Back to Journals » Clinical Epidemiology » Volume 11
Guillain-Barré syndrome in Denmark: validation of diagnostic codes and a population-based nationwide study of the incidence in a 30-year period
Authors Levison LS , Thomsen RW , Christensen DH , Mellemkjær T , Sindrup SH, Andersen H
Received 29 December 2018
Accepted for publication 16 February 2019
Published 18 April 2019 Volume 2019:11 Pages 275—283
DOI https://doi.org/10.2147/CLEP.S199839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Irene Petersen
Lotte Sahin Levison,1 Reimar Wernich Thomsen,2 Diana Hedevang Christensen,2 Thomas Mellemkjær,1 Søren Hein Sindrup,3 Henning Andersen1
1Department of Neurology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 3Department of Neurology, Odense University Hospital, Odense, Denmark
Purpose: To validate the diagnostic codes for Guillain-Barré syndrome (GBS) in the Danish National Patient Registry (DNPR). Secondly, to examine 30-year trends in the incidence of GBS in Denmark.
Patients and methods: We used the DNPR to identify all patients aged 16 and above diagnosed with a primary GBS diagnosis at any Danish department of neurology between 1987 and 2016. Medical files were reviewed according to the clinical criteria of the National Institute of Neurological Disorders and Stroke Committee and classified according to the Brighton criteria. The incidence rate (IR) was calculated based on data from 1987 to 2016 and stratified by season, gender, and age.
Results: Over 30 years, we identified 2,319 patients aged 16 and above in the DNPR. From a validation cohort of 573 patients, we were able to retrieve 425 (74.2%) medical files; 356 GBS diagnoses were confirmed. The overall positive predictive value was 83.8% (95% confidence interval (CI): 80.0–87.0). In 99% of the confirmed patients, the Brighton criteria level 1–3 for GBS were met. The IR was fairly stable over 30 years at 1.77 per 100,000 person years (95% CI: 1.70–1.84). The incidence was higher in the winter season (IR ratio compared with summer: 1.18 (95% CI: 1.09–1.29)), and was strongly associated with male gender (IR ratio vs females: 1.44 (95% CI: 1.33–1.57)). IRs rose with age at diagnosis, particularly after the age of 50 in both men and women and a minor peak was observed for total IR in young adults.
Conclusion: Primary diagnostic codes for GBS at Danish departments of neurology have high validity. The DNPR is a well-suited data source for epidemiological research on GBS. The Danish nationwide 30-year GBS IR is stable over time and similar to GBS IRs reported in other European and North American populations.
Keywords: registries, positive predictive value, international classification of disease codes, epidemiology
Introduction
Guillain-Barré syndrome (GBS) is an acute inflammatory disorder of peripheral nerves characterized by rapidly progressive, symmetric weakness, and areflexia. Respiratory insufficiency develops in about 25% of cases, rendering mechanical ventilation crucial and long-term hospital admission necessary.1
The variable incidence of GBS in different populations may reflect differential genetic susceptibility or exposure to causative pathogens.2,3 A comprehensive review estimated the incidence rates of GBS in North America and Europe at 1–2 per 100,000 person years (PY).2 Given the rarity of the disease, most of the published studies on GBS incidence rate and trends have been performed over long periods.4–8 The use of prospectively obtained administrative health care data may be a valuable and cost-efficient method for large-scale epidemiological research in GBS. Such data are routinely collected for administrative purposes, but the potential lack of completeness and accuracy of diagnoses may question the use for research purposes.9–12
In the present nationwide study, we aimed to examine the quality of the GBS discharge diagnosis in the Danish National Patient Registry (DNPR) by estimating the positive predictive value (PPV), using information from medical records. We furthermore examined the nationwide GBS incidence rate over a 30-year period in Denmark, from 1987 to 2016.
