins of the antecubital fossa. After the blood specimen collection, then transferred into an ethylene diamine tetraacetate (EDTA) test tube and gently mixed to prevent clotting. The blood specimen was analyzed using Sysmex KX-21N automated hematology analyzer to determine complete blood count.
Parasitological Examination
From each study participant, pea-size (1gm) fresh stool samples were collected following the standard operating procedures (SOPs) in clean and labeled leak-proof stool cups. Stool specimens were processed by the wet mount technique. A small amount of stool sample was put on microscopic slides and a drop of normal saline was added. Then, wooden applicator sticks were used to mix the sample and the saline, and cover glass was applied. Finally, using 10x and 40x microscope objectives helminths eggs, larvae, and cysts of protozoan parasites were assessed. Children who were febrile were screened for malaria parasites. Thick and thin blood smear slides were prepared, stained with Giemsa (10%), and examined microscopically for malaria parasites. Malaria was excluded when thick blood films were reported negative after examining 100 fields under 100x oil immersion objective.
Data Quality Control
To ensure data quality, the questionnaire had been prepared in English and translated to the local language (Amharic) and then re-translated back to English to see its consistency and pre-testing was done to ensure its validity. The performance of the automated hematology analyzer was checked by running three levels of (Normal, Low, and High) controls. Known malaria-positive and negative blood smear were used to prepare quality control (QC) materials for thick and thin films to check the quality and performance of the Giemsa stain, as well as stool examination, was performed after checking the working normal saline solution.
Data Analyses and Interpretation
Data were entered into EpiData version 4.6.0.0 statistical software then exported to Statistical Package for Social Sciences (SPSS) version 23 software for analyses. Descriptive statistics (mean, median, frequency, percentage) were used to summarize the characteristics of study participants; and the results were presented in tables and charts. Shapiro–Wilk test was done to check the normality of data. A binary logistic regression model was fitted to identify factors associated with hematological abnormalities. The Hosmer-Lemeshow goodness-of-fit test was used to assess the fitness of the model. Independent variables having a p-value less than or equals to 0.2 in bi-variable analyses were included in the multi-variable analyses to control confounders. Both crude odds ratio (COR) and adjusted odds ratio (AOR) with the corresponding 95% confidence interval (CI) were calculated to show the strength of association. P-value <0.05 in the multi-variable binary logistic regression model was considered as statistically significant.
Ethical Approval and Consent to Participate
Ethical approval was obtained from University of Gondar, College of Medicine and Health Sciences, School of Biomedical and Laboratory Sciences, Research and Ethical Review Committee (Reference number SBMLS/2438/12) before data collection. Permission letter to conduct the study were obtained from the University of Gondar Specialized Referral Hospital Chief Executive Officer. Written informed consent was obtained from the child’s parents. To ensure confidentiality, study subjects were identified using codes, and only authorized persons accessed the collected data. Study participants who had abnormal results were notified to the physician and nurse who are working at the nutritional center for proper patient care.
Results
Socio-Demographic Characteristics
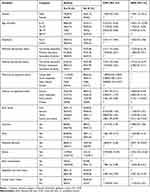
A total of 251 undernourished under-five children were participated in the study; of the participants, 126 (50.2%) were males. The age of study participants ranged from 6 to 59 months. The median interquartile range (IQR) age of the study participant was 19 months (10–35). Regarding their age distribution, 151 (60.2%) were between 6 and 23 months, and 38 (15.1%) were between 24 and 35 months. Parents/guardians of the participating children were interviewed. Out of 251 respondents, 133 (53%) were urban dwellers and 217 (86.5%) were married. Regarding their educational status, 122 (48.6%) of their mothers and 98 (39%) fathers had no formal education. The majority, 199 (79.2%), of mothers were housewives and 108 (43%) of fathers were farmers (Table 1).
