Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Hard-Candy Consumption Does Not Have an Effect on Volume and pH of Gastric Content in Patients Undergoing Elective Gastrointestinal Endoscopic Procedures: A Randomized Controlled Trial
Authors Somnuke P , Kitisin N, Chumklud P, Kunavuttitagool P, Deepinta P, Wadrod A, Prachayakul W, Amornyotin S , Raykateeraroj N
Received 15 July 2022
Accepted for publication 22 November 2022
Published 28 November 2022 Volume 2022:18 Pages 1049—1057
DOI https://doi.org/10.2147/TCRM.S377421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Pawit Somnuke,1 Nuanprae Kitisin,1 Phornprasurt Chumklud,1 Pishsinee Kunavuttitagool,1 Penpuk Deepinta,1 Araya Wadrod,2 Warayu Prachayakul,3 Somchai Amornyotin,1 Nattaya Raykateeraroj1
1Department of Anesthesiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand; 2Department of Nursing, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand; 3Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand
Correspondence: Nattaya Raykateeraroj, Department of Anesthesiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 10700, Thailand, Tel +66 2 419 7990, Fax +66 2 411 3256, Email [email protected]
Purpose: This study aimed to determine the effect of hard candies on gastric content volume and pH in patients undergoing elective esophagogastroduodenoscopy and colonoscopy. Additionally, the study evaluated the difficulty of the procedure, complications, and satisfaction levels of the endoscopist and patient.
Patients and Methods: A randomized controlled study equally recruited 108 outpatients to candy and control groups. The patients in the candy group could consume sugar-free candies within 2 hours before anesthesia, while the controls remained fasted. The endoscopic procedure began under topical pharyngeal anesthesia and intravenous sedation. A blinded endoscopist suctioned the gastric volume through an endoscope. A blinded anesthesia provider tested the gastric pH with a pH meter. The primary outcome variables were gastric volume and pH. The secondary outcome variables were complications, the difficulty of the procedure, and endoscopist and patient satisfaction.
Results: The characteristics of both patient groups were comparable. The mean gastric volume of the candy group (0.43 [0.27– 0.67] mL/kg) was not significantly different from that of the control group (0.32 [0.19– 0.55] mL/kg). The gastric pH of both groups was similar: 1.40 (1.10– 1.70) for the candy group and 1.40 (1.20– 1.90) for the control group. The procedure-difficulty score of the candy group was higher than that of the control group. The satisfaction scores rated by the endoscopist and the patients in both groups were comparable. In addition, most endoscopists and patients in the candy and control groups reported being “very satisfied”. No complications were observed in either group.
Conclusion: Hard candies did not affect gastric volume or pH. Elective gastrointestinal endoscopic procedures in adult patients who preoperatively consume candies could proceed to prevent delays and disruption of workflows.
Keywords: gastric pH, gastric volume, gastrointestinal endoscopy, hard candy, preoperative fasting guidelines
Corrigendum for this paper has been published.
Introduction
Preoperative fasting is a prerequisite in patients scheduled for procedures under anesthesia to mitigate the risk of pulmonary aspiration of gastric content, which could lead to severe complications such as aspiration pneumonitis or Mendelson’s syndrome.1–3 Several studies have reported that severe pulmonary complications are likely to occur if the pH of the gastric content is < 2.54–6 and the volume is ≥ 0.5 mL/kg.5–7
International guidelines recommend that patients refrain from consuming food or drinks for at least 6 to 8 hours before procedures are performed under anesthesia. Nevertheless, some patients admit to having refreshments, such as hard candies and chewing gum, during the fasting period. However, there are some discrepancies between established preoperative fasting guidelines. While the European Society of Anesthesiology allows chewing gum, candy, and smoking prior to procedures,8 the American Society of Anesthesiologists (ASA) has not specified comparable exceptions to the recommended 6 to 8 hours of fasting.1 As a result, some anesthesia providers may be unsure whether they should carry on anesthetizing patients for procedures or postpone the cases until an adequate fasting time is achieved. In the busy environment of the typical endoscopy unit, the postponement or even cancellation of cases where fasting requirements have not been met can delay patient management and disrupt workflows.