Materials and methods
Setting and data sources
Denmark has 5.7 million citizens, and the country is divided into five regions.13 The Danish National Health Service provides all inhabitants with tax-supported health care. Since 1968, all Danish citizens have been registered in the Civil Personal Registry and have been given a unique 10-digit civil registry number (CPR number). This number contains information on birth date and gender, and enables unique identification and matching of registry data at the individual level.14,15 The DNPR was established in 1977 and contains data on all somatic hospital admissions, including CPR number, admission, and discharge dates, hospital department, primary discharge diagnosis code (the primary reason for hospitalization), and supplementary diagnosis codes.16 All neurological hospital care is provided by the five Danish regions through 14 neurological departments, comprising five university hospitals and nine general hospitals. Hospital data are recorded prospectively for reimbursement purposes, independently of specific research questions. Medical diagnoses have been registered in the DNPR using the International Classification of Disease, version 8 (ICD-8) from 1977 through 1993 and the International Classification of Disease, version 10 (ICD-10) from 1994 onwards.17
The study was approved by the Danish Data Protection Agency (record number 1-16-02-817-17) and the Danish Patient Safety Authority (record number 3-3013-2316/1, 3-3013-2316/2).
Study population
Using the DNPR, we identified all patients with discharge diagnoses consistent with GBS: ICD-8: 354.00: Polyradiculitis acuta (21.7% of all the GBS diagnoses) and ICD-10: DG61.0: GBS (78.3% of all the GBS diagnoses) and restricted the population to those with the first GBS discharge diagnosis (N=3,357). To ensure the highest quality of data, we included only cases that fulfilled the following criteria: 1) primary GBS diagnoses, as the potential severity of the disease usually causes admission and thus secondary or tertiary diagnosis will include less accurate diagnoses, and 2) diagnoses made at a department of neurology, as other departments rarely treat GBS and may have insufficient clinical experience with diagnosing adult GBS.11,18 The study was based on data from adult patients (>15 years of age) in the period from 1987 to 2016, thus containing a population of 2,319 unique GBS patients.
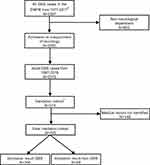
From the study population described above, we aimed to produce a representative sample of the Danish population for validation purpose. We included five neurological departments (including two university hospitals and three general hospitals) in three of the five Danish regions. We sampled two-thirds of the cases from university hospitals and one-third from general hospitals, since the university hospitals treat the majority of GBS cases. From this subsample, we randomly selected 573 cases, constituting our validation cohort. A flowchart of the selection process is shown in Figure 1. Contributing hospitals and departments are listed in Table S1.
Validation
Validation was performed on the final validation cohort, using information from medical records. Medical records were identified using the CPR number and were manually reviewed. All records were reviewed by the same physician (LL), and uncertain cases were reviewed together with a senior neurologist (HA). Neurophysiological examinations were manually reviewed and classified into subtypes according to the criteria presented by Hadden et al19. Cases were categorized as GBS or non-GBS according to the diagnostic criteria of the National Institute of Neurological Disorders and Stroke (NINDS), relying on a combination of clinical features, findings on cerebrospinal fluid (CSF), and nerve conduction studies (NCS). We included the variant syndromes and subsequently classified all cases according to the Brighton criteria. The Brighton criteria were applied in order to add the level of diagnostic certainty (graded 1–4) and to ensure comparability with other GBS studies.20–23
Statistical analysis
PPV was used as a measure of diagnostic validity and estimated as the proportion of GBS cases identified in the final validation cohort that fulfilled the NINDS criteria for GBS according to the medical files. Confidence intervals of 95% (CIs) based on binomial distribution were computed using the Wilson score.24 The analyses were stratified by year of diagnosis, gender, hospital (general vs university hospital), and age at diagnosis to evaluate for any difference in PPV. The average annual GBS incidence was estimated based on the corresponding mid-year population (>15 years of age) of Denmark obtained from Statistics Denmark13 and 95% CIs were calculated assuming a Poisson distribution. Incidence rates were also calculated for three 10-year periods. Differences in incidence rates by season (October–March vs April–September), gender and age were evaluated by calculating incidence rate ratios (IRR). Data were analyzed using STATA version 15.1. We employed joinpoint regression analysis to further examine incidence time trends, which include fitting a series of straight lines to the IR trend.25 Each join-point describes a statistically significant (P<0.05) change in the trend of the slope of the line segment. We evaluated IR variations over time by gender and for the total IR over the 30-year study period, and also for season and for age at diagnosis, using Joinpoint Trend Analysis Software version 4.6.0.0.
