Back to Journals » Research and Reports in Tropical Medicine » Volume 11
Helicobacter pylori Infection and Predictors Among Dyspeptic Adult Patients in Southwest Ethiopia: Cross-Sectional Study
Received 17 September 2020
Accepted for publication 5 November 2020
Published 19 November 2020 Volume 2020:11 Pages 141—147
DOI https://doi.org/10.2147/RRTM.S282557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mario Rodriguez-Perez
Daniel Kahase, Kassahun Haile
Department of Medical Laboratory Science, College of Medicine and Health Science, Wolkite University, Wolkite, Ethiopia
Correspondence: Daniel Kahase
Department of Medical Laboratory Science, College of Medicine and Health Science, Wolkite University, P.O. Box: 07, Wolkite, Ethiopia
Tel +251 912414564
Fax +251 11 322 00 41
Email [email protected]
Background: Globally, H. pylori infection affects approximately 4.4 billion people. The burden of the infection varies within and between countries, with a higher prevalence reported from developing countries including Ethiopia. Thus, this study aimed to determine the magnitude and predictors of H. pylori infection among dyspeptic patients who visited Wachemo University Nigist Eleni Mohammed Memorial Referral Hospital, Southwest Ethiopia.
Methods: Consecutive willing dyspeptic adult patients (n=405) were enrolled in a cross-sectional study done from September to December 18, 2019. Socio-demographic and behavioral characteristics of the study participants were gathered by a pretested structured questionnaire. Stool samples were examined for H. pylori antigens using Wondfo one step H. pylori feces test kit. SPSS version 20 was utilized to compute descriptive statistics, binary and multivariate logistic regression. A p-value of < 0.05 was considered statistically significant.
Results: Mean age of patients was 30 (± 7.4 SD) years, and 56.8% of participants were females. About 51.4% (208/405) of adult dyspeptic patients were infected with H. pylori. Being female gender (AOR꞊ 2.56, CI 95% ꞊1.61– 4.07, p꞊0.001), consumption of alcohol (AOR꞊1.95, CI 95% ꞊1.02– 3.73, p꞊ 0.019) and being undernourished (underweighted) (AOR꞊ 4.59, CI 95%꞊ 1.28– 16.45, p꞊0.019) were independent predictors of H. pylori infection.
Conclusion: In the study area, high (51.4%) magnitude of H. pylori infection was observed in dyspeptic patients and significantly associated with female gender, alcohol consumption, and undernourishment.
Keywords: H. pylori, dyspepsia, predictors, Southwest Ethiopia
Background
H. pylori infection is a major public health problem worldwide. Globally it affects approximately 4.4 billion people.1 The overall prevalence of the infection varies within and between countries, with a higher prevalence generally reported from developing countries; a study indicated 50.8% in developing countries as compared to 34.7% in developed countries.2 Another study revealed 70.1%, 69.4%, and 66.6% burden of infection in Africa, South America, and Western Asia, respectively.1 Whereas a large amount of population was affected in different parts of Ethiopia, the percentage varies from 36.8% to 83.3% across the country.3–8
H. pylori infection is acquired in early childhood through fecal-oral or oral-oral transmission and becomes a chronic infection if left untreated.9 It possesses different mechanisms to colonize the host and can result in several gastrointestinal diseases, including gastritis, peptic ulcer, duodenal ulcer, and gastric cancer.10 Studies by Johnson et al and Ersumo et al reported a significant number of gastric cancer cases from Tikur Anbessa hospital, Addis Ababa, Ethiopia where most patients referred from across the country.11,12
Predisposing factors for H. pylori infection are related to socio-demographic characteristics, sanitation, the lifestyle of the population, genetic predisposition, and socioeconomic status.5,9,13 The diagnosis of H. pylori infection is performed by invasive (rapid urease test, culture, endoscopy and endoscopic biopsy for histopathology) and non-invasive (urea breath tests, stool antigen test, and serological tests) methods.14 Whereas the choice of diagnostic methods generally depends on availability, affordability, differences in test performance, clinical situation, the population prevalence of infection, and factors that may influence the test results, such as the use of anti-secretory treatment and antibiotics.15
A lot of studies in Ethiopia were conducted using IgG and/or IgM antibody rapid tests3,16 which have questionable performance in detecting acute infection and, it can be positive for months to years after eradication making it difficult to discern if it is a current or past infection. This may probably hinder the exact magnitude of the infection in the community, hence the current study was carried out by using a stool antigen test to hasten this problem. Besides, there is a paucity of data in Ethiopia particularly in the Hadiya zone, about the prevalence of H. pylori infection and its associated risk factors. Therefore, this study aimed to determine the burden and its associated risk factors of H. pylori infection at Hadiya zone, Southwest Ethiopia.