Based on direct observation of patients scheduled for esophagogastroduodenoscopy (EGD) at our institution (a tertiary-care, academic hospital), there have been times when patients have consumed hard candies before planned procedures. Cases involving the preprocedural chewing of gum have not yet been observed. This may be because chewing gum is not popular in the Asian population,9 especially in elderly Thai individuals. However, information about the effects of hard-candy consumption before gastrointestinal endoscopic (GIE) procedures is limited. A study by Hamid et al demonstrated that patients who ate lollipops and those in a completely fasted control group had no differences in gastric content volume or the incidence of pulmonary complications resulting from aspiration.10 Therefore, we hypothesized that procedures being performed under anesthesia could be allowed to proceed for patients who ingested some candies beforehand.
Our study primarily aimed to determine the effects of candy on the volume and pH of the gastric content of adult patients undergoing GIE procedures. The secondary outcome variables were complications, the difficulty of the procedures, and endoscopist and patient satisfaction.
Materials and Methods
Study Design
This rater-blinded, randomized, controlled study was conducted at a tertiary-care, university-affiliated teaching hospital. Before starting the research, its protocol was approved by the Siriraj Institutional Review Board, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand (approval number SI188/2564). The study was registered at the Thai Clinical Trial Registry (https://www.thaiclinicaltrials.org/show/TCTR20210625006). The recruited participants were outpatients scheduled for EGD and colonoscopy under anesthesia between October and December 2021. Patients were enrolled if they
- were aged between 18 and 70 years,
- had an American Society of Anesthesiologists (ASA) physical status classification of I to III,
- had undergone fasting following the standard ASA guidelines, and
- were able to comprehend spoken and written Thai.
Patients were excluded if they had a body mass index > 30 kg/m2 or a full stomach condition (esophageal atresia, gastric outlet obstruction, intestinal obstruction, upper gastrointestinal bleeding, or ascites). Patients were informed of their enrollment by telephone the evening before the GIE procedure and were reminded of it in the preprocedural holding area. Written informed consent was obtained in the holding area before the start of the procedure.
The patients were randomly assigned to a “candy group” or a “control group” (full fasting) via computer-generated randomization (www.randomization.com). The patients in the candy group were allowed to consume 3 zero-calorie, sugar-free candies (ingredients: sorbitol, 97.36%; peppermint flavor, 2%; and sucralose, 0.05%) within 2 hours before the start of anesthesia for EGD. The control group received nothing by mouth (NPO). The endoscopists were board-certified gastroenterologists; each had several years of experience to avoid performance bias. The endoscopists were blinded to the patients’ group allocations.
Topical pharyngeal anesthesia with 8 to 10 puffs of 10% lidocaine spray and moderate to deep sedation with 3 to 5 mg/kg/h of intravenous propofol infusion were performed to ensure adequate depth of anesthesia. Oral secretions were cleared by suctioning before endoscope insertion, while gastric content was aspirated under endoscopic visualization into a clean plastic container. Water flushing was prohibited before the gastric content was suctioned. The gastric volume was measured in mL by an appropriate plastic syringe, and the gastric pH was measured by a pH meter (Hanna Instruments SRL, Nusfalau, Salaj, Romania). Normally, the elective patients were scheduled for both EGD and colonoscopy. Therefore, colonoscopy was performed just after the completion of EGD by the same endoscopist under moderate to deep sedation by intravenous propofol. However, no variables were measured during colonoscopy.
Immediately after the EGD procedure ended, the endoscopists rated their overall satisfaction. However, the patients were asked to report their satisfaction later once they had fully recovered in the postanesthesia care unit (indicated by a sedation score of 0) to avoid the delay of the procedure considering that there was a large volume of patients per day. The patients would give the scores based on their satisfaction of the overall procedure. The endoscopist and patient satisfaction scores were on a Likert scale of 1 to 5, where 1 denoted “very dissatisfied” and 5 represented “very satisfied”.