Results
Descriptive data
The final study population included 2,319 adult GBS cases from 1987 to 2016, 1,348 males (58.1%) and 971 females (41.9%). The average age at first GBS diagnosis was 52.7 years (range 16.1–95.8 years). From the validation cohort of 573 adult cases at five hospitals, 425 medical files (74.2%) could be located (Figure 1). The 148 missing medical records were primarily from two hospitals (Aarhus University Hospital and Kolding Hospital: 122 (82.4%) of 148 missing medical records), with ~80% being older records from the period before 2005. In the final validation sample, the average age at diagnosis was 51 years (range 16–94 years) and 56.5% were male, similar to the overall study population. In 356 of 425 cases, the diagnosis was found to be GBS. Of the confirmed cases, 44.9% met the Brighton criteria level 1% and 43.5% were classified as level 2, 10.4% and 1.1% were classified as level 3 and 4, respectively. Variant syndromes accounted for 1.6% encompassing Miller Fisher syndrome only. Of the 69 non-GBS cases, seven initially met the GBS criteria but were later diagnosed with the acute onset of chronic inflammatory demyelinating polyneuropathy (six cases) and mononeuritis multiplex (one case), respectively.
Positive predictive values
The overall PPV was 83.8% (95% CI: 80.0–87.0). Stratifying for gender, year of diagnosis, age at diagnosis and type of hospital, the highest PPV was achieved for diagnoses registered in the most recent calendar period (2007–2016), at age ≥65, and in cases admitted to a university hospital (Table 1). If the Brighton criteria including level 1–3 were applied, the PPV was 82.8% (95% CI: 79.0–86.1).
 | Table 1 Validity of ICD-codes for Guillain-Barré syndrome in the DNPR |
Incidence rates
The incidence rate was calculated for the period 1987–2016: A total of 2,319 unique adult individuals received a GBS diagnosis during this period, and the general adult population accumulated a total of 131,209,065 PY. The overall incidence rate was 1.77 per 100,000 PY (95% CI: 1.70–1.84), incidence rates for three 10-year periods and per year are presented in Table 2. A fairly stable incidence was observed with no differences in IRR when comparing the latest two decades with the first (Table 2) and no statistically significant changes in IR time trend were observed for males, females, or for the total IR. One calendar year, 2011, stood out, however (Figure 2) with an IR of 2.36 (95% CI: 2.09–3.04 per 100,000 PY) and a peak-through ratio versus the lowest incidence in 2008 of 1.84 per 100,000 PY (95% CI: 1.34–2.56). Moreover, an increased incidence in the winter compared to the summer season was observed (IRR: 1.18 (95% CI: 1.09–1.29)) (Figure 3). Throughout the months of diagnosis, we found a statistically significant IR concavity in May (95% CI: March–August), with a peak-through ratio of 1.62 per 100,000 PY (95% CI: 1.32–2.00) when comparing January and May incidences. The IRR for males versus females was clearly increased at 1.44 (95% CI: 1.33–1.57). The incidence rates rose with age, particularly after the age of 50, in both men and women. When stratifying the age at diagnosis in 10-year age groups, we observed a peak in the age group 70–79 years (Table 2). Trends in IRs by continuous age at diagnosis were also evaluated using one-year interval, however, after the age of 85 the total number of cases per year of age was low (range: 0–14), thus these IR estimates were rather uncertain (Figure 4). First, we applied trend analyses for cases diagnosed from 16 to 90 years, which produced two statistically significant peaks at 34 (95% CI: 29–38) years of age and at 72 (95% CI: 65–76) years of age, respectively. Second, we restricted the joinpoint analyses to cases diagnosed from 16 to 85 years of age, and observed two statistically significant IR peaks at the age of 34 (95% CI: 29–38) years of age and at 68 (95% CI: 58–73) years of age, respectively. Among males, we observed two IR peaks, at 35 (95% CI: 29–37) years of age and 72 (95% CI: 57–78) years of age, respectively, with only the peak at 72 years being statistically significant. Among females, only one IR peak was identified at 68 (95% CI: 54–75) years of age, this peak was statistically significant.