Clinical and Feeding Characteristics
Among 251 study participants, 138 (55%) and 141 (56.2%) had diarrhea and fever in the last 2 weeks before data collection, respectively. Edema was present in 14 (5.6%) of children. The majority, 216 (86.1%), of children were exclusively breastfed in the first 6 months of their life. Forty-three (17.1%) and 6 (2.4%) of the children were infected with intestinal and malaria parasites, respectively. Out of 43 children infected with intestinal parasites, 14 (32.5%) and 8 (18.6%) of children were infected with Ascaris lumbricoides and Hookworm parasites, respectively (Table 2).
 |
Table 2 Clinical and Feeding Characteristics of Undernourished Under-Five Children Attending at the University of Gondar Specialized Referral Hospital, Northwest Ethiopia, 2020 |
Magnitude of Hematological Abnormalities
The altitude adjusted Hb level among undernourished children ranges from 1.9g/dl to 16.4 g/dl, with a median (IQR) value of 10.08 g/dl (9.5–11.8). The overall magnitude of anemia as defined by Hb levels lower than 11 g/dl adjusted for altitude above sea level was found to be 53.4% (95% CI: 47.2–59.6%). Of them, 74 (55.2%) were males and 76 (56.7%) were from rural dwellers. The highest frequency of anemia was among children aged groups 6–23 months (73.1%) while the least was among children age 36 to 47 months (5.2%). The range of WBC count was 2.2 to 25×103 cells/μL, with a median (IQR) value of 9×103cells/μL (6.5–12.6). Leukopenia was detected in 2.8% (95% CI: 0.74–4.84%), whereas leukocytosis was detected in 26.7% (95% CI: 21.18–32.20%) of the children. The range of platelet count was 27 to 781×103 cells/μL, with a mean (±SD) value of 349.3±145.03×103 cells/μL. Thrombocytopenia was detected in 8% (95% CI: 4.59–11.34%), whereas thrombocytosis was detected in 23.9% (95% CI: 18.59–29.22%) of the children (Table 3 and Figure 1).
 |
Table 3 Hematological Parameters of Undernourished Under-Five Children Attending at the University of Gondar Specialized Referral Hospital, Northwest Ethiopia, 2020 |
 |
Figure 1 Magnitude of hematological abnormalities among undernourished under-five children, University of Gondar Specialized Referral Hospital, 2020. |
Severity and Morphological Classification of Anemia
The severity of anemia was classified based on their Hb levels. Of 134 anemic children, 17.2% were found to be severely anemic, whereas 44% and 38.8% were moderately and mildly anemic, respectively. Morphologically based on their MCV obtained from the complete blood count result, the most common type of anemia was microcytic anemia (73.1%), followed by normocytic anemia (25.4%). Two children (1.5%) had macrocytic anemia (Figure 2).
 |
Figure 2 Type of anemia among undernourished under-five children, University of Gondar Specialized Referral Hospital, 2020. |
Anemia and Associated Factors
To determine the association, bi-variable binary logistic regression analyses was done. Based on the analyses, children aged 6–23 months, male in sex, being infected with intestinal parasite, being fourth and above in birth order, fever, having edema, not eating meat, not eating vegetables and fruits, being rural in residence, being children of fathers with no formal education, and being children of fathers who are farmers, and daily laborer was significantly associated with the magnitude of anemia. However, in the multi-variable binary logistic regression analyses model, being male (AOR= 1.99; 95% CI: 1.10–3.61), being aged between 6 and 23 months (AOR= 5.45; 95% CI: 2.13–13.93), being fourth and above in birth order (AOR= 3.01; 95% CI: 1.20–7.54), intestinal parasite infestation (AOR= 2.87; 95% CI: 1.25–6.63), a child with edema (AOR= 5.33; 95% CI: 1.21–23.56), not eating vegetables and fruits (AOR= 2.05; 95% CI: 1.03–4.10) and parental occupation (daily laborer) (AOR= 3.13; 95% CI: 1.14–8.55) have remained associated factors with anemia in undernourished under-five children (Table 4).
