Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Graft outcomes following immunosuppressive therapy with different combinations in kidney transplant recipients: a nationwide cohort study
Authors Tsai YF , Liu FC, Kuo CF , Chung TT, Yu HP
Received 1 February 2018
Accepted for publication 20 April 2018
Published 12 June 2018 Volume 2018:14 Pages 1099—1110
DOI https://doi.org/10.2147/TCRM.S164323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Yung-Fong Tsai,1,2,* Fu-Chao Liu,1,2,* Chang-Fu Kuo,3,4 Ting-Ting Chung,3 Huang-Ping Yu1,2,5
1Department of Anesthesiology, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 2College of Medicine, Chang Gung University, Taoyuan, Taiwan; 3Division of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 4Office for Big Data Research, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 5Department of Anesthesiology, Xiamen Chang Gung Hospital, Xiamen, China
*These authors contributed equally to this work
Background: Immunosuppression plays an essential role to overcome immune-related allograft rejection, but it also causes some nephrotoxicity. This study aimed to investigate how the immunosuppressant combinations affect graft outcomes in kidney transplant recipients.
Methods: A nationwide population-based cohort study using the Taiwan National Health Insurance Database was conducted. A total of 3,441 kidney transplant recipients who underwent kidney transplantation during the targeted period were included. The effects on graft outcomes contributed by conventional immunosuppressants, including corticosteroid, calcineurin inhibitors, antimetabolite purine antagonists, and mammalian target of rapamycin inhibitors, were compared.
Results: A total of 423 graft failures developed after the index date. Therapy regimens incorporated with purine antagonists had a comparable reduction of graft failure among four main drug groups regardless of whether they were given as monotherapy or in combination (adjusted hazard ratio: 0.52, 95% confidence interval: 0.42–0.63). Corticosteroid was found to have inferior effects among four groups (adjusted hazard ratio: 1.67, 95% confidence interval: 1.28–2.21). Furthermore, all 15 arrangements of mutually exclusive treatment combinations were analyzed by referencing with corticosteroid monotherapy. As referenced with steroid-based treatment, regimens incorporated with purine antagonists all have superior advantage on graft survival regardless of whether given in monotherapy (65% of graft failure reduced), dual therapy (48%–67% reduced), or quadruple therapy (43% reduced). In all triple therapies, only corticosteroid combined with calcineurin inhibitor and purine antagonist demonstrated superior protection on graft survival (52% of graft failure reduced).
Conclusion: The results may recommend several superior regimens for contributing to graft survival, and for supporting a steroid-minimizing strategy in immunosuppression maintenance.
Keywords: chronic rejection, corticosteroid, graft survival, purine antagonist, steroid-minimizing strategy
Introduction
Kidney transplantation for patients with end-stage renal disease is an effective option to improve the quality and length of life, and is globally increasing with time.1 Allograft rejections, including acute and chronic rejections, seriously limit the function and survival of transplant grafts.2,3 With the advancement of immunosuppressant therapy, acute rejection is dramatically decreased; however, chronic rejection is cause for long-term damage within a decade of transplant, and rates are poor for long-term survival.1,4 Chronic rejection happens relatively more frequently than does acute rejection, and is responsible for the majority of graft failure after transplantation.3
Immunosuppressive therapy is imperative to reduce the likelihood of immune-related injury to immunologically nonidentical kidney allograft and to prevent allograft rejection and functional failure. Immunosuppressive treatments for maintenance usually consist of multiple classes of agents which provide different mechanisms of action. A combination of immunosuppressants will be steadily maintained within the first few months after kidney transplantation. As the risks of acute and chronic rejection decrease over time, the maintenance immunosuppression is decreased to reduce the associated risks of infection and posttransplant malignancy.5 In some cases, modification of immunosuppression regimens is needed when drug toxicity or posttransplant complications develop. Almost all kidney transplant recipients (KTRs) are maintained on immunosuppressive therapy because of the risk of chronic rejection or late acute rejection. The best policy is to maximize the overall effectiveness of graft survival and to minimize the drawbacks of immunosuppressive combinations. The optimal maintenance regimen for kidney transplantation is not well established at present.