 | Table 2 GBS Incidence rates and incidence rate ratios per 100,000 person years |
 | Figure 2 Annual GBS incidence per 100,000 from 1987 to 2016 in males and females.Abbreviation: GBS, Guillain-Barré syndrome. |
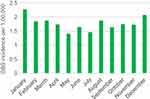 | Figure 3 Monthly GBS incidence per 100,000 during the period from 1987 to 2016. Abbreviation: GBS, Guillain-Barré syndrome. |
 | Figure 4 Incidence per 100,000 during the period from 1987 to 2016 by age at diagnosis.Abbreviation: GBS, Guillain-Barré syndrome. |
Discussion
In our validation study, the vast majority of the GBS diagnoses were confirmed by review of medical records, yielding a high PPV. This supports the use of the DNPR in epidemiological studies of GBS in Denmark. Our data showed a slightly higher PPV in the age group ≥65 years, in cases admitted at university hospitals, in cases diagnosed after the year 2007 and among males. These data are fairly similar to those from an American GBS validation study of the ICD-9 code 357.0.11 They found a PPV of 70% when requiring the GBS code to be in a primary position on an inpatient claim and the diagnosis based on a neurologist consultation. Lower PPVs were obtained in earlier studies of the ICD-9 hospital discharge diagnosis, where primary inpatient GBS code and neurological department admission was not compulsory.26–28 Further, the ICD-9 code of 357.0 included acute infectious or post-infectious polyneuritis, acute idiopathic polyneuritis, and febrile polyneuritis, and did not have a separate diagnostic GBS coding.
We did not examine the sensitivity of GBS coding in our study. In a recent study, our group retrieved all medical records from patients coded with GBS and adjacent diagnoses in a nationwide cohort restricted to the period September 2012 to December 2015. This study included both adults and children; both primary, secondary and tertiary ICD-10 diagnoses of GBS (DG61.0); adjacent codes for other inflammatory neuropathies (DG61.1, DG61.8, and DG61.9); and both patients contacting the emergency room, hospital outpatient specialist clinic visits, and inpatient hospital admissions.18 Of 299 validated GBS cases, 5.4% were found in the subgroup of patients with adjacent diagnostic codes (DG61.1, DG61.8, and DG61.9), and 7.4% had been admitted at non-neurological departments. The PPV was much lower at non-neurological departments (19.9%) compared with departments of neurology (63.5%). These results suggest that due to the lack of completeness, we may have underestimated the GBS incidence slightly. However, in future analytical epidemiological studies on GBS risk and prognostic factors, validity of the diagnosis is of high importance, and a patient population with primary GBS diagnoses from neurological departments may be well-suited.29
We found an overall incidence rate of 1.77 per 100,000 PY (95% CI: 1.70–1.84) over the 30-year period. Al-Hakem et al, reported a slightly lower incidence rate of 1.59 per 100,000 PY, most likely explained by the inclusion of children, who are shown to have lower GBS IR.18,30 The aetiological explanations responsible for the IR peak in 2011 are not clear, however, no concomitant increase in influenza vaccinations or campylobacter infections were reported by Statens Serum Institut, an auspices of the Danish Ministry of Health.31 With regard to the slightly higher incidence observed during the winter, previous studies have shown ambiguous results. A recent French nationwide study of 9,391 patients hospitalized with a principal GBS diagnosis between 2008 and 2013 found a statistically significantly higher incidence in the winter than in the summer, in line with our findings.3,32 The seasonal variation may be caused by concomitant increased incidence of infections in the winter.32 The higher incidence in males as compared to females and the increasing incidence with age is consistent with findings in most other studies.2 Also, a bimodal pattern of incidence by age with peaks occurring in young adults and the elderly, indicated by our data, has been described in western populations.33,34
The strengths of the present study include the use of national registry-based data, which minimizes self-selection bias to certain clinics, and allowed us to sample patients at the date of the first diagnosis, so length-time bias is avoided. All Danish residents have free access (tax-funded) to medical care including hospital admission and treatment, and all medical care is registered in one nationwide system, which minimizes risk of selection problems. Data in the registries are recorded by the treating physicians and collected mainly for administrative use, and therefore unrelated to research purposes. Therefore, the risk of recall and nonresponse bias is low.