Methods
Study Design, Study Area and Study Period
A cross-sectional study was conducted at Wachemo University Nigist Eleni Mohammed Memorial Referral Hospital (WUNEMMRH), Hosanna town, Hadiya Zone, Southern Ethiopia from September to December 18/2019. Hosanna town is located about 232 km far from the capital city of Ethiopia, Addis Ababa. WUNEMMRH is the largest public hospital in the Hadiya Zone and it provides teaching, diagnostic, and referral services for more than 3.2 million people. It gives service in four major departments. Each department has its own inpatient, outpatient, and referral clinics.
Study Population
The study population was dyspeptic adult patients who gave stool samples and interviewed during the study period at the outpatient department.
Sample Size Determination and Sampling Technique
The sample size was computed by using the single population proportion formula, taking 52.2% prevalence of H. pylori infection in symptomatic patients from a previous review study done in Ethiopia,17 and by considering a 95% confidence interval (CI), 5% margin of error and 6% nonresponse rate. Accordingly, the minimum sample size was determined to be 406. However, one participant was not included due to incomplete data and the reset 405 consecutive willing dyspeptic adult patients were enrolled in this study.
Data Collection
At the outpatient department (OPD) data on socio-demographic (gender, age, monthly income, marital status, residence, occupational status educational status), behavioral (smoking, alcohol intake, dietary habit) characteristics of the study participants were collected using a pre-tested structured questionnaire through face-to-face interviews. Body mass index (BMI) data of the study participants were measured during the study period. BMI ≤ 18.5 kg/m2 was classified as underweight; BMI = 18.6–24.9 kg/m2 as normal weight; and BMI = 25–29.9 kg/m2 as overweight.18
Laboratory Analysis
Approximately 2 grams of stool sample was collected from each study participant using clean, dry, leak-proof, and wide-mouthed containers. H. pylori stool antigen was detected by Wondfo one step H. pylori feces test kit (Guangzhou Wondfo Biotech, China) which has test sensitivity of 99.1% and specificity of 99.2%. Briefly, stool sample was emulsified and added to the sample well of the test cassette. If the sample contains H. pylori antigens, the antigen will bind to the antibody coated on the colloidal gold particles to form antigen-antibody-gold complexes. These complexes pass through the nitrocellulose membrane by capillary action toward the test region and control region. As the complexes reach the test region on which anti-H. pylori monoclonal antibodies are immobilized, they bind to the monoclonal antibody and form a colored line. A second red control line will always appear in the result window to indicate that the test has been correctly performed and the test device functions properly.19
The intestinal parasite was examined using a bright-field compound microscope under a 10x objective. Both examinations were performed dully according to the standard operating procedure of the hospital and the manufacturer’s instruction.
Data Analysis and Interpretation
The completed questionnaire was assured for completeness and integrity by the principal investigators. SPSS version 20 software (SPSS INC, Chicago, IL, USA) was utilized for statistical analysis. Socio-demographic data, behavioral and physical characteristics of study participants were reported by descriptive statistics. Binary logistic regression analysis was performed to examine the presence of an association between H. pylori stool antigen positivity and different independent predictors. Subsequently, possible predictors with a P-value of < 0.2 in binary logistic regression were selected for further analysis with multiple logistic regression models. At last, a P-value of < 0.05 was declared as statistically significant.
Results
Socio-Demographic, Behavioral and Dietary Characteristics of the Study Population
A total of four hundred five symptomatic dyspeptic adult patients enrolled with a predominance of females 230 (56.8%). The mean (±SD) age of the study population was 30 ± 7.4 years with a range of 18 to 49 years. About 38% of the study participants fell in the age group of 27–34 years. Most of the participants were rural dwellers 236 (56.8%), secondary 121 (29.9%) in their educational status and farmer 109 (26.9%) with regard to occupational status (Table 1).