Leukocytosis, Thrombocytosis, Thrombocytopenia, and Their Associated Factors
Both bi-variable and multi-variable binary logistic regression analyses were done to assess associated factors for leukocytosis. The variables that were statistically associated with leukocytosis were only child ages between 36 and 47 months (AOR= 4.05; 95% CI: 1.13–14.55) (Table 5). To assess factors associated with thrombocytopenia and thrombocytosis, both bi-variable and multi-variable binary logistic regression analyses were done. Accordingly, any of the variables did not show statistically significant association in the multi-variable binary logistic regression model.
Discussion
The hematopoietic tissues have a high rate of cell renewal and proliferation that require a sufficient supply of nutrients, and may thus be altered by a deficiency of nutrients.1 Experimental studies suggested that hematological abnormalities during PEM occur due to alterations of hematopoiesis in the BM, hematopoietic progenitors’ cell cycle,10 lymphohematopoietic organs,12 and alteration of the production of colony-stimulating factor by macrophages.11
In the current study, anemia was the most prevalent hematological abnormalities in undernourished children (53.4% (95% CI: 47.2–59.6)). This finding was in agreement with previous literature.14,16,30 According to WHO, anemia is considered a severe public health problem if the magnitude is ≥40%.36 Accordingly, the magnitude of anemia in the current study indicates that anemia is a severe public health problem among undernourished under-five children in the study area. Low intake of iron-rich foods and diminished nutrient absorption caused by changes in the gastrointestinal epithelium of undernourished individuals contribute towards the development of anemia.40 Besides, an interaction between adaptation to inadequate food intake and the impact of other stresses associated with infection or dietary imbalance may lead to anemia.41
The magnitude of anemia in this study (53.4% (95% CI: 47.2–59.6)) was in line with similar studies done in Sri Lanka (55.5%)42 and Bangladesh (56.5%).43 However, it was lower than studies done in Ethiopia (61% in stunted, 64.3% in underweighted, and 68.2% in wasted children),17 India (84%,44 90%,45 91%,9 and 95%22), and Brazil (88%).46 In contrast, it was higher than study conducted in Ethiopia (41.43%).47 The possible explanation for the difference could be attributed to variations in the socio-demographic characteristics, sample size, and study design. The high intensity of parasitic infections and non-regular consumption of vegetables and fruits might partly explain the high magnitude of anemia in this study.
The level of severity of anemia revealed that 44%, 38.8%, and 17.2% had mild, moderate, severe anemia, respectively. The magnitude of severe life-threatening anemia in the current study was comparable to the results of the studies reported from Nigeria (24%),30 India (19%)28, and Turkey (17.6%).16 However, it was higher than the study done in Sri Lanka (0.7%)42 while, it was lower than studies conducted in India (67% and 52%) of the participants had severe anemia.22,45 The difference might be variations in socio-demographic characteristics of the study participants, sample size, study design, and study period. Besides, it may also be related to variation in the local prevalence of parasitic infections (hookworms and malaria) and dietary habits.
Morphological classification of anemia showed that 73.1% had microcytic anemia followed by normocytic anemia (25.4%). Similarly, a study done in India showed that microcytic anemia (55%) was the predominant type of anemia in undernourished children.44 In contrast, Dwivedi et al reported that macrocytic anemia was the most common type of anemia in children with SAM.28 The depletion of iron is the possible reason for the high percentage of microcytic anemia.48
In the current study, the second common hematological abnormalities were leukocytosis, which was observed in 26.7% (95% CI: 21.2–32.20). Even though the percentage of leukocytosis is not reported, different studies confirm that leukocytosis a common feature in undernourished children. Findings from previous studies in India,9,22 Nigeria,30 and Sudan27 showed that the mean value of WBC count was higher in undernourished children when compared with their counterparts. The reason for the increment of leukocyte count may be related to infections like gastroenteritis and respiratory tract infections which are commonly seen in PEM.9,49 On the other hand, leukopenia occurred in 2.8% (95% CI: 0.74–4.8) of the study participants. A similar finding has been reported previously in several studies.28,50,51 Experimental studies suggest that leukopenia in malnutrition occurs due to the spleen and bone marrow hypoplasia which compromise hematopoiesis.52 This may cause immune suppression and increase susceptibility to infection. As Dwivedi et al state leukopenia may also be attributed to vitamin B12 deficiency in children with acute malnutrition.28