In the present study, the Brighton criteria were as suitable as the NINDS criteria to identify the GBS patients. The Brighton criteria contribute with information on the levels of diagnostic certainty depending on patient characteristics and the availability of the data. The predominant cause for not reaching level 1 was normal or missing information of the protein concentration in CSF (36.0%) and normal or missing information of the NCS (30.3%). The Brighton level distribution in the present study is comparable to that in a previous European GBS cohort of 494 patients.22
Limitations
Our study has a number of limitations to be considered. First, our validation study was performed using data from only three of the five regions in Denmark. Regional differences in diagnostic practice may in theory affect the PPV, but owing to the uniform nature of the Danish health care system, the structure of record keeping, and the inclusion of neurological departments at both general and university hospitals, we consider the results to be generalizable for the entire country. Second, because we did not include data on undiagnosed patients with GBS in this study, we were unable to estimate the negative predictive value, sensitivity, and specificity of the GBS diagnosis.35 However, due to the acute and severe symptoms and clinical signs in GBS, only few patients likely go undiagnosed, and misdiagnosis eg, with adjacent ICD-10 diagnostic codes seems rather infrequent.18 Third, a number of potential cases were excluded from validation in our study because their medical records were missing. These records were unavailable for review mainly due to administrative reasons such as destruction of paper archives as the medical records were digitalized. Fourth, the Brighton criteria are sensitive to lack of sufficient documentation of the key diagnostic characteristics: Objective clinical findings, CSF analysis, and NCS, as missing information in these regard would classify more cases in the level 4 group. Thus, it is especially important when the size of the level 4 group is not negligible compared to the group of analyzed cases. However, only four persons were classified as Brighton level 4 (1.1%) among our GBS cases.2,22,36 Finally, a potential limitation may arise from the focus on the adult population and study variables may perform differently in a childhood population, and thus should be a focus in future studies.
Conclusion
This study shows that primary GBS discharge diagnosis codes from neurological departments in Denmark have high validity. The Danish GBS incidence rate over 30 years is remarkably stable and similar to GBS incidence rates reported in other western populations, with important risk increases related to the winter season, male gender, and higher age. Our findings support the use of the DNPR as a valuable data source for epidemiological research on GBS.
Acknowledgments
We thank biostatistician Lisbeth Munksgård Baggesen for skillful assistance in the data management process and the assistance in the employment of Joinpoint Trend Analysis Software. This work received funding from teacher Svend Aage Nielsen Wacherhausens Foundation, Aase and Ejnar Danielsen Foundation and A.P. Møller Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–482. doi:10.1038/nrneurol.2014.121
2. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barre syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123–133. doi:10.1159/000324710
3. Delannoy A, Rudant J, Chaignot C, Bolgert F, Mikaeloff Y, Weill A. Guillain-Barre syndrome in France: a nationwide epidemiological analysis based on hospital discharge data (2008–2013). J Peripher Nerv Syst. 2017;22(1):51–58. doi:10.1111/jns.12202
4. Bak P. Guillain-Barre syndrome in a Danish county. Neurology. 1985;35(2):207–211.
5. Congia S, Melis M, Carboni MA. Epidemiologic and clinical features of the Guillain-Barre’ syndrome in Sardinia in the 1961–1980 period. Acta Neurol (Napoli). 1989;11(1):15–20.
6. Winner SJ, Evans JG. Age-specific incidence of Guillain-Barre syndrome in Oxfordshire. Q J Med. 1990;77(284):1297–1304.
7. Govoni V, Granieri E, Casetta I, et al. The incidence of Guillain-Barre syndrome in Ferrara, Italy: is the disease really increasing?. J Neurol Sci. 1996;137(1):62–68.
8. Cuadrado JI, de Pedro-Cuesta J, Ara JR, et al. Guillain-Barre syndrome in Spain, 1985–1997: epidemiological and public health views. Eur Neurol. 2001;46(2):83–91. doi:10.1159/000050769
9. Jiang GX, de Pedro-Cuesta J, Fredrikson S. Guillain-Barre syndrome in south-west Stockholm, 1973–1991, 1. Quality of registered hospital diagnoses and incidence. Acta Neurol Scand. 1995;91(2):109–117.