Sample Size Calculation
The sample size was calculated by PASS sample size software (version 8.0; NCSS LLC, Kaysville, UT, USA). A previous report indicated that the gastric volume of patients who complied with standard preoperative ASA fasting guidelines was 20 ± 15 mL (mean ± SD).11 As the average body weight of a Thai adult was approximately 60 kg according to a previous report,12 the gastric volume that would cause pulmonary aspiration was ≥ 30 mL, given that the critical volume was ≥ 0.5 mL/kg.5–7 Assuming noninferiority for gastric volume with a test power of 0.9, an equivalent margin of 10, an actual difference of 0, SD of 15, alpha error of 0.025, and beta error of 0.09, the calculated sample size was 49 per group. With the addition of a 10% dropout rate, the final sample size per group was 54.
Statistical Analysis
The participants’ baseline characteristics were reported using descriptive statistics. The Kolmogorov–Smirnov test confirmed the normality of the data. Parametric data were reported as frequency (n), percentage, and mean ± standard deviation (SD) and compared using Student’s unpaired t-test or Fisher’s exact test for nominal and categorical data. Nonparametric data are reported as the median and interquartile range and were compared using the Mann–Whitney U-test. All data were demonstrated with an alpha error < 5%, and a probability (P) value < 0.05 was considered statistically significant. The statistical analyses were performed with PASW Statistics for Windows, version 18.0 (SPSS Inc, Chicago, IL, USA) and MedCalc Statistical Software, version 19.6.4 (MedCalc Software Ltd, Ostend, Belgium; https://medcalc.org; 2021).
Results
The study recruited 108 outpatients scheduled for EGD and colonoscopies. Of these, 54 patients were assigned to the candy group, and the remaining 54 patients were placed in the control group (Figure 1). The demographic characteristics of the groups were comparable. The mean age of the overall participants was 57.73 ± 9.53 years (mean ± SD), and most participants were female and classified as ASA II. The two groups had no statistically significant differences in their comorbidities (eg hypertension, type 2 diabetes mellitus, dyslipidemia, coronary artery disease, chronic kidney disease, and history of stroke). Approximately 40% of the participants in each group had been prescribed proton-pump inhibitors. The median NPO time was 11 hours. In the candy group, patients took some zero-calorie sugar-free candies within 2 hours (mean, 29.24 ± 21.66 min) before the start of anesthesia. There was no difference in the total procedural time of EGD and colonoscopy (Table 1).
 |
Table 1 Patient Characteristics, Proton-Pump Inhibitor Use, Indications for Endoscopy, Procedural Time, and Total Anesthesia Time |
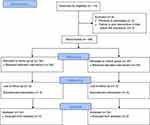 |
Figure 1 Flow diagram. |
All patients received topical pharyngeal anesthesia. The dosage was 8 to 10 puffs of 10% xylocaine spray; 4 to 5 puffs at a time; administered twice, with an interval of 3 to 5 min. Loss of gag reflex was ensured before applying intravenous sedation with propofol infusion. As the gastric content was visualized with the endoscope, it was entirely aspirated to the container. The median gastric volume of the participants was 23 (12.02–36.00) mL, with the volume of the candy group being significantly greater than that of the control group (28.5 vs 20.00 mL; P < 0.05). When adjusted to mL/kg, the median gastric volume of all participants was 0.36 (0.20–0.57) mL/kg. The medians of the candy and control groups were not significantly different (0.43 [0.27–0.67] mL/kg and 0.32 [0.19–0.55] mL/kg, respectively). The median difference in the gastric volume of the 2 groups was 6.00 (0.00–13.00) mL or 0.08 (–0.02–0.19) mL/kg (median [95% CI]). The overall gastric pH of the participants was 1.4, with the groups having similar values.