An increment of platelet count was observed in 23.9% (95% CI: 18.59–29.22) of the children. Similar studies were done in Turkey,31 India,9 and Sudan27 reported that higher mean platelet count in children with malnutrition. The possible reason for thrombocytosis during malnutrition may be associated with infection. For example during respiratory tract infection, there are increasing levels of inflammatory cytokines which heightens the production of thrombocytes.33 In contrast, thrombocytopenia was observed by 8% (95% CI: 4.59–11.34). The decrement of platelet count in the current study was in agreement with previous studies.22,30,31 The possible reason for the decrement of platelet count in undernourished children attributed to a decrease in BM activities and platelet destruction during infection.30,31
In this study, a child whose ages between 6 and 23 months was found to be 5.45 times more prone to anemia as compared to an older age counterpart (AOR= 5.45; 95% CI (2.13–13.93)). This is in line with studies done in Bangladesh43 and Sri Lanka.42 The possible reason for the high magnitude of anemia in this age group may be related to the low concentration of iron in breast milk which is insufficient to meet the daily iron requirements of the child.53 Being male in gender and being fourth and above in birth order were also associated with anemia in this study (AOR= 1.99; 95% CI: 1.10–3.61) and (AOR= 3.01; 95% CI: 1.20–7.54) respectively. This is similar to studies done in India.54,55 The possible explanation could be due to state of the rapid growth of male children in the first months of life which increases their micronutrient requirement including iron.56,57 Increasing birth order increases the anemic status of the children. This may be related to maternal depletion of micronutrients which have few significant effects on the iron status of the newborn.55
Children being infected by intestinal parasites are 2.87 times more likely to have anemia than children who are free from parasitic infection (AOR= 2.87; 95% CI: 1.25–6.63). It is in agreement with similar study done in India.58 The reason might be intestinal parasites suck blood and also damage the intestinal wall, causing blood leakage. It may also relate to the parasite directly induces iron deficiency through blood loss by mechanical rupture of host capillaries and arterioles.59 During infection or inflammation, there is a shortened cell survival, impaired response to erythropoietin, and impaired iron availability due to inadequate release of iron by the reticuloendothelial cells and reduced absorption from the gut.15,60 The other factor associated with anemia in undernourished children was edema (AOR= 5.33; 95% CI: 1.21–23.56). This might be related to excessive consumption of cow’s milk that is frequently associated with iron deficiency and intestinal bleeding.61,62
Not eating vegetables and fruits were found to be related to a risk of acquiring anemia (AOR= 2.05; 95% CI: 1.03–4.10). This may be related to fruits and vegetables serve as a good source of non-heme iron. Besides, Vitamin C which originates from fruits and vegetables potentiates absorption of non-heme iron that is found in legumes and other plant-based meals.63 Also children whose paternal occupation is daily laborer are 3.13 more likely to have anemia than children whose father is a non-daily laborer (AOR= 3.13; 95% CI: 1.14–8.55). The reason might be children from poor households are less likely to get iron-rich foods.64 On the other hand, children ages 36–47 months are 4.05 times more likely to develop leukocytosis (AOR= 4.05; 95% CI: 1.13–14.55). This might be children experience numerous infectious episodes in their childhood period.65 Unlike anemia, thrombocytopenia and thrombocytosis were not significantly associated with explanatory variables. The possible explanation could be attributed to a small number of cases of children with these abnormalities and small sample size; therefore, the number of observations in each category of explanatory variables becomes small and these observations would have low power to predict association.
The present study had some limitations: Since the study design was cross-sectional, it is difficult to establish a cause–effect relationship between hematological abnormalities and associated factors as it is temporal association. Analyzing stool samples by direct wet mount technique potentially limits this study. Moreover, micronutrient deficiency such as iron, folate, and vitamin B12 which is a major predisposing factor for anemia was not assessed. The study does not screen children for sickle cell disease due to the unavailability of testing materials.