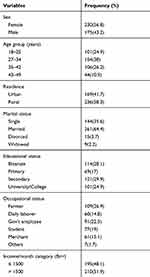 |
Table 1 Socio-Demographic Characteristics of 405 Study Population |
The proportion of alcohol consumer at least once a weak and cigarette smoker accounted for 61 (15.1%) and 44 (10.9%) of the study population respectively. An assessment of the food habits of the study participants brought out 213 (52.6%) had a habit of drinking coffee at least once a day and 137 (34%) had a habit of drinking raw milk at least once a week (Table 2).
 |
Table 2 Helicobacter pylori Stool Antigen Positivity Among Different Socio-Demographic Characteristics of 405 Study Participants, 2019 |
Physical Characteristics and Intestinal Parasite Status of the Study Population
Microscopic examination of stool revealed that 19.5% (79/405) of the study participants were infected with different types of medically important intestinal parasites. G.lamblia (43%), A.lumbricoides (27.8%) and E. histolytic/dispar (26.6%) were the dominant parasites respectively. Regarding BMI, the majority (82.5%, 334/405) of the study population fall in the normal followed by overweight (11.9%, 48/405) BMI category (Table 2).
Magnitude of H. pylori Infection and Independent Predictors
The overall magnitude of H. pylori infection among dyspeptic adult patients was 51.4% (208/405, CI 95%꞊ 46.4–56.3) (Table 2). The proportion of H. pylori infection was higher in females (59.1%) than males (41.1%) and the difference was statistically significant (p < 0.001). High prevalence of H. pylori infection (55.7%) was obtained in the age group of 35–42 years old followed by 27–34 years old (53.2%) participants (p ꞊ 0.146) (Table 3).
 |
Table 3 Helicobacter pylori Stool Antigen Positivity Against Behavioral, Dietary Habit, Physical Characteristics and Intestinal Parasite Status of 405 Study Participants, 2019 |
Being married, being illiterate (COR꞊1.91, CI 95%꞊1.11–3.29, p꞊0.020) and primary (COR꞊2.12, CI 95%꞊1.04–3.61, p꞊0.038) in educational status were observed as predictors of H. pylori infection in bivariate analysis. However, in multivariate analysis, primary level and secondary level education of the participants showed a borderline association (p꞊0.070 versus p꞊0.050) (Table 4).
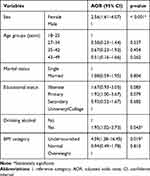 |
Table 4 Multivariate Analysis of Selected Possible Predictors Associated with Stool Antigen Positivity of H. pylori |
A significantly high infection rate was also observed in undernourished study participants (82.6%) as compared to overweighed participants (AOR꞊4.59, CI 95% ꞊1.28–16.45, p꞊ 0.019). In addition, drinking alcohol had a positive relation to H. pylori infection (AOR꞊ 1.95, CI 95% ꞊ 1.02–3.73, p꞊ 0.019) (Table 4). However, other studied independent factors such as age group, monthly income, residence, occupational status, smoking cigarette, dietary habit, and intestinal parasite status had shown no statistically significant association with H. pylori infection (Table 3).
Discussion
A significant burden of H. pylori infection is reported in most of the world with large amounts of variation existed among regions even within the same country.1 The current study demonstrated an overall magnitude of H. pylori stool antigen positivity in adults (≥18 years old) with dyspepsia as 51.4%. This magnitude was comparable with the work of Melese et al, 52.2%17 and Kibru et al, 52.4%.5 In the contrary, lower burden of H. pylori infection was reported from Dessie 30.4%,19 Addis Ababa 36.8%,8 Gonder, Ethiopia 37.6%6 and China 42%.20 Those differences could reflect variation in lifestyle, geographic area and socio-economic status of the study participants.
However, a high magnitude of H. pylori was documented in studies done in Gonder, Ethiopia (65.7%),16 and Hawassa, South Ethiopia (83.3%).3 In addition, reports from Pakistan (57%),21 Cameron (64.39%)13 and Kenya (71%)22 indicated high H. pylori infection rate. Possibly the source of the difference could be due to variation in the study design, laboratory diagnosis technique (antibody detection test from blood) and personal hygiene of the study population.