Since 1980, cyclosporine has been given to KTRs to suppress immunologic reactions, resulting in a boost in the success rate of organ transplantation.6,7 The dosage and time length of steroid use were slowly reduced to decrease systemic side effects and complications from the immunosuppressant itself. Calcineurin inhibitors are found to induce nephrotoxicity that could be a contributor to chronic rejection.8,9 It is essential to adjust the suitable concentration of immunosuppressive regimens and to develop novel agents in transplantation medicine. The protocol of multiple immunosuppressants is designed with less adverse effects and better therapeutic ratios.10
The conventional regimens include corticosteroid, calcineurin inhibitors, antimetabolite purine antagonists, and less routinely used mammalian target of rapamycin inhibitors (mTORIs).11 The first three immunosuppressants are the most commonly used maintenance combination by most transplant centers.12 The purpose of immunosuppression modifications is to reduce the side effects related to immunosuppressants, and keep the protective effects needed for graft survival. The combinations of regimens may be prescribed differently due to recipient tolerance, comorbidity, physician experience, and geographic area. Until now, there is still no general comparison for all immunosuppressant combinations on long-term observation. It is important to evaluate the best drug combinations of maintenance immunosuppression that provide superior prevention of chronic rejection and protection of graft survival. The purpose of the present study was to compare the protective benefits to chronic graft survival by different immunosuppressant combinations in kidney transplantation.
Methods
Data source and study population
Our population-based open cohort studied KTRs in Taiwan who received kidney transplant surgery between 1996 and 2011. We used the Taiwan National Health Insurance Research Database (NHIRD) as our primary data source, which routinely collects health information from the entire Taiwan National Health Insurance (NHI) beneficiaries since 1995. The NHI is a single-payer system, and all residents in Taiwan must enroll in this NHI by law, resulting in a coverage rate of over 99.6%.13 The NHIRD includes demographic data and diagnoses, medical procedures, operations, and prescriptions in primary and specialist care as well as information for other programs, including dental services, maternity, rehabilitation, pharmacy, and preventive care. All the information within the database is linked by a unique personal identification, which is encrypted before the database is released for research use. All retrieved data from the NHIRD were de-identified and computerized. The diagnostic coding system in NHIRD followed the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The validity, representativeness, and clinical consistency of this database have been reported.14
The study protocol was according to the guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital and the NHIRD research committee. All data on individual information and medical records that we received from the NHIRD in the study cohort were anonymized and de-identified; therefore, informed consent from the KTRs was unavailable due to privacy policy.
Inclusion and exclusion criteria of the study population
We initially identified 3,882 KTRs who received kidney transplantation from 1996 to 2011 in Taiwan (Figure 1). The selected KTRs were included on the basis of the operation code of kidney transplant surgery (ICD-9-CM coded 55.69) within this period. All the KTRs were treated with immunosuppressants for the purpose of offsetting the immunological rejection to increase graft survival. We excluded 29 patients who were not coded any prescription drugs for immunosuppression during the full cohort period. The main aim of present study was to evaluate the protective effects of different immunosuppressant combinations on graft failure. To avoid the interferences from surgical-related or postoperative complication-related graft dysfunctions, KTRs who survived more than 6 months after transplantation were identified (Figure 2). We excluded 110 patients who died within 6 months after transplantation, and 301 patients who received no immunosuppressant therapy for 6 months after transplant surgery. In order to reduce the effects from factors other than immunosuppressants on chronic rejection, the targeted period of observation was defined as the period since beginning 6 months after kidney transplantation. In addition, one patient was excluded due to incomplete coding for birth date and gender. Finally, 3,441 KTRs were included in our cohort study. In this study, the ascertainment of organ transplantation was considered to be essentially 100% complete since the costs of kidney transplantation are fully covered by the NHI program.
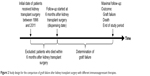  | Figure 2 Study design for the comparison of graft failure after kidney transplant surgery with different immunosuppressant therapies. |
Definition of graft failure in the study cohort
Only KTRs who survived more than 6 months after transplant were enrolled. The primary outcome of interest was graft failure, which was defined as requirement of another kidney transplant surgery, or for regular hemodialysis more than 3 months postoperatively. This was because some KTRs may remain on hemodialysis as a transitional treatment soon after kidney transplantation and wait for functioning of the donated kidney. The hemodialysis treatment was identified from the outpatient and inpatient files. Among 3,441 KTRs in the cohort, 423 patients were diagnosed with graft failure in the observation period, and 3,018 recipients were without graft failure.