Conclusion and Recommendation
In the current study anemia was the major hematological abnormality identified, followed by leukocytosis and thrombocytosis. Anemia among undernourished under-five children is a severe public health problem in the study area. Child ages 6–23 months, being male, high birth order, father’s occupation (daily laborer), not eating vegetables and fruits, intestinal parasite infection, and edema was associated with anemia in undernourished children. On the other hand, only age of the child was associated with leukocytosis in undernourished under-five children. Therefore, improving the sanitation of children, deworming, food supplementation and strengthen and setting prevention programs, developing treatment protocols for anemia may help to reduce the burden of anemia, childhood mortality, morbidity, and its long-term implications. Moreover, case-control and longitudinal research are encouraged to see and describe the trend of hematological changes in undernourished children.
Abbreviations
AOR, adjusted odd ratio; CD, cluster of differentiation; CI, confidence interval; COR, crude odd ratio; EDTA, ethylene diamine tetra acetic acid; HAZ, height for age Z-score; Hb, hemoglobin; Hct, hematocrit; IQR, interquartile range; MCH, mean hemoglobin concentration; MCHC, mean cell hemoglobin concentration; MCV, mean cell volume; OR, odd ratio; PEM, protein energy malnutrition; RBC, red blood cell; SAM, severe acute malnutrition; SD, standard deviation; WAZ, weight for age Z-score; WBC, white blood cell; WHO, World Health Organizations; WHZ, weight for height Z-score.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The study was approved by the Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. The study was also done as per the declaration of Helsinki.
Acknowledgments
We would like to acknowledge University of Gondar, College of Medicine and Health Sciences, School of Biomedical and Laboratory Sciences, Department of Hematology and Immunohematology for their financial support to conduct the study. We would like to thank children and their parents/guardians for their willingness to participate in this study. Finally, our special thanks goes to laboratory and nutritional rehabilitation center nursing staff members of the University of Gondar Specialized Referral Hospital for their cooperation during data collection.
Author Contributions
All authors made substantial contributions to conceptualization and design, data acquisition, data analysis and interpretation, took part in drafting of the initial manuscript and revising it critically for final approval of the version to be published. All authors agreed to submit to the current journal and agreed to be accountable for all aspects of the work.
Funding
University of Gondar.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Borelli P, Blatt SL, Rogero MM, Fock RA. Haematological alterations in protein malnutrition. Rev Bras Hematol Hemoter. 2004;26(1):49–56. doi:10.1590/S1516-84842004000100010
2. Blaney S, Menasria L, Main B, et al. Determinants of undernutrition among young children living in Soth Nikum district, Siem Reap, Cambodia. Nutrients. 2019;11(3):685. doi:10.3390/nu11030685
3. Santos EW, Oliveira DC, Silva GB, et al. Hematological alterations in protein malnutrition. Nutr Rev. 2017;75(11):909–919. doi:10.1093/nutrit/nux041
4. WHO. UNICEF/WHO/The World Bank group joint child malnutrition estimates: levels and trends in child malnutrition: key findings of the 2020 edition; 2020. Available from: https://apps.who.int/iris/handle/10665/331621.
5. Ethiopia: mini demographic and health survey 2019. Available from: https://dhsprogram.com/pubs/pdf/PR120/PR120.pdf.
6. WHO, Unicef. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: Joint Statement by the World Health Organization and the United Nations Children’s Fund. 2009.
7. Mary E. Protein energy malnutrition, pathophysiology, clinical consequences and treatment. Nutr Paediatr. 2008;171–184.
8. McKenna SL, Cotter TG. Functional aspects of apoptosis in hematopoiesis and consequences of failure. Adv Cancer Res. 1997;71(7):121–164.
9. Gohain EK, Pathak K, Choudhury B, Case Control A. Study of hematological changes in children with protein energy malnutrition attending Gauhati medical college and hospital. J Appl Dent. 2016;15(10):25–29.