The endoscopists gave a rating of “very easy” to approximately three-quarters of the GIE procedures in the control group but to only around half of those in the candy group (Table 2). The patients with the history of PPI use (n = 44) had significantly higher gastric pH than the ones without PPI (n =64) (1.6 (13.-2.7) vs 1.3 (1.1–1.7), P = 0.012). Subgroup analysis was performed to determine the impact of PPI therapy on gastric pH. The gastric pH of non-PPI patients was not different between the candy and control group (1.3 (1.1–1.6) vs 1.3 (1.1–1.7), P = 0.909). Also, the gastric pH was not different between the PPI users in candy and control groups (1.5 (1.3–2.3) vs 1.6 (1.3–2.8), P = 0.363) (Table 3). Neither group had intraprocedural complications (such as oxygen desaturation or pulmonary aspiration). The endoscopists and patients also rated overall satisfaction. The median satisfaction scores of the endoscopists and the patients in the candy and control groups did not differ. Most endoscopists and patients reported being “very satisfied” (Table 4). The endoscopist and patient satisfaction scoring sheet was provided in Supplementary Data.
 |
Table 2 Patients’ Gastric Volume and pH |
 |
Table 3 The Impact of Proton-Pump Inhibitor on Gastric pH |
 |
Table 4 Complications, Difficulty of Procedure, and Endoscopist and Patient Satisfaction |
Discussion
There is no agreement between standard international guidelines about the time patients should refrain from consuming chewing gum or hard candies before procedures requiring anesthesia. Numerous studies have investigated the change in gastric content volume and acidity following chewing gum relative to the effects of standard fasting. However, the results of these investigations varied. Whereas some studies reported that gastric content volume increased due to chewing gum,11,13,14 other researchers disagreed, finding that the volume was similar to that of NPO patients.10,15–17 On the other hand, there is consensus among investigators regarding the acidity of gastric content. All studies agreed that gastric pH was not affected by chewing gum, with the pH after chewing being similar to that of NPO patients.10,11,13–15,17
Regarding preoperative hard-candy sucking, there are few studies on its effect on the characteristics of gastric content. Interestingly, 1 study that investigated this aspect with a particular type of sugar candy, lollipops, found no difference in gastric volume or pH compared with a standard fasting group.10 However, the sample size in the study was small, and the result has yet to be confirmed.
Our study explored the effects of hard-candy consumption during the preoperative period. We focused on hard candies in the form of lozenges rather than lollipops or candy sticks, which are less culturally familiar and less commercially available in Thailand. The patients were allowed to suck and chew the candies to imitate the actual behavior of candy ingestion. A significantly greater gastric content volume was observed in the candy group than in the control group. This finding might be because hard candy can stimulate saliva production,18 and candy chewing itself also encourages saliva production via the movement of the masticatory muscles.19 This increased saliva was naturally swallowed into the stomach by the patients during their wait in the preoperative holding area.
We found that the gastric content volume of the standard fasting patients (20 mL) equaled that reported by another study.11 Although the volume for the candy group was higher than that for the control group, it was still less than the presumed value for pulmonary aspiration risk that we had initially hypothesized (30 mL). Intriguingly, when the gastric volume was converted to mL/kg, the volumes of the candy and control groups were < 0.5 mL/kg each, indicating that the patients were safe from aspiration complications. Nevertheless, recent publications have suggested that pulmonary aspiration might occur when the gastric volume is larger than previously believed. The upper limit of the normal gastric volume in standard-fasted patients might be as high as 1.5 mL/kg.20–22 Even in elective patients undergoing preoperative fasting according to recognized guidelines, 4.5% of the patients still had > 1.5 mL/kg of gastric volume in the stomach.20 The gastric pH value was similar between groups and corresponded with previous reports.10,15 Taken together, these data show that administering hard candies before endoscopic procedures under anesthesia is safe. The absence of adverse events in our study supports this conclusion.
Regarding the endoscopists’ satisfaction, they rated a significantly smaller proportion of the GIE procedures in the candy group as “very easy” than in the control group. The endoscopists reported that the esophagus and stomach of the candy group patients appeared frothier than those of the control group patients.