Exposure data
The exposures of interest for KTRs were the four main types of immunosuppressants including corticosteroid (prednisolone), calcineurin inhibitors, purine antagonists, and mTORIs. Calcineurin inhibitors include cyclosporine and tacrolimus. Purine antagonists include azathioprine and mycophenolate mofetil. mTORIs include sirolimus and everolimus. All individual prescriptions for all types of immunosuppressants were extracted. We did two different analyses. First, we analyzed the usage of any type of immunosuppressant drug as a binary exposure variable for each treatment period, regardless of whether it was in combination with any other drug. Second, we grouped different combinations of four immunosuppressant types into 15 mutually exclusive groups for mono-, dual-, triple-, and quadruple-therapy combinations. The duration of each treatment period was defined from the earliest date of prescription to the latest date of that specific drug use for the group of treatment. The follow-up time was partitioned into different treatment periods, and each period corresponded to the specific type or combination of immunosuppressants. It could be partitioned as a no treatment period if no immunosuppressant was prescribed. If another group of immunosuppression drugs was added before the previous treatment was stopped, the treatment period of the initial treatment was defined from the earliest date of the initial treatment to the earliest date of the next treatment. This would be partitioned as two different treatment periods. We assumed that medications may still have withdrawal effects after drug use was stopped. The events would be added for 30 days after the last date on stopping drug use, and it was attributed to the previous treatment rather than counting as unexposed time.
Confounding variables
Besides the immunologic contributors, nonimmunologic ones are also considered to potentially contribute to acute or chronic kidney graft loss. The covariates included patient demographics, comorbidities, and co-medications. Patient demographics included age, gender, place of residence, occupation, and socioeconomic level. A place of residence for each individual was categorized according to the level of urbanization.15 Patients’ occupations were classified into five categories, and their socioeconomic levels were categorized into five specific income quintiles. Age was calculated at the start of every treatment. The place of residence, occupation, and income level were defined at the year of the relevant treatment period.
We analyzed comorbidities to represent individual health status, including malignancy, hypertension, cerebrovascular disease, congestive heart failure, thrombosis, myocardial infarct, peripheral vascular disease, diabetes mellitus, hyperlipidaemia, liver disease, ulcer disease, chronic pulmonary disease, autoimmune disease (psoriasis, ankylosing spondylitis, myasthenia gravis, multiple sclerosis, and rheumatologic disease), dementia, hemiplegia or paraplegia, and psychosis.16 Each comorbidity was evaluated at the start of each treatment period.
The analyses of prescription co-medications included nonsteroid anti-inflammatory drugs (NSAIDs), anticoagulants, aspirin, anticonvulsants, lipid-lowering agents (ie, statins, fibrates, and other), antihypertensives (ie, angiotensin-converting enzyme inhibitors/angiotensin-receptor antagonists, beta blockers, calcium channel blockers, diuretics, and other), nitrates, insulin, other hypoglycemic agents, and vitamin D. We defined patients with co-medication status when they were treated with that specific drug at the relevant period of immunosuppression drug treatment.
Statistical analysis
The Cox proportional hazards model was used to estimate the risk of graft failure among KTRs between immunosuppressant exposure and non-exposure. Hazard ratio (HR) was also adjusted by the aforementioned confounding variables. The counting process model was adopted in this study, and the exposure to immunosuppressant was treated as time varying exposures. We calculated unadjusted and adjusted HRs of the four immunosuppressant types; the exposures in each type were binary variable (use or other immunosuppressant use). We also calculated unadjusted and adjusted HRs in the mutually exclusive combinations group. We conducted two analyses: one analysis was to compare each immunosuppression drug group of patients used with the other three types of immunosuppression drug groups, and the other analysis was to compare each treatment combination group referenced with the corticosteroid alone group. All tests of statistical hypothesis were done on the two-sided 5% level of significance. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of the KTRs
In total, this cohort included 3,441 KTRs who received immunosuppressant treatment for 6 months after kidney transplantation. All cohort patients received prescriptions for at least one immunosuppressant during the follow-up period. Of the cohort, 3,350 KTRs (97.36%) were prescribed corticosteroid, 2,562 KTRs (74.46%) were prescribed calcineurin inhibitors, 3,188 KTRs (92.65) were prescribed purine antagonists, and 1,797 KTRs (52.22%) were prescribed mTORIs (Table 1). We compared the characteristics of KTRs who were prescribed each of the four types of immunosuppressants during follow-up. The comorbidity status was identified at the start of the first treatment period. Co-medication exposure was defined at the first point of treatment of that specific immunosuppression drug. All the baseline characteristics, comorbidities, and co-medication usages among the four groups were similar with no statistical significance.