In this study, a high proportion of female participants (59.1%, 136/230) were infected and being female was a predictor for H. pylori infection (P<0.001). This was supported by studies done in Assosa, Ethiopia7 and Iran.23 However, the exact mechanism of why being female significantly associated with H. pylori infection remains unclear. Unlike our result, one meta-analysis study indicated that male sex was associated with a greater prevalence of H. pylori infection, both in children and adults.24 But several studies reported that there is no sex preferences during H. pylori infection.5,6,8,17,19 This all might show the absence of consensus on the concept that males or females more likely to be infected.
Based on our findings, age had not significant influence (p ˃ 0.05) on H. pylori infection which is comparable to previous reports from Ethiopia.3,8 Other studies showed a significant association between age and H. pylori infection.3,5,16 These differences might be influenced by the participants’ age range, in our case, it was 18–49 years. The role of educational status differentially put patients with a low educational level at higher risk of infection was also shown by others5,8 though this could not be revealed in the current and similar previous studies.3,19
Interestingly, our result depicted alcohol consumers were significantly at risk of acquiring H. pylori infection which is in line with the report of Seid et al, and Melese et al,17,19 This might be explained by the result of a study that found alcohol concentrations of greater than 10% increases the mucosa’s permeability by damaging the mucosal barrier.25 In contrast, some authors revealed alcohol consumption as a protective factor against active H. pylori infection5,26 and others report no relation in between.6,7 The reason for the contradiction of these results could be due to the fact that H. pylori colonization may be affected by multiple factors and type, amount, and duration of alcohol consumption.
Studies revealed that overweight and obese patients had significantly a higher rate of H. pylori infection compared to BMI-healthy individuals.20,21 However, in our observation undernourishment (low BMI) was another factor that significantly predicted H. pylori infection, given 82.6% of the underweight were infected which is in agreement with the work of Kibru et al.5 This association could be due to the fact that H. pylori affect iron and vitamin B12 absorption and hormones implicated in the regulation of appetite and growth negatively.27,28
This study has some limitations. First, it was limited to adult patients with dyspepsia which may not infer to the prevalence of H. pylori infection of the general population. Second, alcohol consumption data with regard to type, amount, and duration was not gathered. Third, the study was limited to a single institution and a certain population but the information reported could be a significant contribution to the existing knowledge on the burden of H. pylori infection and predictors.
Conclusion
Our study showed that the prevalence of H. pylori among our study population was high (51.4%). Among the studied associated factors, it was significantly greater in females (59.1%), alcohol consumers (60.7%), and malnourished patients (82.6%). Therefore, interventions against H. pylori infection prevention in the study area need to be scaled up to diminish the burden.
Abbreviations
AOR, adjusted odds ratio; BMI, body mass index; COR, crude odds ratio; WUNEMMRH, Wachemo University Nigist Eleni Mohammed Memorial Hospital.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was reviewed and approved by the Ethical Committee of the Department of Medical Laboratory Sciences of Jima University. It was sought while we were students at Jima University. Participation was voluntary and each study subject involved in the study gave written informed consent. The study was carried out in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable.
Acknowledgments
We acknowledge the support of the staff of the WUNEMMRH and our study participants for their good cooperation.
Author Contributions
Daniel Kahase conceived, designed the study and drafted the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429.
2. Sjomina O, Pavlova J, Niv Y, Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23:6–11. doi:10.1111/hel.12514
3. Tadesse E, Daka D, Yemane D, Shimelis T. Seroprevalence of Helicobacter pylori infection and its related risk factors in symptomatic patients in southern Ethiopia. BMC Res Notes. 2014;7(834):1–5.
4. Negash M, Baynes HW, Geremew D. Helicobacter pylori infection and its risk factors: a prospective cross-sectional study in resource-limited settings of Northwest Ethiopia. Can J Infect Dis and Med. 2018;2018.
5. Kibru D, Gelaw B, Alemu A, Addis Z. Helicobacter pylori infection and its association with anemia among adult dyspeptic patients attending Butajira Hospital, Ethiopia. BMC Infect Dis. 2014;14(1):1–7. doi:10.1186/s12879-014-0656-3
6. Kasew D, Abebe A, Munea U, et al. Magnitude of Helicobacter pylori among dyspeptic patients attending at University of Gondar Hospital, Gondar, Northwest Ethiopia. Ethiop J Health Sci. 2017;27(6):571. doi:10.4314/ejhs.v27i6.2
7. Dilnessa T, Amentie M. Original article prevalence of Helicobacter pylori and risk factors among dyspepsia and non-dyspepsia adults at Assosa General Hospital, West Ethiopia: a comparative study. Ethiop J Health Dev. 2017;31(1).