Although international guidelines suggest that the fasting period should be 6 to 8 hours,1,8 in practice, patients might fast much longer. The average fasting duration for adult elective operations was reported to be between 9 and 12 hours;23 however, durations > 20 hours have been found at our institution. The disadvantages of prolonged fasting periods are thirst, hunger, anxiety, increased risk of postoperative nausea and vomiting, and patient dissatisfaction.24–26 The current investigation demonstrated that most patients were very satisfied with the EGD process and anesthesia despite consuming candies to relieve thirst or possible unpleasant symptoms before the procedure. However, 1 patient in our control group reported being “somewhat dissatisfied” after experiencing postprocedural dizziness.
Our work had many strengths. For example, there were healthy patients and individuals with several comorbidities to generate generalizability. In addition, bias was reduced by using certified endoscopists who had a diversity of experience. From our results, the gastric pH was influenced by the use of PPI as the PPI users had significantly higher pH. In contrast, candy itself did not affect gastric pH because there was no difference between the gastric pH of the non-PPI patients who were in the candy and control groups. Furthermore, the patients with prescribed proton-pump inhibitors were equally stratified between the groups to minimize interference from gastric pH interpretation. Moreover, our study is the first to report endoscopist and patient satisfaction for intervention and control groups. Finally, compared with a previous report on preoperative lollipop sucking where a gastric tube was placed for gastric volume measurement,10 we used direct endoscopic visualization for better accuracy.
One of the limitations of our study was that we did not include sugar-containing candies for fear that they might alter the blood sugar levels of diabetic participants. Nonetheless, previous work demonstrated that sugar affects neither gastric volume nor saliva production.15,19 Additionally, regarding patient satisfaction, we did not specifically ask whether they felt satisfied with having candies or maintaining a fasting state. Therefore, the satisfaction score might not directly reflect the benefits or drawbacks of preoperative candy consumption.
Conclusion
This study found that consuming hard candies did not affect the pH of gastric content and did not result in the accumulation of gastric content volume to the level of aspiration risk. Although not mentioned in the ASA guidelines for preoperative fasting, hard-candy consumption before elective EGD or other surgical procedures could be allowed. Scheduled operations should not be delayed or canceled if the patients have consumed hard candies during the nominal fasting period. Future directions would be to incorporate information regarding candy consumption into institutional and national guidelines for preoperative patient preparation.
Data Sharing Statement
The participant data are not publicly available. The data presented in this study are available on request from the Institutional Review Board and the corresponding author.
Acknowledgments
The authors thank Ms. Chayanan Thanakiattiwibun, Research Assistant, Integrated Perioperative Geriatric Excellent Research Center, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand for her contribution to the administrative work and data analysis. The authors also thank Assistant Professor Chulaluk Komoltri, Statistician, Division of Clinical Epidemiology, Department of Research Development, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand for data analysis and advice on data interpretation. The authors are also indebted to Mr David Park for the English-language editing of this paper.
Funding
This study received funding from the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (IO: R016531057). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. American Society of Anesthesiology. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376–393.
2. Engelhardt T, Webster NR. Pulmonary aspiration of gastric contents in anaesthesia. Br J Anaesth. 1999;83:453–460. doi:10.1093/bja/83.3.453
3. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Surv Anesthesiol. 1994;38:185. doi:10.1097/00132586-199406000-00059
4. Teabeaut JR. Aspiration of gastric contents: an experimental study. Am J Clin Pathol. 1952;28:51.
5. Exarhos ND, Logan WD, Abbott OA, Hatcher CR. The importance of pH and volume in tracheobronchial aspiration. Dis Chest. 1965;47:167–169. doi:10.1378/chest.47.2.167
6. Kinni ME, Stout MM. Aspiration pneumonitis: predisposing conditions and prevention. J Oral Maxillofac Surg. 1986;44:378–384. doi:10.1016/S0278-2391(86)80033-7
7. Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859–868. doi:10.1213/00000539-197453060-00010
8. Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28(8):556–569. doi:10.1097/EJA.0b013e3283495ba1
9. Consumption frequency of chewing gums in Japan 2017. Statista; 2022. Available from: https://www.statista.com/statistics/860380/japan-chewing-gum-consumption-frequency/.