Comparing immunosuppressants to find relative advantage on graft protection
Immunosuppression is induced for reducing immunologic-related graft loss after organ transplantation. All KTRs in our cohort were given at least one type of immunosuppressant during follow-up. We compared the important outcome associated with the four main categories including, corticosteroid, calcineurin inhibitors, purine antagonists, and mTORIs. The HR of graft failure in KTRs treated with each immunosuppressant type was compared with KTRs prescribed with other types of immunosuppressants. The exposures of immunosuppressant types in Table 2 are not mutually exclusive, and they may include any use of other types as dual, triple, or quadruple therapy. HR was adjusted by multiple covariances such as the use of co-medications and the potential confounders listed in Table 1 to rule out nonimmunologic contributions. The results of adjusted HR showed the overall relative advantages between the different types of immunosuppressants, and they are not indicated to increase graft failure rates. Among the main exposures of interest, purine antagonists were shown to have a significantly superior advantage for reducing 48% of graft loss (adjusted HR: 0.52, 95% confidence interval [CI]: 0.42–0.63) compared with other types (Table 2). Corticosteroid (adjusted HR: 1.67, 95% CI: 1.28–2.21) and mTORIs (adjusted HR: 1.25, 95% CI: 1.00–1.56) showed an inferior protection of graft survival in KTRs.
Comparisons of graft survival associated with different treatment combinations
All treatment strategies can be further broken down in detail into 15 mutually exclusive combinations. Treatment periods included 5.7 million person-years in total. The corresponding adjusted HRs for each different combination were compared with monotherapy with corticosteroid (Table 3). For monotherapy comparison, purine antagonists showed a superior protection of 65% reduction in graft loss compared with corticosteroid (adjusted HR: 0.35, 95% CI: 0.18–0.63). Calcineurin inhibitors decreased 53% of graft loss compared with corticosteroid (adjusted HR: 0.47, 95% CI: 0.19–0.98).
For dual therapy, corticosteroid combined with purine antagonists reduced 48% of graft failure. Calcineurin inhibitors combined with purine antagonists reduced 63% of graft failure. Calcineurin inhibitors combined with mTORIs reduced 74% of graft failure. Purine antagonists combined with mTORIs reduced 67% of graft failure. For triple combinations, only corticosteroid combined with calcineurin inhibitors and purine antagonists reduced 52% of graft failure. Quadruple therapy with a four-drug combination was also shown to reduce graft failure by 43%.
We also further analyzed all the patients throughout the observation period after kidney transplantation. These results included KTRs with acute rejection, chronic rejection and surgical-related mortality, and the results are listed in Tables S1 and S2.
Discussion
As conventional immunosuppressant therapy improves, the 1-year survival rate of kidney grafts has increased from 82.5% to 91.2% due to the reduction of acute rejection.6,7 However, chronic rejection and long-term survival of allograft remain a difficult problem. Chronic rejection is the most common cause of allograft failure in kidney transplantation in recent decades.3 The present study reported the important differences between diverse immunosuppressant combinations and their protective benefits to graft survival against chronic rejection in KTRs after kidney transplant surgery. Many published studies were either clinical trials limited to shorter observation periods and smaller sample sizes, or one that focused on few targeted drugs.17–20 Our cohort study provided the important comparisons of graft protection by different immunosuppressant combinations in KTRs based in a nationwide population. Because KTRs may stay on hemodialysis while waiting for the donated kidney to function in the period right after kidney transplantation, graft failure was defined solely during the period beginning 6 months after kidney transplantation.