10. Borelli P, Barros FEV, Nakajima K, et al. Protein-energy malnutrition halts hemopoietic progenitor cells in the G0/G1 cell cycle stage, thereby altering cell production rates. Braz J Med Biol Res. 2009;42(6):523–530. doi:10.1590/S0100-879X2009000600008
11. Oliveira D, Hastreiter AA, Borelli P, Fock RA. The influence of protein malnutrition on the production of GM-CSF and M-CSF by macrophages. Braz J Pharm Sci. 2016;52(3):375–382. doi:10.1590/s1984-82502016000300003
12. Fock RA, Blatt SL, Beutler B, et al. Study of lymphocyte subpopulations in bone marrow in a model of protein–energy malnutrition. Nutrition. 2010;26(10):1021–1028. doi:10.1016/j.nut.2009.08.026
13. Borelli P, Blatt S, Pereira J, et al. Reduction of erythroid progenitors in protein–energy malnutrition. Br J Nutr. 2007;97(2):307–314. doi:10.1017/S0007114507172731
14. El Nawawy A, Barakat S, El Walily T, Moneim A, Deghady A, Hussein M. Evaluation of erythropoiesis in protein energy malnutrition. East Mediterr Health J. 2002;(8):2–3.
15. Warrier R, Dole M, Warrier J, Suskind R. The anemia of malnutrition. In: Nestle Nutrition Workshop Series (USA). 1990:61–72.
16. Özkale M, Sipahi T. Hematologic and bone marrow changes in children with protein-energy malnutrition. Pediatr Hematol Oncol J. 2014;31(4):349–358. doi:10.3109/08880018.2013.813098
17. Tekile AK, Woya AA, Basha GW. Prevalence of malnutrition and associated factors among under-five children in Ethiopia: evidence from the 2016 Ethiopia demographic and health survey. BMC Res Notes. 2019;12(1):391. doi:10.1186/s13104-019-4444-4
18. Girum T, Kote M, Tariku B, Bekele H. Survival status and predictors of mortality among severely acute malnourished children< 5 years of age admitted to stabilization centers in gedeo zone: a retrospective cohort study. Ther Clin Risk Manag. 2017;13:101.
19. Jarso H, Workicho A, Alemseged F. Survival status and predictors of mortality in severely malnourished children admitted to Jimma University specialized hospital from 2010 to 2012, Jimma, Ethiopia: a retrospective longitudinal study. BMC Pediatr. 2015;15(1):76. doi:10.1186/s12887-015-0398-4
20. Jáuregui-Lobera I. Iron deficiency and cognitive functions. Neuropsych Dis Treat. 2014;10:2087. doi:10.2147/NDT.S72491
21. Yaikhomba T, Poswal L, Goyal S. Assessment of iron, folate and vitamin B12 status in severe acute malnutrition. Indian J Pediatr. 2015;82(6):511–514. doi:10.1007/s12098-014-1600-7
22. Arun Kumar Arya PK, Midha T, Singh M, Singh M. Hematological profile of children with severe acute malnutrition: a tertiary care centre experience. Int J Contemp Pediatr. 2017;4(5):1577–1580. doi:10.18203/2349-3291.ijcp20173072
23. Gibson R. Strategies for preventing micronutrient deficiencies in developing countries. Asia Pac J Clin Nutr. 2004;13.
24. Stoltzfus RJ, Chway HM, Montresor A, et al. Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr. 2004;134(2):348–356. doi:10.1093/jn/134.2.348
25. Buitrón D, Hurtig A-K, San MS. Nutritional status of Naporuna children under five in the Amazon region of Ecuador. Pan Am j Public Health. 2004;15(3):151–159.
26. Espinoza M, Perelli J, Olmos R, Bertin P, Jara V, Ramírez P. Nutritional assessment as predictor of complications after hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2016;38(1):7–14. doi:10.1016/j.bjhh.2015.10.002
27. Basheir HM, Hamza KM. Hematological parameters of malnourished sudanese children under 5 years–Khartoum state–2011. J Clin Med. 2015;1(4):152–156.