8. Shiferaw G, Abera D. Magnitude of Helicobacter pylori and associated risk factors among symptomatic patients attending at jasmin internal medicine and pediatrics specialized private clinic in Addis Ababa city, Ethiopia. BMC Infect Dis. 2019;19(1):1–6. doi:10.1186/s12879-019-3753-5
9. Van DYTHP, De JR. Transmission of Helicobacter pylori: a role for food? Bull World Health Organ. 2001;79(99):455–460.
10. Mitchell H, Diped H, Katelaris P, Frcp F.Epidemiology, clinical impacts and current clinical management of Helicobacter pylori infection.Med J Aust.2016;204. doi:10.5694/mja16.00104.
11. Johnson O, Ersumo T, Ali A. Gastric carcinoma at Tikur Anbessa Hospital, Addis Ababa. East Afr Med J. 2000;77(1):27–30.
12. Ersumo T, Johnson O, Ali A. Gastrointestinal tract cancer: a five year study in a teaching central referral hospital, Ethiopia. Ethiop Med J. 2000;38(2):93–103.
13. Brigitte L, Mabeku K, Larissa M, Ngamga N, Leundji H. Potential risk factors and prevalence of Helicobacter pylori infection among adult patients with dyspepsia symptoms in cameroon. BMC Infect Dis. 2018;18(278):1–11.
14. Kalali B, Formichella L, Gerhard M. Diagnosis of Helicobacter pylori: changes towards the future. Diseases. 2015;3(3):122–135. doi:10.3390/diseases3030122
15. Hunt RH, France FM. Helicobacter Pylori in developing countries. J Gastrointestin Liver Dis. 2011;20(3):299–304.
16. Mathewos B, Moges B, Dagnew M. Seroprevalence and trend of Helicobacter pylori infection in Gondar University Hospital among. BMC Res Notes. 2013;346(6):2–5.
17. Melese A, Genet C, Zeleke B, Andualem T. Helicobacter pylori infections in Ethiopia; prevalence and associated factors: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):1–15. doi:10.1186/s12876-018-0927-3
18. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190.
19. Seid A, Demsiss W. Feco-prevalence and risk factors of Helicobacter pylori infection among symptomatic patients at dessie referral. BMC Infect Dis. 2018;18(260):1–9.
20. Xu X, Li W, Qin L, Yang W, Id GY, Wei Q. Relationship between Helicobacter pylori infection and obesity in Chinese adults: a systematic review with meta-analysis. PLoS One. 2019;14(9):1–13.
21. Siddiqui B, Yakoob J, Abbas Z, Azmat R, Fatima SS, Awan S. Distribution of Helicobacter pylori infection and abnormal body- mass index (BMI) in a developing country. J Infect Dev Ctries. 2018;12(5):342–346. doi:10.3855/jidc.10051
22. Shmuely H, Obure S, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection. Emerg Infect Dis. 2003;9(9):1103–1107. doi:10.3201/eid0909.020374
23. Agah S. Female gender and Helicobacter pylori infection, the most important predisposition factors in a cohort of gastric cancer: a longitudinal study. Caspian J Intern Med. 2016;7(2):136–141.
24. Ibrahim A, Morais S, Ferro A, Lunet N. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: systematic review and meta-analysis of 244 studies. Dig Liver Dis. 2017;49(7):742–749. doi:10.1016/j.dld.2017.03.019
25. Bode JC. Alcohol and the gastrointestinal tract. Adv Int Med Pediatr. 1992;45:1–75.
26. Kuepper-Nybelen J, Thefeld W, Rothenbacher D, Brenner H. Patterns of alcohol consumption and Helicobacter pylori infection: results of a population based study from Germany among 6545 adults. Aliment Pharmacol Ther. 2005;21(1):57–64. doi:10.1111/j.1365-2036.2004.02276.x
27. Abdel Y, Abdel L, Mohamed R, Mohamed R, Ahmed M. Helicobacter pylori and its hematological effect. EJIM. 2019;332–342.
28. Franceschi F, Annalisa T, Teresa DR, et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. 2014;20(36):12809–12817. doi:10.3748/wjg.v20.i36.12809
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