Chronic rejection can induce progressive loss of graft function after 3 months posttransplantation, and most KTRs could be histologically proofed of chronic allograft nephropathy. Acute rejection episodes usually occurred within the first 3 months. Some acute rejections that develop after 2 to 6 months have the greatest impact on the risk of chronic rejection.3 To reduce the effects from factors other than immunosuppressants on chronic rejection, such as surgical-related or graft-related confounding bias, we studied the protective effects of immunosuppressants solely in the period beginning 6 months after kidney transplantation, and this research was focused on chronic rejection with less influence of acute rejection.
The protective effects on graft contributed by conventional immunosuppressants including corticosteroid, calcineurin inhibitors, antimetabolite purine antagonists, and mTORIs were compared. Overall, our study indicated that a treatment regimen that incorporated purine antagonists had a comparable reduction of graft failure among the four main drug groups regardless of whether it was monotherapy or in combination (adjusted HR: 0.52, 95% CI: 0.42–0.63) (Table 2). In contrast, corticosteroid and mTORIs showed an inferior protection on chronic rejection among the four targeted classes. Furthermore, an advanced analysis was studied to compare the differences among treatment combinations that were prescribed as monotherapy or multiple therapies with other drugs. We analyzed all arrangements of mutually exclusive treatment combinations using monotherapy with corticosteroid as a reference because it is the most commonly used immunosuppressant (97.36%). Most immunosuppressive protocols for KTRs usually include a large dosage of steroid as the fundamental composite of the regimen. The results of our study indicated that purine antagonists, azathioprine and mycophenolate mofetil, have an advantage on reducing graft loss compared with steroid-based treatment. Purine antagonists showed more protection against chronic rejection no matter whether they were prescribed as monotherapy or multiple combinations, despite adjustments for risk factors at baseline. However, the risk of graft failure when prescribed in combination with purine antagonists is similar to the risk with purine antagonist monotherapy. In the monotherapy analysis, calcineurin inhibitors and purine antagonists showed the superior advantage for graft survival, but mTORIs did not.
Comparing the effects of all dual-therapy combinations, only a calcineurin inhibitor combined with an mTORI and any purine antagonist-based combinations showed a superior advantage on graft survival as compared with steroid-based treatment (Table 3). The dual-therapy regimens of corticosteroid combined with a calcineurin inhibitor (adjusted HR: 1.47, 95% CI: 0.98–2.20) or mTORI (adjusted HR: 1.05, 95% CI: 0.58–1.83) showed no difference of graft protection compared with monotherapy using corticosteroid. We further analyzed the intragroup comparisons in dual- and triple-therapy combinations respectively by regrouping them according to whether they were incorporating with or without corticosteroid (Table S3). In dual therapy groups, the combination without corticosteroid revealed less risk of graft failure then the combination with corticosteroid administration. This result supported the steroid-avoidance strategy and was generally compatible with previous data of intergroup comparisons in Table 3.
In triple-therapy combinations, only corticosteroid combined with a calcineurin inhibitor and purine antagonist was obviously superior to steroid-based treatment (adjusted HR: 0.48, 95% CI: 0.33–0.70), but any other triple-therapy combinations were not. Our findings also showed that the conventional triple immunosuppression protocols markedly improved long-term graft outcome compared with steroid monotherapy. Furthermore, in intragroup analyses of triple therapy, the combination without corticosteroid showed no difference in the risk of graft failure with the combinations with corticosteroid administration (Table S3). In addition, quadruple-therapy combinations also showed better protection on chronic rejection compared with the steroid-based treatment (adjusted HR: 0.57, 95% CI: 0.34–0.94).