28. Dwivedi D, Singh V, Singh J, Sharma S. Study of anaemia in children with severe acute malnutrition. J Nepal Paediatr Soc. 2017;37(3):250–253. doi:10.3126/jnps.v37i3.18480
29. Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002;56(S3):S46. doi:10.1038/sj.ejcn.1601485
30. Saka A, Saka M, Ojuawo A, et al. Haematological profile in children with protein energy malnutrition in North Central Nigeria. Glob J Med Res. 2012;12(4):1–7.
31. Üner A, Çalişkan Ü, Öner AF, Koç H, Kasap AF. Platelet functions in patients with protein-energy malnutrition. Clin Appl Thromb Hemost. 2001;7(4):286–288. doi:10.1177/107602960100700406
32. Speth C, Löffler J, Krappmann S, Lass-Flörl C, Rambach G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013;8(11):1431–1451. doi:10.2217/fmb.13.104
33. Choudhury J. Thrombocytosis in under-five children with lower respiratory tract infection. Ann Med Health Sci Res. 2017;7(6):47–51.
34. Gordon DM, Frenning S, Draper HR, Kokeb M. Prevalence and burden of diseases presenting to a general pediatrics ward in Gondar, Ethiopia. J Trop Pediatrics. 2013;59(5):350–357. doi:10.1093/tropej/fmt031
35. Wagnew F, Dejenu G, Eshetie S, Alebel A, Worku W, Abajobir AA. Treatment cure rate and its predictors among children with severe acute malnutrition in northwest Ethiopia: a retrospective record review. PLoS One. 2019;14(2):e0211628. doi:10.1371/journal.pone.0211628
36. WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. World Health Organization; 2011.
37. Janus J, Moerschel SK. Evaluation of anemia in children. Am Fam Physician. 2010;81(12):1462–1471.
38. Geletaw T, Tadesse MZ, Demisse AG. Hematologic abnormalities and associated factors among HIV infected children pre-and post-antiretroviral treatment, North West Ethiopia. J Blood Med. 2017;4(8):99–105. doi:10.2147/JBM.S137067
39. Wollo E. Risk factors for child under-nutrition with a human rights edge in rural villages of North Wollo, Ethiopia. East Afr Med J. 2005;82:625–630.
40. Leal LP, Batista Filho M, Lira P, Figueiroa JN, Osório MM. Prevalence of anemia and associated factors in children aged 6–59 months in Pernambuco, Northeastern Brazil. Rev Saude Publica. 2011;45(3):457–466. doi:10.1590/S0034-89102011000300003
41. Badham J, Zimmermann MB, Kraemer K. The Guidebook Nutritional Anemia: Task Force Sight and Life. 2007:228.
42. Keerthiwansa J, Gajealan S, Sivaraja S, Subashini K. Malnutrition and anaemia among hospitalised children in Vavuniya. Ceylon Med J. 2014;59(4):141–143. doi:10.4038/cmj.v59i4.7869
43. Rahman MS, Mushfiquee M, Masud MS, Howlader T. Association between malnutrition and anemia in under-five children and women of reproductive age: evidence from Bangladesh demographic and health survey 2011. PLoS One. 2019;14(7):e0219170. doi:10.1371/journal.pone.0219170
44. Nayana N, Sreenivas N, Jayaram S. Study of anemia among protein energy malnourished children in Mysore. Int J Evid Based Healthc. 2015;2(9):1198–1210. doi:10.18410/jebmh/2015/174
45. Thakur N, Chandra J, Pemde H, Singh V. Anemia in severe acute malnutrition. Nutrition. 2014;30(4):440–442. doi:10.1016/j.nut.2013.09.011
46. de Fátima Costa Caminha M, da Figueira MAS, Falbo AR, de Araújo de Amorim RC, Gallindo TC, Filho MB. Co-existence of micronutrient deficiencies in hospitalized children with severe malnutrition treated according to the WHO protocol. Trop Doct. 2011;41(4):230–232. doi:10.1258/td.2011.100140
47. Akalu TY, Baraki AG, Wolde HF, et al. Anemia and determinants among severely malnourished children admitted to Amhara regional referral hospitals, Northwest Ethiopia. Open J Nutr Food Sci. 2020;2(1):1007.