10. Hamid K, Masoud L, Reza FH, Mehran G, Karmella K. Comparison of different non-pharmacological preoperative preparations on gastric fluid volume and acidity: a randomized controlled trial. Anaesth Pain Intensive Care. 2019;16:165–168.
11. Soreide E, Holst-Larsen H, Veel T, Steen PA. The effects of chewing gum on gastric content prior to induction of general anesthesia. Anesth Analg. 1995;80(5):985–989. doi:10.1097/00000539-199505000-00023
12. “Size Thailand”: the standard sizes of Thai people. Thailand Board of Investment; 2017. Available from: https://www.boi.go.th/un/boi_event_detail?module=news&topic_id=4099.
13. Goudra BG, Singh PM, Carlin A, et al. Effect of gum chewing on the volume and pH of gastric contents: a prospective randomized study. Dig Dis Sci. 2015;60:979–983. doi:10.1007/s10620-014-3404-z
14. Ouanes JP, Bicket MC, Togioka B, Tomas VG, Wu CL, Murphy JD. The role of perioperative chewing gum on gastric fluid volume and gastric pH: a meta-analysis. J Clin Anesth. 2015;27:146–152. doi:10.1016/j.jclinane.2014.07.005
15. Dubin SA, Jense HG, McCranie JM, Zubar V. Sugarless gum chewing before surgery does not increase gastric fluid volume or acidity. Can J Anaesth. 1994;41:603–606. doi:10.1007/BF03010000
16. Bouvet L, Loubradou E, Desgranges FP, Chassard D. Effect of gum chewing on gastric volume and emptying: a prospective randomized crossover study. Br J Anaesth. 2017;119:928. doi:10.1093/bja/aex270
17. Best GW, Fanning SB, Robertson IK, Blackford D, Mitchell BL. Assessing the effect of sugar-free chewing gum use on the residual gastric volume of patients fasting for gastroscopy: a randomised controlled trial. Anaesth Intensive Care. 2019;47:541–547. doi:10.1177/0310057X19886881
18. Alghsoon S, von Rosenvinge E. Effect of hard candy on salivary production. Am J Gastroenterol. 2021;16:S1354. doi:10.14309/01.ajg.0000786680.31593.4f
19. Brudevold F, Kashket S, Kent RL. The effect of sucrose and fat in cookies on salivation and oral retention in humans. J Dent Res. 1990;69:1278–1282. doi:10.1177/00220345900690061101
20. Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118:363–371. doi:10.1093/bja/aew435
21. Van de Putte P, Perlas A. The link between gastric volume and aspiration risk. In search of the holy grail? Anaesthesia. 2018;73:274–279. doi:10.1111/anae.14164
22. Ohashi Y, Walker JC, Zhang F, et al. Preoperative gastric residual volumes in fasted patients measured by bedside ultrasound: a prospective observational study. Anaesth Intensive Care. 2018;46:608–613. doi:10.1177/0310057X1804600612
23. Tosun B, Yava A, Açıkel C. Evaluating the effects of preoperative fasting and fluid limitation. Int J Nurs Pract. 2015;21:156–165. doi:10.1111/ijn.12239
24. Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344–1350. doi:10.1097/00000539-200111000-00063
25. Dennhardt N, Beck C, Huber D, et al. Optimized preoperative fasting times decrease ketone body concentration and stabilize mean arterial blood pressure during induction of anesthesia in children younger than 36 months: a prospective observational cohort study. Pediatr Anesth. 2016;26:838–843. doi:10.1111/pan.12943
26. Frykholm P, Schindler E, Sümpelmann R, Walker R, Weiss M. Preoperative fasting in children: review of existing guidelines and recent developments. Br J Anaesth. 2018;120:469–474. doi:10.1016/j.bja.2017.11.080
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