KTRs treated with immunosuppressive protocols that include large doses of steroid may be prompted to steroid-related morbidity.21,22 Long-term use of high-dose steroids induces a few serious side effects and steroid-induced morbidity, including hypertension, hyperlipidemia, new-onset diabetes mellitus, cataract, osteoporosis, and cardiovascular events.23 For the sake of minimization of these side effects, strategies for steroid avoidance or rapid discontinuation were advocated and attempted recently.24,25 Infection is the second most prevalent cause of mortality in KTRs with a functioning graft.26 Interestingly, a steroid-avoidance protocol was reported to decrease cytomegalovirus (CMV) infection and steroid-related infections.25 It is desirable to seek a suitable immunosuppressant combination that can decrease infection rate without loss of graft function. However, recent studies have shown conflicting results. Study trials of late steroid minimization in selected KTRs showed an increase of acute rejection and a decrease of graft survival.27–29 Some modified trials rapidly tapered the steroid dosage and stopped within 1 week. The results showed that the acute rejection rate was increased, but no differences in graft or patient survival rates were noted when compared with long-term steroid maintenance.23,30 Moreover, rapid discontinuation of steroid was related to decreases in the rates of cardiovascular events and new-onset diabetes mellitus.31,32 A recently updated review reported that steroid avoidance and withdrawal for KTRs increase the rate of acute rejection but showed no difference in graft and patient survival for 5-year follow-up after kidney transplantation.23 However, the long-term benefits of steroid minimization strategies remain unclear at present. Our study is valuable in providing the long-term evaluations of graft survival with different immunosuppressant combinations. Some steroid-free regimens have lower chronic rejection rates compared with monotherapy with steroids. How best to tailor an effective regimen and plan the timing of withdrawal without compromising efficacy would be an attractive topic for further investigation.
There are some limitations in our study. First, the NHIRD could not provide pathological data of renal biopsy, serum creatinine concentration, or the mean estimated GFR levels. We defined graft failure as the primary outcome, and the definition of diagnosis criteria was the requirement for another kidney transplant surgery or for regular hemodialysis more than 3 months postoperatively. The subclinical progressive fibrosis and transplant vasculopathy in some grafts may be underdiagnosed. Second, human leukocyte antigen matching can avoid a lot of graft rejections and provide a better long-term survival rate.2 However, our data were unable to analyze the degree of histoincompatibility between graft and recipient. Otherwise, the confounding variables were incomplete with few donor or graft factors examined such as donor sex, age, and cold ischemic time of graft which NHIRD could not provide. Third, the use of a particular immunosuppressive agent also introduced a significant degree of clinician’s bias.
Conclusion
We have presented a multitude of immunosuppressant combination options that provide better prevention of chronic rejection and improved graft survival in KTRs. We have also provided some optimal options to achieve an excellent steroid-avoidance regimen in immunosuppression maintenance. Since long-term benefits of steroid minimization strategies remain unclear, our study is valuable to provide the long-term evaluations of graft survival with different immunosuppressant combinations.
Acknowledgments
This study was based in part on data from the NHIRD provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent the views of the Bureau of National Health Insurance, Department of Health nor of the National Health Research Institutes. The authors would like to thank the data management and statistical assistance from the Center for Big Data Analytics and Statistics of the Chang Gung Memorial Hospital, Linkou branch. This work was supported by the National Science Council of Taiwan (project NMRPG3E0241) and Chang Gung Memorial Hospital (project CORPG3E0132) and was supported by the University of Nottingham in methodology and infrastructure.
Disclosure
The authors report no conflicts of interest in this work.