10. Shui IM, Rett MD, Weintraub E, et al. Guillain-Barre syndrome incidence in a large United States cohort (2000–2009). Neuroepidemiology. 2012;39(2):109–115. doi:10.1159/000339248
11. Funch D, Holick C, Velentgas P, et al. Algorithms for identification of Guillain-Barre syndrome among adolescents in claims databases. Vaccine. 2013;31(16):2075–2079. doi:10.1016/j.vaccine.2013.02.009
12. Huang WC, Lu CL, Chen SC. A 15-year nationwide epidemiological analysis of Guillain-Barre syndrome in Taiwan. Neuroepidemiology. 2015;44(4):249–254. doi:10.1159/000430917
13. Statistik Denmark. 2018. Available from:
14. Epidemiology FL. When an entire country is a cohort. Science (New York, NY). 2000;287(5462):2398–2399. doi:10.1126/science.287.5462.2398
15. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. doi:10.1177/1403494810387965
16. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
17. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi:10.1177/1403494811401482
18. Al-Hakem H, Sindrup SH, Andersen H, et al. Guillain-Barre syndrome in Denmark: a population-based study on epidemiology, diagnosis and clinical severity. J Neurol. 2019;266(2):440–449.
19. Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Ann Neurol. 1998;44(5):780–788. doi:10.1002/ana.410440512
20. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barre syndrome. Ann Neurol. 1990;27(Suppl):S21–S24.
21. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barre syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. doi:10.1016/j.vaccine.2010.06.003
22. Fokke C, van Den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33–43. doi:10.1093/brain/awt285
23. Wakerley BR, Yuki N. Mimics and chameleons in Guillain-Barre and Miller Fisher syndromes. Pract Neurol. 2015;15(2):90–99. doi:10.1136/practneurol-2014-000937
24. Wilson EB. Probable Inference the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–212. doi:10.1080/01621459.1927.10502953
25. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351.
26. Koobatian TJ, Birkhead GS, Schramm MM, Vogt RL. The use of hospital discharge data for public health surveillance of Guillain-Barre syndrome. Ann Neurol. 1991;30(4):618–621. doi:10.1002/ana.410300418
27. Bogliun G, Beghi E. Validity of hospital discharge diagnoses for public health surveillance of the Guillain-Barre syndrome. Neurol Sci. 2002;23(3):113–117. doi:10.1007/s100720200036
28. Lee CD, Jones TF. Hospital discharge database optimization in Guillain-Barre syndrome surveillance. Muscle Nerve. 2012;46(1):60–62. doi:10.1002/mus.23261
29. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. doi:10.1016/j.jclinepi.2004.10.012
30. Rabie M, Nevo Y. Childhood acute and chronic immune-mediated polyradiculoneuropathies. Eur J Paediatr Neurol. 2009;13(3):209–218. doi:10.1016/j.ejpn.2008.04.009
31.
32. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barre syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150–163. doi:10.1159/000184748
33. Rees JH, Thompson RD, Smeeton NC, Hughes RA. Epidemiological study of Guillain-Barre syndrome in south east England. J Neurol Neurosurg Psychiatry. 1998;64(1):74–77.
34. Kaplan JE, Katona P, Hurwitz ES, Schonberger LB. Guillain-Barre syndrome in the United States, 1979–1980 and 1980–1981. Lack of an association with influenza vaccination. JAMA. 1982; 248(6):698–700.
35. Loong TW. Understanding sensitivity and specificity with the right side of the brain. BMJ (Clini Res Ed). 2003;327(7417):716–719. doi:10.1136/bmj.327.7417.716
36. Roodbol J, de Wit MY, van Den Berg B, et al. Diagnosis of Guillain-Barre syndrome in children and validation of the Brighton criteria. J Neurol. 2017;264(5):856–861. doi:10.1007/s00415-017-8429-8
Supplementary material
 | Table S1 Contributing hospitals and departments |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.