48. Simbauranga RH, Kamugisha E, Hokororo A, Kidenya BR, Makani J. Prevalence and factors associated with severe anaemia amongst under-five children hospitalized at Bugando Medical Centre, Mwanza, Tanzania. BMC Hematol. 2015;15(1):13. doi:10.1186/s12878-015-0033-5
49. Page A-L, de Rekeneire N, Sayadi S, et al. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One. 2013;8(7):e68699. doi:10.1371/journal.pone.0068699
50. Edozien JC, Khan MR, Waslien CI. Human protein deficiency: results of a Nigerian village study. T. J Nutr. 1976;106(3):312–328. doi:10.1093/jn/106.3.312
51. Fondu P, Hariga-Muller C, Mozes N, Neve J, Van Steirteghem A, Mandelbaum I. Protein-energy malnutrition and anemia in Kivu. Am J Clin Nutr. 1978;31(1):46–56. doi:10.1093/ajcn/31.1.46
52. Cunha MCR, da Silva Lima F, Vinolo MAR, et al. Protein malnutrition induces bone marrow mesenchymal stem cells commitment to adipogenic differentiation leading to hematopoietic failure. PLoS One. 2013;8(3):e58872. doi:10.1371/journal.pone.0058872
53. Onyemaobi G, Onimawo I. Anaemia prevalence among under-five children in Imo State, Nigeria. Aust J Basic Appl Sci. 2011;5(2):122–126.
54. Goswmai S, Das KK. Socio‐economic and demographic determinants of childhood anemia. J Pediatr. 2015;91(5):471–477. doi:10.1016/j.jped.2014.09.009
55. Ray S, Chandra J, Bhattacharjee J, Sharma S, Agarwala A. Determinants of nutritional anaemia in children less than five years age. Int J Contemp Pediatr. 2018;3(2):403–408.
56. Santos R, Gonzalez ESC, Albuquerque E, et al. Prevalence of anemia in under five-year-old children in a children’s hospital in Recife, Brazil. Rev Bras Hematol Hemoter. 2011;33(2):100–104. doi:10.5581/1516-8484.20110028
57. Chaparro CM. Setting the stage for child health and development: prevention of iron deficiency in early infancy. J Nutr. 2008;138(12):2529–2533. doi:10.1093/jn/138.12.2529
58. Rahman MA, Mannan M, Rahman MH. Influence of infection on iron profile in severely malnourished children. Indian J Pediatr. 2009;76(9):907. doi:10.1007/s12098-009-0098-x
59. Odebunmi J, Adefioye O, Adeyeba O. Hookworm infection among school children in Vom, Plateau State, Nigeria. Am Eurasian J Sci Res. 2007;1:39–42.
60. Cherayil BJ. The role of iron in the immune response to bacterial infection. Immunol Res. 2011;50(1):1–9. doi:10.1007/s12026-010-8199-1
61. Bondi SA, Lieuw K. Excessive cow’s milk consumption and iron deficiency in toddlers: two unusual presentations and review. Infant Child Adolesc Nutr. 2009;1(3):133–139. doi:10.1177/1941406409335481
62. Sullivan PB. Cows’ milk induced intestinal bleeding in infancy. Arch Dis Child. 1993;68(2):240. doi:10.1136/adc.68.2.240
63. Amsalu S, Tigabu Z. Risk factors for ever acute malnutrition in children under the age of five: a case-control study. Ethiop J Health Dev. 2008;22(1):21–25. doi:10.4314/ejhd.v22i1.10058
64. Gebreegziabiher G, Etana B, Niggusie D. Determinants of anemia among children aged 6–59 months living in Kilte Awulaelo Woreda, northern Ethiopia. Anemia. 2014;2014:245870. doi:10.1155/2014/245870
65. Grüber C, Keil T, Kulig M, et al. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatr Allergy Immunol. 2008;19(6):505–512. doi:10.1111/j.1399-3038.2007.00688.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.