References
Chapman JR. What are the key challenges we face in kidney transplantation today? Transplant Res. 2013;2(Suppl. 1):S1. | ||
Bhatti AB, Usman M. Chronic renal transplant rejection and possible anti-proliferative drug targets. Cureus. 2015;7(11):e376. | ||
Joosten SA, Sijpkens YW, van Kooten C, Paul LC. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 2005;68(1):1–13. | ||
Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, Mclntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–612. | ||
Tsai YF, Chen HP, Liu FC, et al. Nationwide population-based study reveals increased malignancy risk in taiwanese liver transplant recipients. Oncotarget. 2016;7(50):83784–83794. | ||
Mayer AD, Dmitrewski J, Squifflet JP, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64(3):436–443. | ||
Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63(7):977–983. | ||
Gheith OA, Bakr MA, Fouda MA, et al. Steroid and azathioprine versus steroid, cyclosporine, and azathioprine therapies in primary haplo-identical living donor kidney transplantation: twenty-year experience. Iran J Kidney Dis. 2008;2(1):34–39. | ||
Weir MR, Ward MT, Blahut SA, et al. Long-term impact of discontinued or reduced calcineurin inhibitor in patients with chronic allograft nephropathy. Kidney Int. 2001;59(4):1567–1573. | ||
Gallon L, Perico N, Dimitrov BD, et al. Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. Am J Transplant. 2006;6(7):1617–1623. | ||
Lim MA, Kohli J, Bloom RD. Immunosuppression for kidney transplantation: where are we now and where are we going? Transplant Rev (Orlando). 2017;31(1):10–17. | ||
Wong W, Venetz JP, Tolkoff-Rubin N, Pascual M. 2005 immunosuppressive strategies in kidney transplantation: which role for the calcineurin inhibitors? Transplantation. 2005;80(3):289–296. | ||
National Health Insurance Administration Ministry of Health and Welfare (NHIA) Organization [webpage on the Internet]. 2016. Available from: https://www.nhi.gov.tw/english/Content_List.aspx?n=EF2C14B2B87D7E2E&topn=ED4A30E51A609E49 | ||
Yu ST, Chang HY, Lin MC, Lin YH. Agreement between self-reported and health insurance claims on utilization of health care: a population study. J Clin Epidemiol. 2009;62(12):1316–1322. | ||
Liu CY, Hung YT, Chuang YJ, Chen YJ, Weng WS, Liu JS. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manage. 2006;4(1):1–22. | ||
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. | ||
Gheith OA, Bakr MA, Fouda MA, Shokeir AA, Sobh M, Ghoneim M. Prospective randomized study of azathioprine vs cyclosporine based therapy in primary haplo-identical living-donor kidney transplantation: 20-year experience. Clin Exp Nephrol. 2007;11(2):151–155. | ||
Noreikaite A, Saint-Marcoux F, Marquet P, Kadusevicius E, Stankevvicius E. Influence of cyclosporine and everolimus on the main mycophenolate mofetil pharmacokinetic parameters: cross-sectional study. Medicine (Baltimore). 2017;96(13):e6469. | ||
Qazi Y, Shaffer D, Kaplan B, et al. Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data. Am J Transplant. 2017;17(5):1358–1369. | ||
Thierry A, Le Meur Y, Ecotiere L, et al. Minimization of maintenance immunosuppressive therapy after renal transplantation comparing cyclosporine A/azathioprine or cyclosporine A/mycophenolate mofetil bitherapy to cyclosporine A monotherapy: a 10-year postrandomization follow-up study. Transpl Int. 2016;29(1):23–33. | ||
Citterio F. Steroid side effects and their impact on transplantation outcome. Transplantation. 2001;72(12 Suppl):S75–S80. | ||
Vanrenterghem YF, Claes K, Montagnino G, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85(2):209–216. | ||
Haller MC, Royuela A, Nagler EV, Pascual J, Webster AC. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2016;(8):CD005632. | ||
Rizzari MD, Suszynski TM, Gillingham KJ, et al. Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol. 2012;7(3):494–503. | ||
Thierry A, Mourad G, Buchler M, et al. Steroid avoidance with early intensified dosing of enteric-coated mycophenolate sodium: a randomized multicentre trial in kidney transplant recipients. Nephrol Dial Transplant. 2012;27(9):3651–3659. | ||
Kahwaji J, Bunnapradist S, Hsu JW, Idroos ML, Dudek R. Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation. 2011;91(2):225–230. | ||
Kasiske BL, Chakkera HA, Louis TA, Ma JZ. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol. 2000;11(10):1910–1917. | ||
Pascual J, Quereda C, Zamora J, et al. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: a meta-analysis of randomized, controlled trials. Transplantation. 2004;78(10):1548–1556. | ||
Vanrenterghem Y, Lebranchu Y, Hene R, Oppenheimer F, Ekberg H. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation. 2000;70(9):1352–1359. | ||
Luan FL, Steffick DE, Ojo AO. Steroid-free maintenance immunosuppression in kidney transplantation: is it time to consider it as a standard therapy? Kidney Int. 2009;76(8):825–830. | ||
Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89(1):1–14. | ||
Luan FL, Steffick DE, Ojo AO. New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation. 2011;91(3):334–341. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







